Study of in situ forming sulfobutyl ether β-cyclodextrin/self-assembling peptide hydrogel and its release profile of dexamethasone in vitro
-
1
University of Alberta, Department of Chemical and Materials Engineering, Canada
-
2
National Research Council (Canada), National Institute for Nanotechnology, Canada
Introduction: The self-assembling peptide (RADA)4 is capable of forming bioactive scaffolds in situ for different biomedical applications. However, a universal way to control the delivery small hydrophobic drugs (ie. anti-inflammatory, anti-cancer, anti-bacterial drugs etc.) while maintaining a hydrogel structure is rarely reported. Cyclodextrins (CDs) have a relatively nonpolar cavity and a hydrophilic exterior, and have been used for solubilizing hydrophobic drugs in pharmaceutical applications for many years. Sulfobutyl ether β-cyclodextrin (SBE-β-CD; Captisol®) is a derivatized form of β-cyclodextrin with a range of six to seven sulfobutyl ether groups. In this work, the influence of SBE-β-CD on (RADA)4 peptide nanofiber conformation and the thermodynamic interactions between each molecule have been determined. A new strategy of using anionic SBE-β-CD as carrier to load hydrophobic drug in the peptide self-assembly nanofiber system is investigated.
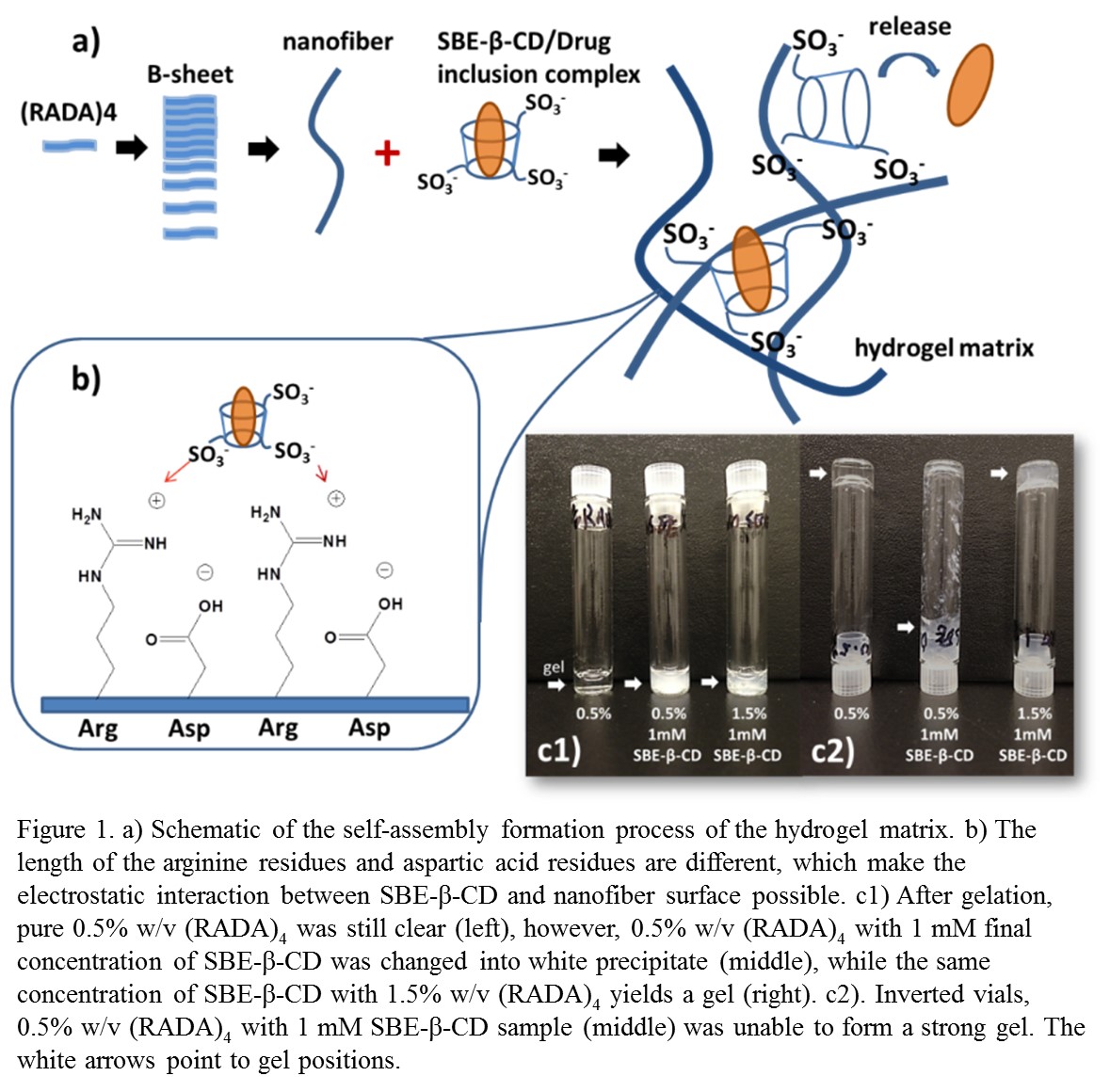
Material and Methods: (RADA)4 peptide (≥95% purity) was purchased from RS Synthesis (USA). SBE-β-CD was donated by Ligand Pharmaceuticals, Inc. (USA). β-cyclodextrin (β-CD) and Dexamethasone (Dex) was purchased from Sigma (USA).
