Introduction: Protein aggregation is a process by which misfolded proteins polymerizes into aggregates and form fibrous structures with a β-sheet conformation of misfolded proteins, known as amyloids. It leads to an array of effects, including mitochondrial dysfunction and cell death, and can trigger serious neurodegenerative conditions[1]. Over the years, a large number of compounds have been used to prevent aggregation, but with minimal success[2]. Moreover, polymers, especially synthetic polymers, have not been employed a lot for such work with high efficiencies. In this study, I have prepared a zwitterionic polymer, poly-sulfobetaine (poly-SPB), with varying degrees of hydrophobicity and molecular weights. Zwitterionic polymers have both positive and negative charges linearly arrayed on the same repeating unit of the polymer backbone and as such displays unique and excellent properties. Poly-SPB showed remarkable efficiency against protein aggregation and was found to preserve protein’s secondary structure. Poly-SPB was then transformed into both self-assembled nanogel and core-shell nanogel and it was seen that formation of nanogel enhanced its efficiency manifold.
Materials and Methods: Polymers were synthesized by reversible addition fragmentation chain transfer (RAFT) polymerization and polymers with different molecular weights and functionalities were prepared[3]. Various analyses were carried out to investigate protein (lysozyme was used as model protein) aggregation inhibition; like visual and spectrophotometric analysis, suppression of amyloid formation, residual enzyme activity and effect on the protein’s secondary structure by NMR, infrared and circular dichroism spectroscopy. Nanogel was synthesized by using a radical cross-linker and poly-SPB. Efficiency of the nanogel was compared with that of the linear polymer.
