Introduction: Recent studies reveal many biomaterials can activate immune and inflammatory pathways even in the absence of other immune cues[1],[2]. However, if and how this activation is altered as polymeric carriers degrade has not been studied. Poly(beta-amino esters) (PBAEs) are a class of rapidly degrading cationic polymers being leveraged as vaccines carriers that we used to study how polymer form (e.g., soluble/free, particle) and extent of degradation influence immunogenicity of these carriers in cells and mice.
Materials and Methods: A prototypical PBAE, Poly1, was synthesized. Following degradation in buffer, molecular weight (MW) was determined by GPC. Poly1 particles were formed by condensation with anionic polymer to mimic a common DNA vaccine modality. Particles were assembled from intact Poly1 and degraded, or, for mechanistic studies, from pre-degraded Poly1 with fragments of distinct MWs. Primary dendritic cells (DCs) were incubated with free Poly1 or particles, then toxicity and surface activation were quantified. Antigen presentation was measured by incubating DCs with PBAEs, and a model antigen (SIINFEKL), then using an antibody that binds SIINFEKL presented via MHCI. In mice, Poly1 was introduced into inguinal lymph nodes (LNs) – tissues that coordinate immune response – to support direct assessment of the impact on the local LN environment[3],[4]. After 24hrs, LN cell counts and activation were quantified.
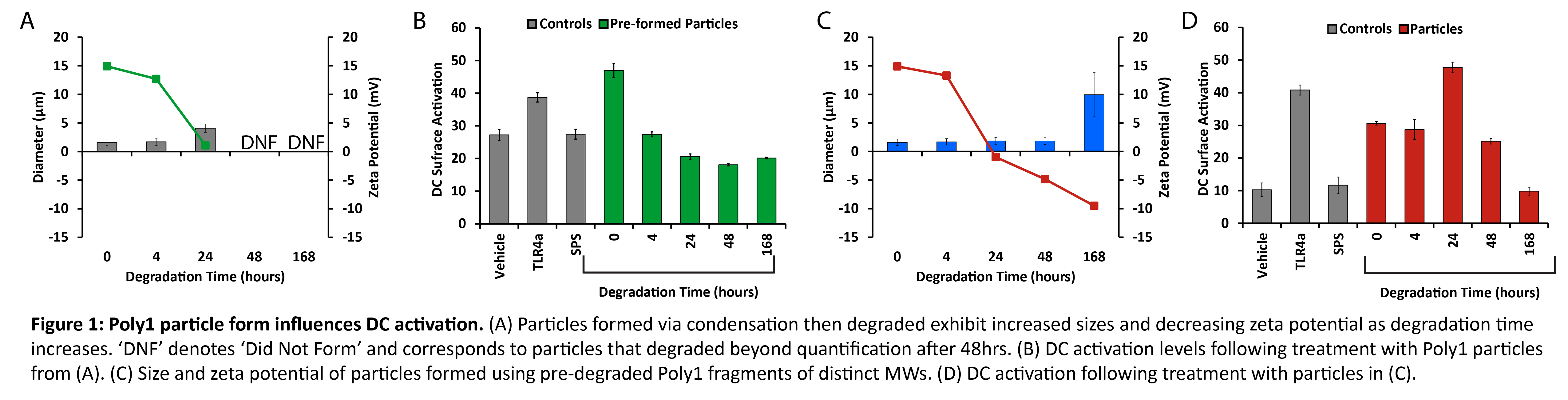
Results and Discussion: Poly1 - with a starting MW of 3.9kDa - degraded rapidly, exhibiting a MW half-life of ~17.7hrs. Treatment with free Poly1 did not activate DCs at any MW. Poly1 particles formed from intact polymer degraded over 48hrs with increasing particle size and more negative surface charges (Fig. 1A). These particles strongly activated DCs at early stages of degradation then became inactive at greater extents of degradation (Fig. 1B). To investigate these trends in a more controlled manner, particles were formed using pre-degraded Poly1 fragments. Depending on the MW, increased size and negative surface charge again correlated with increased degradation time (Fig. 1C). Particles also drove strong DC activation that generally waned as MW decreased (Fig. 1D). These effects correlated with high levels of antigen presentation when DCs were treated with particles corresponding to early degradation times, while free Poly1 again exhibited no activity (Fig. 2A). In mice, Poly1 particles significantly increased both the number of LN resident cells and their activation state (Fig. 2B,C).