The influence of SBE-β-CD on peptide’s secondary structure and hydrogel formation was evaluated by AFM, circular dichroism (CD) and zeta potential analyzer. The binding affinities between model drug Dex, SBE-β-CD, and (RADA)4 was characterized using isothermal titration calorimetry (ITC). In vitro release of Dex from SBE-β-CD/(RADA)4 hydrogel was measure using UV-Vis.
Results and Discussion: The ionic interaction between SBE-β-CD and (RADA)4 peptide dramatically affects the nanofiber formation and subsequent gel stability; where gel stability is related to the SBE-β-CD/(RADA)4 ratio. Excessive amounts of SBE-β-CD seem to reduce the β-sheet formation of peptide, resulting in a white precipitation instead of transparent hydrogel. The ITC results suggest that the dissociation of SBE-β-CD/Dex inclusion complexes dominate the overall release rate. The different concentration of SBE-β-CD and (RADA)4 peptide significantly affect the release kinetics. Generally, higher concentration of both SBE-β-CD and (RADA)4 peptide is supposed to perform a slower release of drug. This observed phenomenon may related to the nanofiber cross-linking structure.
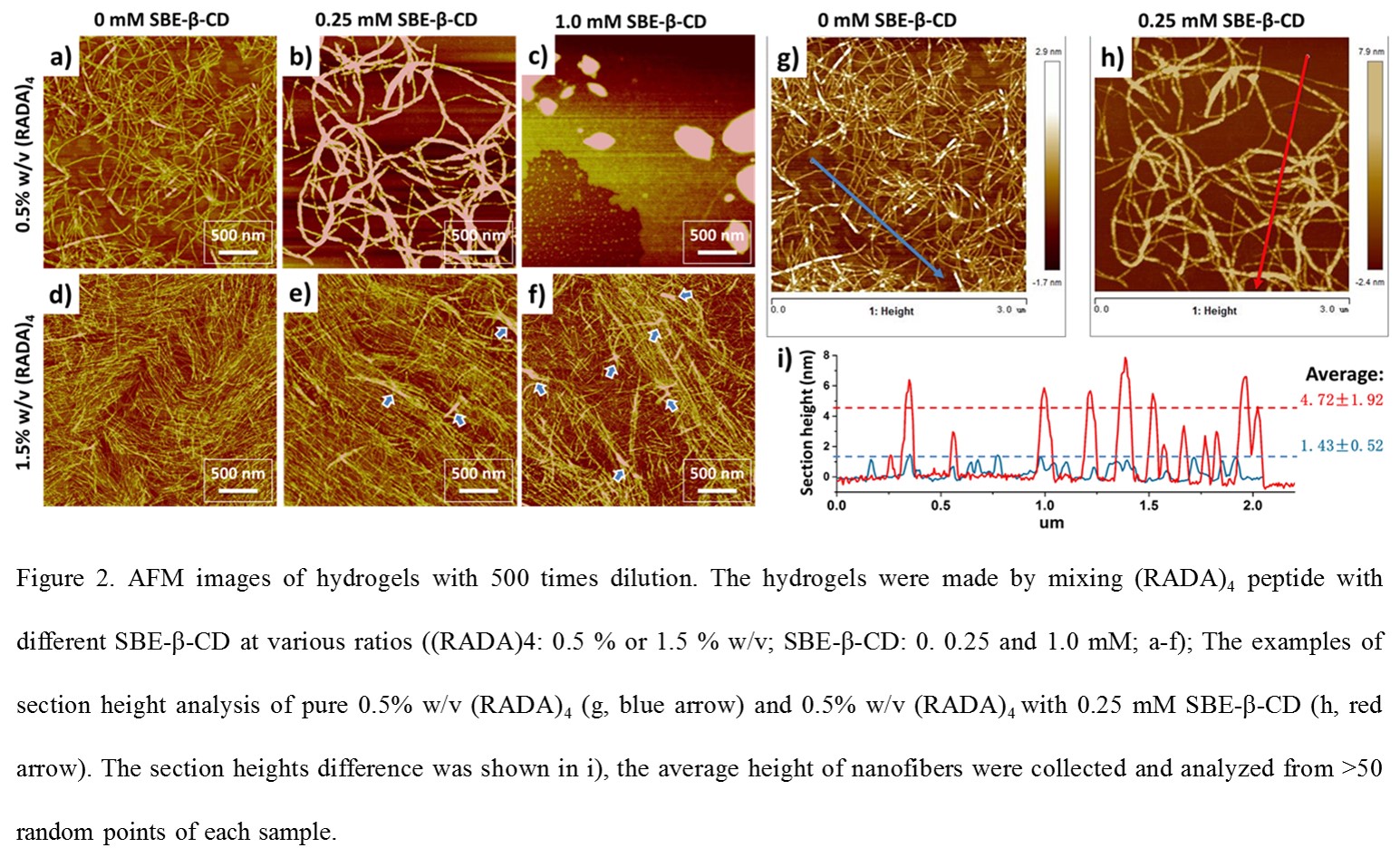
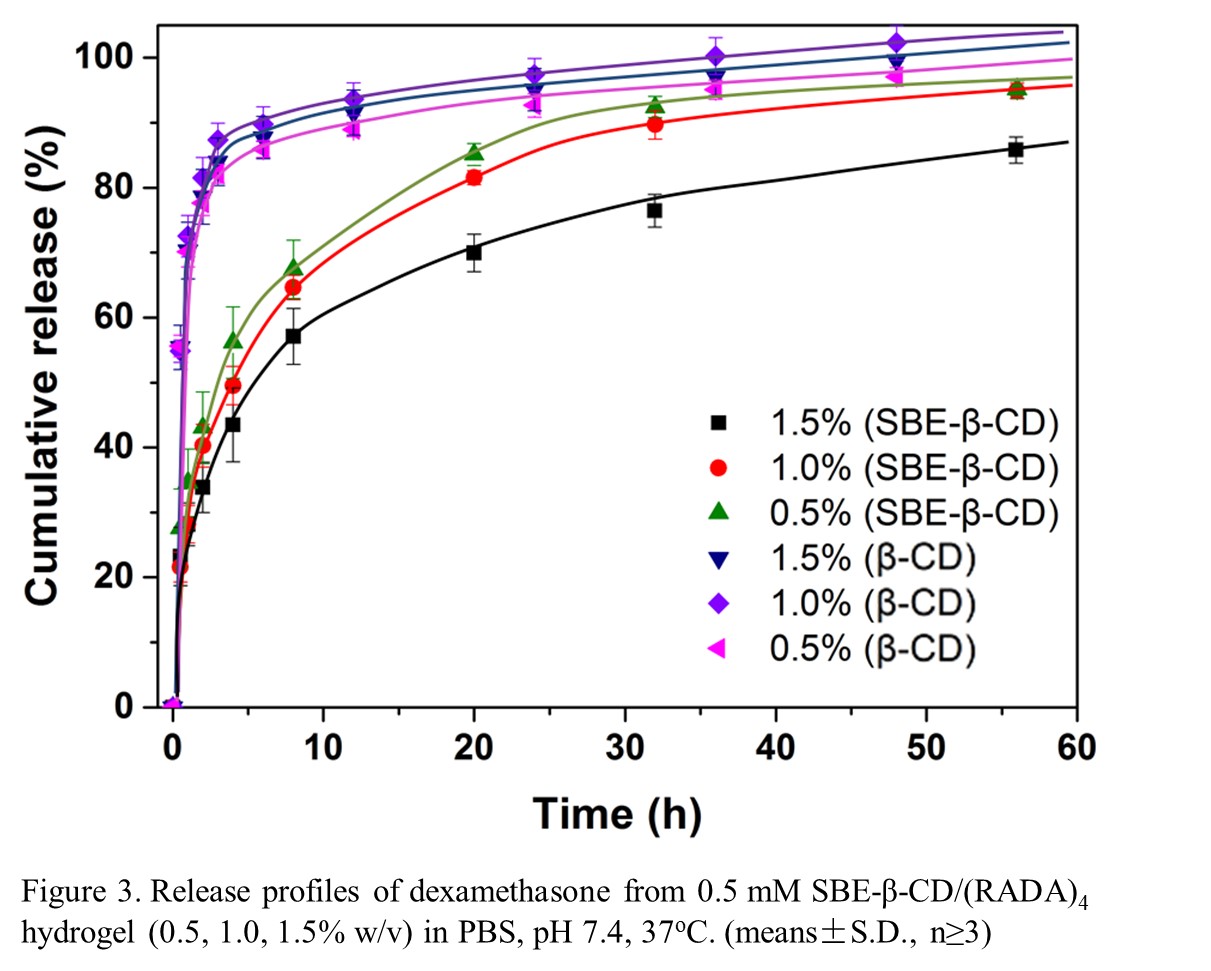
Conclusion: This work discussed the basis of the SBE-β-CD/self-assembling peptide based in situ hydrogel and leading to application prospects of other hydrophobic drugs delivery, such as anti-cancer or anti-infection therapies.
China Scholarship Council (CSC)
Keywords:
self-assembly,
Scaffold,
delivery,
gel
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Extracellular matrices for therapeutic delivery
Citation:
Lu
L and
Unsworth
LD
(2016). Study of in situ forming sulfobutyl ether β-cyclodextrin/self-assembling peptide hydrogel and its release profile of dexamethasone in vitro.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01768
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Lei Lu, University of Alberta, Department of Chemical and Materials Engineering, Edmonton, AB, Canada, Email1
Dr. Larry D Unsworth, University of Alberta, Department of Chemical and Materials Engineering, Edmonton, AB, Canada, larry.unsworth@ualberta.ca