Introduction: Hyaluronic acid (HA) is one of the major glycosaminoglycans (GAG) of the extracellular matrix consisting of the β-(1-4)-linked disaccharide D-glucuronyl-β-(1-3)-N-acetyl-D-glucosamine as repeating unit (Fig. 1). Implants coated with sulfated HAs show improved healing of dermal wounds and regeneration of bone[1]-[3]. Biological activities of sulfated GAGs are exerted by the binding of the sulfated carbohydrates to numerous mediator proteins including growth factors, enzymes, and enzyme inhibitors. While polymeric GAGs have been studied broadly, little is known about binding affinities of sulfated GAG oligosaccharides, molecular structures of GAG-protein complexes[4], and the precise structure-activity relationships of sulfated GAGs. Thus, we have developed oligo-HA probes with defined sulfation patterns and with suitable labels for fluorescent imaging, anisotropy measurements, paramagnetic probes and protein pull-down studies. Aim of the study was the identification of defined sulfated oligo-HAs for the construction of rationally improved artificial extracellular matrices (aECM).
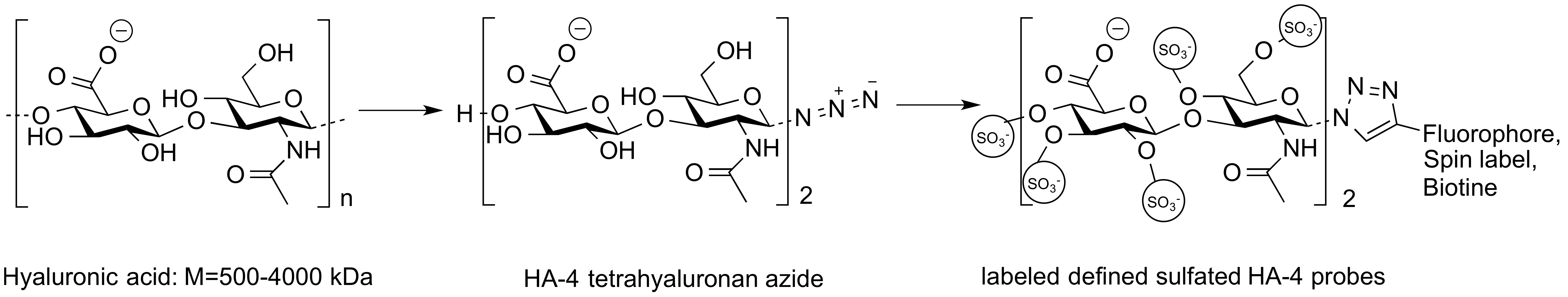
Figure 1: Defined sulfated oligo-HA probes.
Materials and Methods: Tetra- and hexa-HAs (HA-4 and HA-6) were synthesized on gram scale applying a newly developed chemo-enzymatic protocol. The reducing end was fixed as a glycosyl azide, which was subsequently employed in anomeric ligation reactions for the attachment of various labels to furnish fluorescent, metal-chelating, and biotinylated probes using dipolar cycloaddition (“click reaction”). Labeled HA-4 probes were prepared with different defined sulfation patterns (sHA-4) from the 6,6´-disaccharide to the nona-saccharide. Binding of defined sHA-4 probes to proteins was determined by fluorescent anisotropy titration and by surface plasmon resonance furnishing directly the respective KD values. A spin-labeled sHA-4 probe was used for structure elucidation of protein complexes using paramagnetic resonance enhancement NMR. sHA-4 probes were also investigated in functional assays in cells and a biotinylated, sHA-4 probe was used for proteome-wide analysis of their interaction partners by protein pull-down and MS/MS analysis.
Results and Discussion: Defined sHA-oligosaccharides, which become readily accessible through the developed protocols, were found to bind to numerous mediator and regulatory proteins with KD values in the nM to µM range. Binding depended critically on the sulfation pattern: in some instances the binding of sHA-4 was even superior to that of sulfated HA-polysaccharides. sHA-4-EDTA enabled the elucidation of its complex with interleukin-10 via paramagnetic resonance enhancement NMR, sHA-4 rhodamine enabled the cellular imaging of the probe and finally, sHA-4 azide was found to be active in a cellular model of wound healing.
Conclusion: Defined sulfated HA-oligosaccharides such as sHA-4 are the active components of sulfated GAG polymers, and are essential for the potent recognition of proteins and thus for sequestering proteins to the wound healing site. These oligosaccharides are promising candidates for the construction of rationally tailored biomaterials, which improve and support healing of injured skin and bones.
References:
[1] van der Smissen A et al., Biomaterials. 32:8938-8946, 2011.
[2] Salbach, J et al., Biomaterials. 33: 8418-29, 2012.
[3] Hintze, V et al., Biomacromolecules, 10: 3290-3297, 2009.
[4] Pichert, A et al., Glycobiology, 22: 134-45, 2012.