Echinoderm connective tissue as a dynamic mechanically responsive biomaterial: insights from synchrotron small-angle X-ray scattering with in situ tensile testing
-
1
Queen Mary University of London, School of Engineering and Materials Science, United Kingdom
-
2
Queen Mary University of London, School of Biological and Chemical Sciences, United Kingdom
-
3
Diamond Light Source Ltd., Beamline I22, United Kingdom
-
4
European Synchrotron Radiation Facility, Beamline ID02, France
Introduction: Mutable collagenous tissue (MCT) of echinoderms is a remarkable example of a biological material which has the unique property of being able to change its stiffness and extensibility within an extremely short time scale[1] via neural mechanisms[2]. The precise mechanisms are not well understood at the nanoscale, although they are believed to involve noncollagenous proteins such as tensilin and stiparin[1]. Using synchrotron small-angle X-ray scattering to probe fibrillar structure during in situ tensile testing of MCT, we investigate the fibrillar mechanics of chemically-induced stiffened and softened sea cucumber dermis.
Materials and Methods: Body-wall tissue from the black sea cucumber (Holothuria leucospilota) was dissected, retaining the collagenous part, for tensile testing combined with X-ray scattering. To induce the normal, stiff and soft state in the tissue, samples were incubated in artificial seawater (ASW), high potassium [K+] artificial seawater (KASW), and calcium-free artificial seawater (CaF-ASW). Samples were tested in a custom-built tensile tester installed in the ID02 beamline (European Synchrotron Radiation Facility, Grenoble, France). Simultaneous with tensile testing of the sea-cucumber tissue to failure, SAXS images were acquired (wavelength 0. 995 Å) with a FReLoN detector every 0.5-seconds. The fibril D-period was obtained by fitting the 5th order meridional peak in the radial SAXS intensity profile I(q)[3],[4]. Fibril strain εF was measured from percentage shifts in D-period from its unstressed value. The direction and degree of fibril orientation was obtained from the angular SAXS intensity profile of the 5th order meridional peak.
Results and Discussion: Macroscopically, CaF-ASW treated samples had ~4 times lower maximum stress compared to ASW while KASW had ~4 times higher (Figure 1). Concurrently, the maximum fibril strain developed in each state is highly different, with the fibrils in CaF-ASW having much lower extension (~0.16%) compared to the ASW (~0.60%), and the KASW much higher (~1.0%) (Figure 1). When taken together with the larger absolute tissue strain in CaF-ASW, we see that the εF/εT is much smaller in the softened state. Furthermore, fibrils undergo a pronounced reorientation into the loading direction (Figure 2: a,b), characterized by a narrowing of the width of the I(χ) profile and increase in peak intensity. The rate of reorientation is much slower for the CaF-ASW softened tissue.
