Introduction: Development of translatable, synthetically engineered hydrogels for percutaneous delivery remains challenging in part due to crosslinking kinetics (i.e. too fast can clog delivery device, too slow leads to material dispersion prior to crosslinking). To address this, we utilize supramolecular assembly to localize hydrogels at the injection site with subsequent covalent crosslinking to achieve desired biophysical properties to limit left ventricular (LV) remodeling and mitral regurgitation (MR) following myocardial infarct (MI).
Materials and Methods: Hyaluronic acid (HA) was modified by adamantane (Ad-HA) or β-cyclodextrin (CD-HA) and mixed to form guest-host (GH) hydrogels. For dual-crosslinking (DC), thiolated Ad-HA and methacrylated CD-HA were prepared by sequential reaction with all modifications at approximately 25%[1]. In vitro, degradation was monitored by uronic acid assay and elastic modulus (E) determined in compression (10%/min) for DC and in shear (1% strain, 1.6 Hz) for GH. Infarct (10-20% of LV, posterolateral) was induced in sheep by coronary ligation. Animals received 16 injections (0.3 mL ea.) of saline, GH, or DC hydrogel (4.5 wt%) into the infarct (Fig 1A). Outcomes were measured by echocardiographic MR grading (0, 8 wk) and MRI (0, 2, 8 wk) post-MI (n≥6 per group).
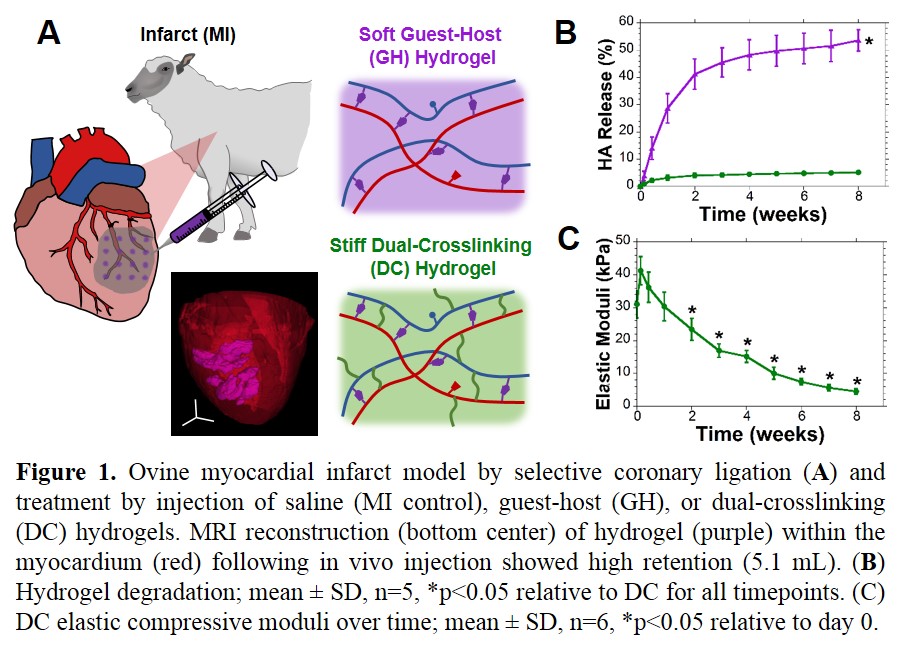
Results: GH hydrogels were observed to degrade more rapidly than DC hydrogels (Fig 1B). GH hydrogels were initially soft (E = 800 Pa), not measured serially due to erosion. DC hydrogels were also initially soft, enabling injection within 12 hr of hydrogel formation (retained at 4°C, pH 5) but attained moduli of 41.2±4.3 kPa within one day at physiological conditions with progressive softening (Fig 1C). Following injection in vivo, MRI showed high retention (Fig 1A). Between treatment groups, no differences were observed in baseline infarct size, MR, or MRI analysis. Longitudinal examination of infarct thickness showed improvements with hydrogel treatment (MI: 3.4 ± 0.2, GH: 5.5 ± 0.2, DC: 10.0 ± 0.8 mm) at 8 wk. LV volume progressively increased and ejection fraction decreased in MI, but were improved by DC treatment (Fig 2A,B). Progression of MR was arrested by DC treatment, relative to untreated MI (Fig 2C).
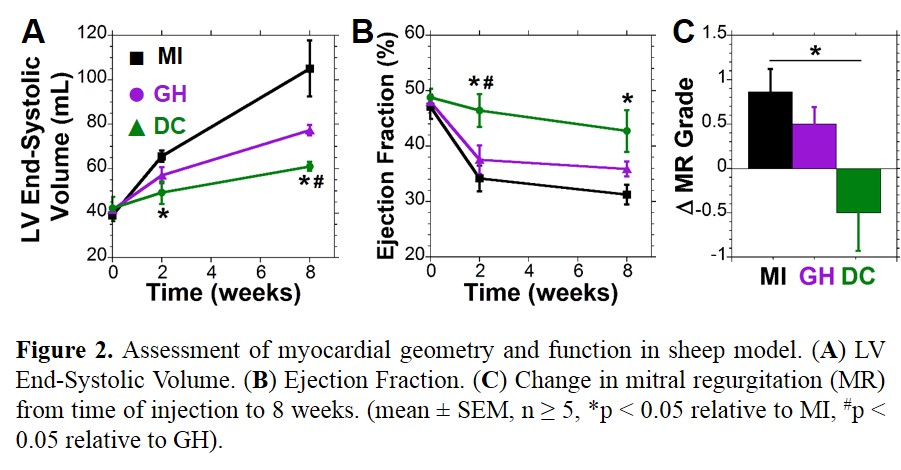
Discussion: DC hydrogels were investigated for their ability to induce mechanical stabilization following MI, with GH serving as a soft material control. Longitudinal analysis by MRI showed preservation of myocardial thickness, LV volume, and function indicating prevention of LV remodeling. Moreover, MR typically associated with such posterolateral MI was abated through hydrogel treatment—likely a result of wall bulking and displacement of the papillary[2].
Conclusions: Results demonstrate the capacity for DC hydrogels to be delivered to MI with retention by supramolecular assembly and covalent crosslinking on clinical timescales. Further, hydrogels were employed in a large animal model of MI with positive results toward prevention of LV remodeling and corresponding functional improvement.
Financial support provided by NIH Grant R01HL111090, a Predoctoral Fellowship (C.B.R.) and Established Investigator Award (J.A.B.) from the American Heart Association.
References:
[1] Rodell, CB et al. Advanced Functional Materials. 2015.
[2] Wenk, JF et al. Annals of Thoracic Surgery. 2010.