Introduction: Nitric oxide (NO) is an intracellular and intercellular messenger that plays an important role in cellular events in physiological and pathophysiological processes [1]. Macrophages produce large amount of NO as a pro-inflammatory marker compared with other cells. Non-invasive detection system of NO is highly required to realize an early therapeutic treatment considering the process of pathophysiological changes. Diaminorhodamine (DAR)-4M is one of the sensitive NO-detecting fluorescent dyes, but the in vivo use is hampered by the low molecular weight and water-insolubility. It has been reported that the hydrophobic derivatives of gelatin are effective in water-solubilization of water-insoluble and low-molecular-weight drugs and enhancing their biological activity [2]. Therefore, in this study, the water-solublization of DAR-4M by the hydrophobic derivative of gelatin was attempted to design an imaging agent for NO detection.
Materials and Methods: Various amounts of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) with an active ester were reacted to gelatin with an isoelectric point of 5.0 and the weight-average molecular weight of 10,000 to obtain DSPE-grafted gelatin derivatives (DSPE-g-gelatin) with different grafting extents. DMSO solution containing DSPE-g-gelatin and DAR-4M was dialyzed against double-distilled water and purified by centrifugation to obtain the DAR-4M water-solubilized by DSPE-g-gelatin micelle (DAR-4M micelle).
A mouse model of acute interstitial nephritis was induced by the intraperitoneal injection of aristolochic acid (AA) [3]. DAR-4M micelle (DAR-4M concentration: 10 mM) was intravenously injected to the mice 1, 4, 7, 14, 21, and 35 days after AA injection. The mice were sacrificed and the inflamed kidneys were isolated 1 hr after micelle injection. Fluorescence imaging of kidneys was performed with the IVIS Spectrum.
Results: The amount of DAR-4M water-solubilized in the micelles became larger with an increase in the extent of DSPE introduced. The apparent size of DAR-4M micelle was almost 100 nm. Fluorescent intensity in the kidneys of AA-induced mice injected with the DAR-4M micelle was higher than that of normal mice and injected with free DAR-4M and the extent became large with time after AA induction (Figure 1).
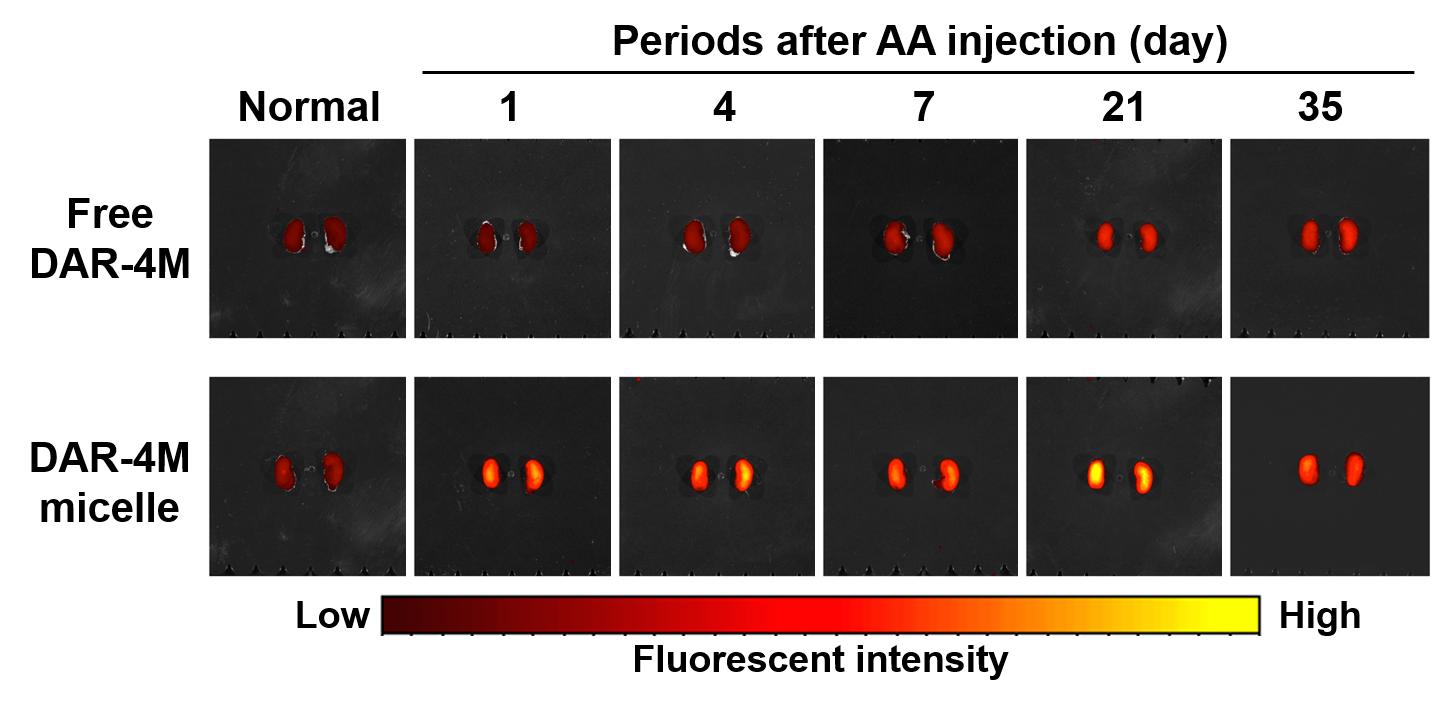
Figure 1. Fluorescent images of isolated kidneys of mice 1 hr after intravenous injection of free DAR-4M or the micelle prepared from DSPE-10. Mice were induced with or without AA and kept for 1, 4, 7, 21, 35 days.
Discussion: Efficient visualization of inflamed kidney by the intravenous injection of the DAR-4M micelle can be explained as follows. The DAR-4M water-solubilized by DSPE-g-gelatin would be effectively delivered to the inflamed kidney. Then, the enough amount of DAR-4M would react with NO produced by macrophages in the inflamed kidney. This explanation is supported by the experimental result that the number of macrophages increased with time for nephritis mice induced with AA.
Conclusion: In vivo delivery and ex vivo imaging of a water-insoluble fluorescent probe were achieved through the water-solubilization with the micelle formation of DSPE-g-gelatin.
References:
[1] Murad F. Angew Chem Int Edit. 1999;38:1857-68.
[2] Tanigo T, et al. Journal of controlled release2010;143:201-6.
[3] Sato N. et al. The Journal of pharmacy and pharmacology. 2004;56:221-9.