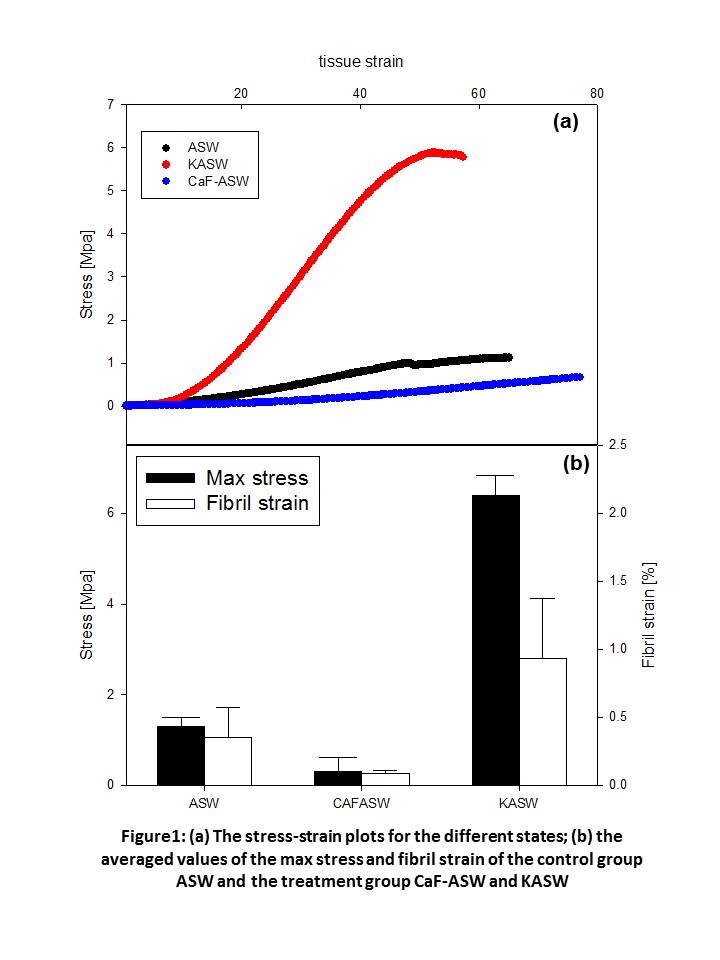

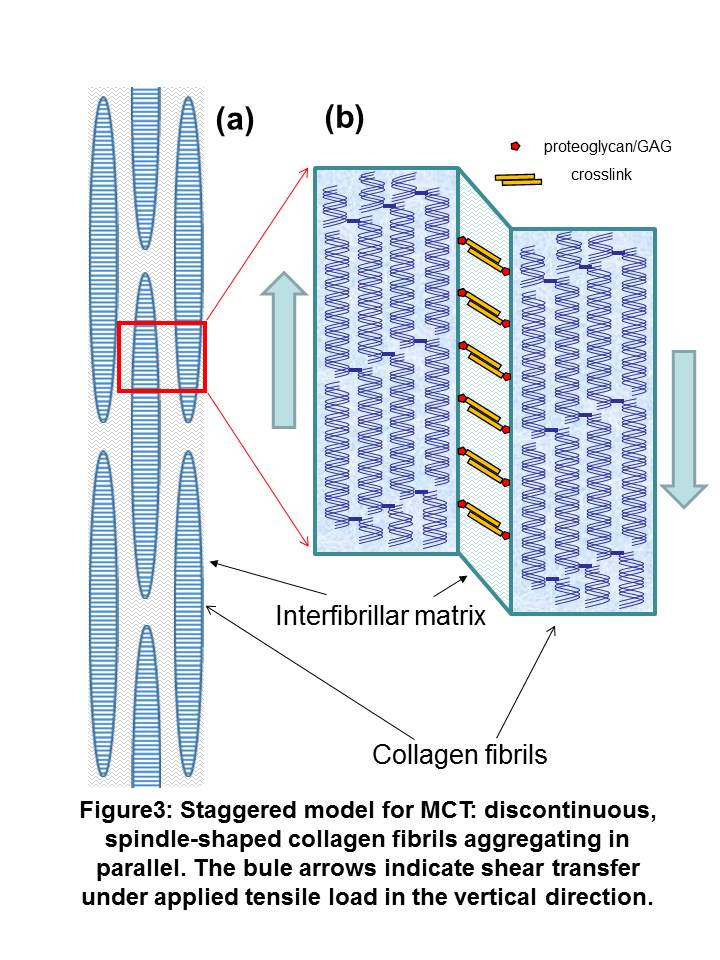
Conclusion: Insight into how the altered fibrillar mechanics observed can explain mutability can be obtained by considering MCT at the nanoscale to consist of collagen fibrils separated by a hydrated interfibrillar matrix of proteoglycans and noncollagenous proteins (Figure 3). Tensile strains in fibrils developed by shear-transfer in the interfibrillar matrix. The altered maximum fibril strains in KASW and CaF-ASW treated MCT suggest that the main mechanism for mechanical mutability in MCT is an alteration of the degree of crosslinking and shear stiffness in the interfibrillar matrix rather than a change in the properties of the collagen fibrils themselves. Our results will help in understanding the structure-function relations of MCT, and can serve as a source for designing new dynamic biomaterials, which would have potential applications in biomedical engineering.
References:
[1] Wilkie I. Mutable collagenous tissue: overview and biotechnological perspective. Echinodermata: Springer; 2005. p. 221-50.
[2] Barbaglio A. The mechanically adaptive connective tissue of echinoderms: Its potential for bio-innovation in applied technology and ecology. 2011.
[3] Gupta H, Seto J, Krauss S, Boesecke P, Screen H. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. Journal of structural biology. 2010;169(2):183-91.
[4] Screen H, Seto J, Krauss S, Boesecke P, Gupta H. Extrafibrillar diffusion and intrafibrillar swelling at the nanoscale are associated with stress relaxation in the soft collagenous matrix tissue of tendons. Soft Matter. 2011;7(23):11243-51.
Keywords:
Tissue Engineering,
hierarchy,
biomaterial
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Mechanical properties of biomaterials
Citation:
Mo
J,
Elphick
MR,
Egertová
M,
Blowes
L,
Terrill
N,
Prévost
S and
Gupta
HS
(2016). Echinoderm connective tissue as a dynamic mechanically responsive biomaterial: insights from synchrotron small-angle X-ray scattering with in situ tensile testing.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01793
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Maurice R Elphick, Queen Mary University of London, School of Biological and Chemical Sciences, London, United Kingdom, m.r.elphick@qmul.ac.uk
Dr. Nick Terrill, Diamond Light Source Ltd., Beamline I22, Oxfordshire, United Kingdom, nick.terrill@diamond.ac.uk
Dr. Sylvain Prévost, European Synchrotron Radiation Facility, Beamline ID02, Grenoble, France, sylvain.prevost@esrf.fr
Dr. Himadri S Gupta, Queen Mary University of London, School of Engineering and Materials Science, London, United Kingdom, h.gupta@qmul.ac.uk