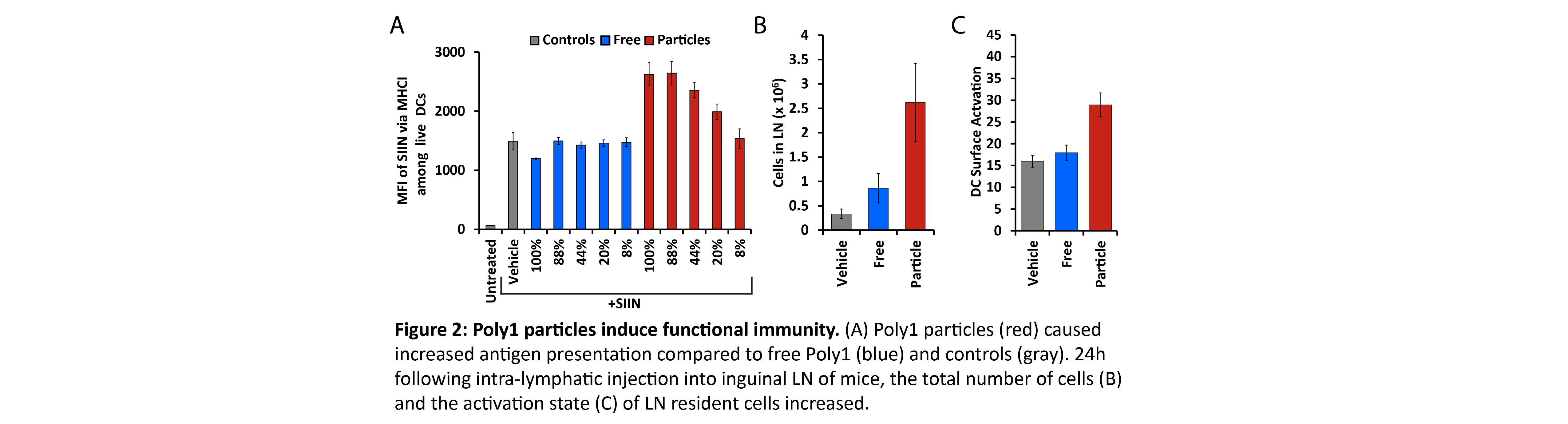
Conclusion: These studies demonstrate free Poly1 does not exhibit intrinsic immunogenicity, while in both cells and mice, Poly1 particles activate DCs and increase antigen presentation in a MW dependent fashion. This discovery – that the inherent immunogenic properties of common biomaterials can evolve during degradation – could inform the design of future vaccine carriers that are not just passive carriers but that also actively direct immune response.
References:
[1] J. I. Andorko, K. L. Hess, and C. M. Jewell, "Harnessing Biomaterials to Engineer the Lymph Node Microenvironment for Immunity or Tolerance," AAPS J, p. 1-16, 2014.
[2] F. A. Sharp, D. Ruane, B. Claass, E. Creagh, J. Harris, P. Malyala, et al., "Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome," Proc. Natl. Acad. Sci. U.S.A., vol. 106, p. 870-875, 2009.
[3] C. M. Jewell, S. C. B. Lopez, and D. J. Irvine, "In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles," Proc. Natl. Acad. Sci. U.S.A., vol. 108, p. 15745-15750, 2011.
[4] J. I. Andorko, L. H. Tostanoski, E. Solano, M. Mukhamedova, and C. M. Jewell, "Intra-lymph Node Injection of Biodegradable Polymer Particles," J. Vis. Exp., p. e50984, 2014.