Results and Discussion: Protein aggregation studies revealed that poly-SPB has extremely high efficiency against protein aggregation with enzyme retaining more than 85% efficiency, even after prolonged heat treatment. Transmission electron microscopy images revealed the formation of amyloid fibrils on heating, which was then quantitatively analysed by employing Thioflavin T. The results demonstrated that poly-SPB effectively inhibits the formation of amyloid fibrils and its efficiency was found to be higher than some of the commercially available reagents also. Conformational analysis revealed that poly-SPB stabilizes protein and preserves its higher-order structure (Fig. 1).
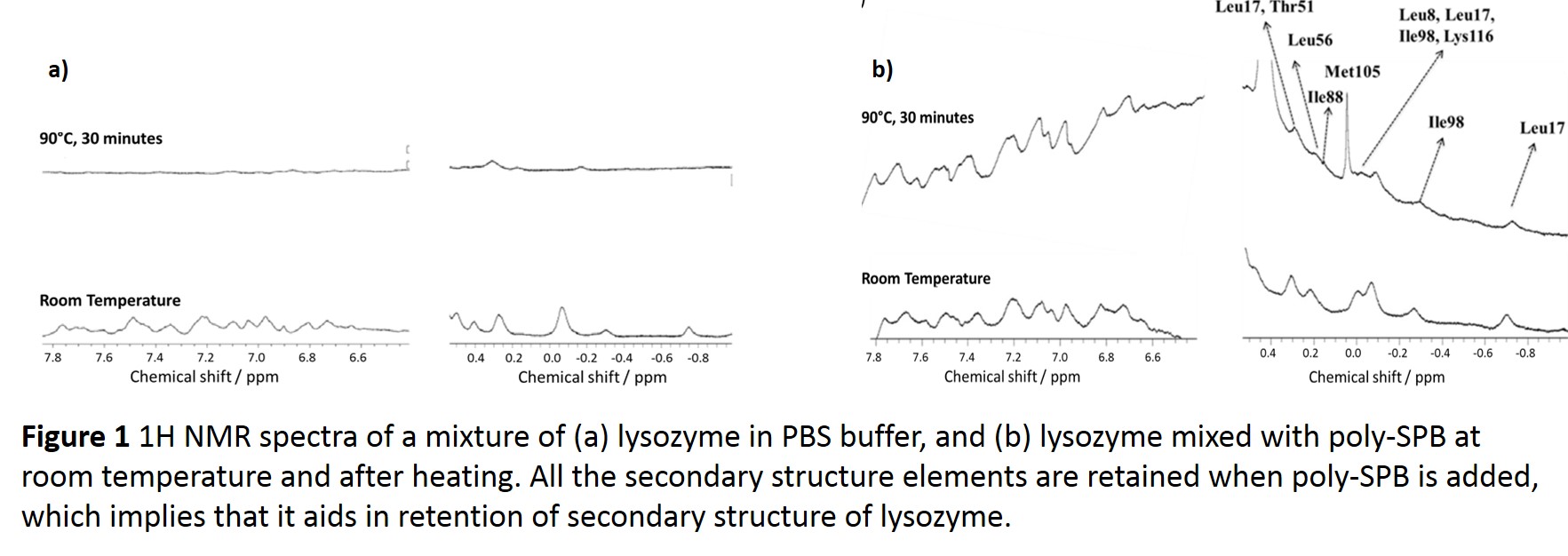
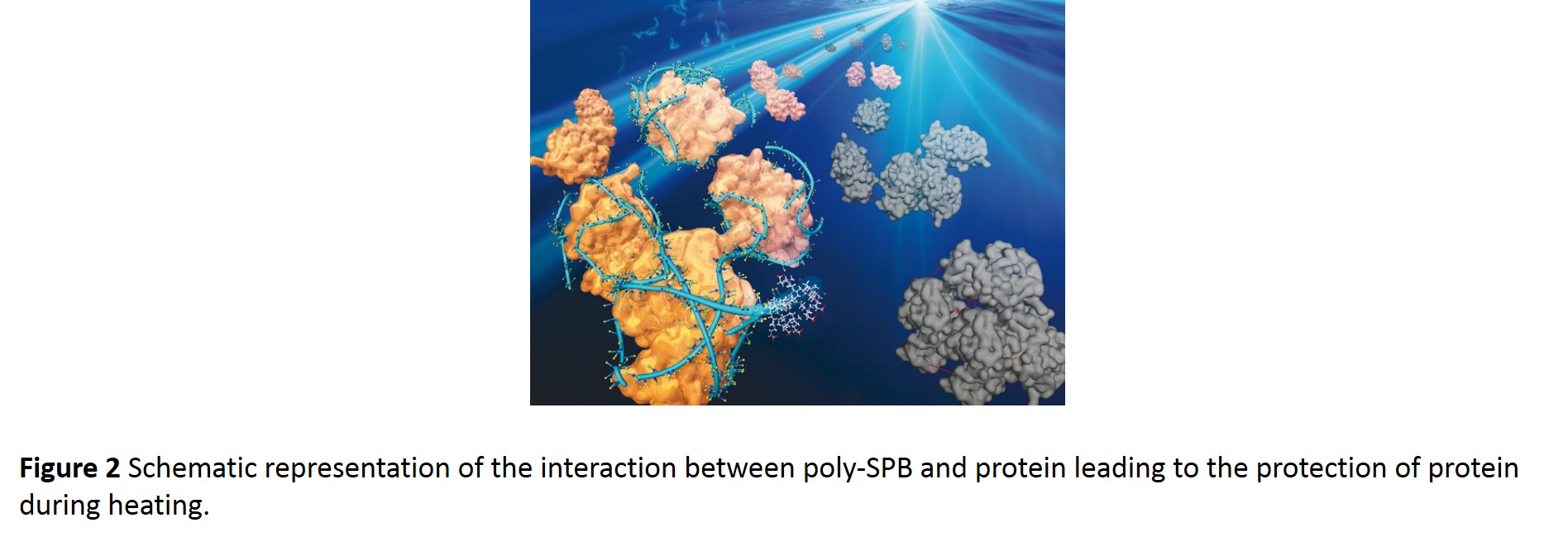
Poly-SPB shows weak and reversible interaction with proteins and thus acts as a molecular shield, reducing collisions between aggregating species and maintaining the water structure; thereby preventing aggregation (Fig. 2).
The formation of poly-SPB nanogel significantly increased its activity with much lower concentration required for aggregation inhibition; suggesting it acts as molecular chaperones. Introduction of hydrophobicity to the nanogel resulted in increased activity.
Conclusions: Zwitterionic polyampholytes are efficient inhibitors of thermal aggregation of protein. Polymer backbone is extremely active against protein aggregation, it inhibits amyloid formation and stabilizes the protein’s secondary structure by acting as molecular shields. Formation of nanogel induced additional activity, higher efficiency as well as more biocompatibility.
This study was supported in part by a Grant-in-Aid, KAKENHI (25870267) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References:
[1] C.A. Ross and M.A. Poirier, "Protein aggregation and neurodegenerative disease," Nature Medicine. Vol. 10, 2004.
[2] H. Yamaguchi and M. Miyazaki, "Refolding Techniques for Recovering Biologically Active Recombinant Proteins from Inclusion Bodies," Biomolecules. Vol. 4, 2014
[3] R. Rajan and K. Matsumura, "A zwitterionic polymer as a novel inhibitor of protein aggregation," Journal of Materials Chemistry B, Vol. 3, 2015.