Introduction: Cell sheet tissue engineering strategies have restricted clinical applicability due to delayed extracellular matrix (ECM) deposition and consequent prolonged production time. Scaffold-free tissue production in vitro can be enhanced by macromolecular crowding (MMC)[1], a biophysical phenomenon that governs the intra- and extra-cellular milieu of multicellular organisms[2]. Although MMC has been proven to be an effective tool in enhancing ECM deposition in permanently differentiated cells[1], its effectiveness in mesenchymal stem cell (MSC) culture has still to be fully assessed. It is hypothesised that MSCs cultured under MMC and low oxygen tension (2%)[3] conditions will maintain their phenotype and function, resulting in multipotent and ECM-rich cell sheets for a variety of clinical targets.
Materials and Methods: Human bone marrow MSCs were cultured for 7 and 14 days with 100 µg/ml of carrageenan (MMC) at 20% and 2% oxygen tension at 37ºC and 5% CO2 in a humidified atmosphere. Collagen I deposition was assessed using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Phenotypic assessment was performed by flow cytometry for the expression of cell surface markers CD90, CD105, CD73, CD44 and lack of expression of CD34, CD11b, CD19, CD45 and HLA-DR. Staining for Oil Red O, Alizarin Red S and Safranin O / Fast green was used to assess adipogenic, osteogenic and chondrogenic differentiation.
Results and Discussion: Collagen I deposition is increased at both oxygen tensions, showing that MMC is effective independently of the oxygen tension (Figure 1). Expression of phenotypic markers was assessed and no differences were found in mesenchymal surface marker expression. Differentiation potential was also assessed and it was seen that adipogenic differentiation potential was reduced at 2% oxygen tension, while chondrogenic differentiation was increased in the presence of MMC and no differences were found for osteogenic differentiation (Figure 2).
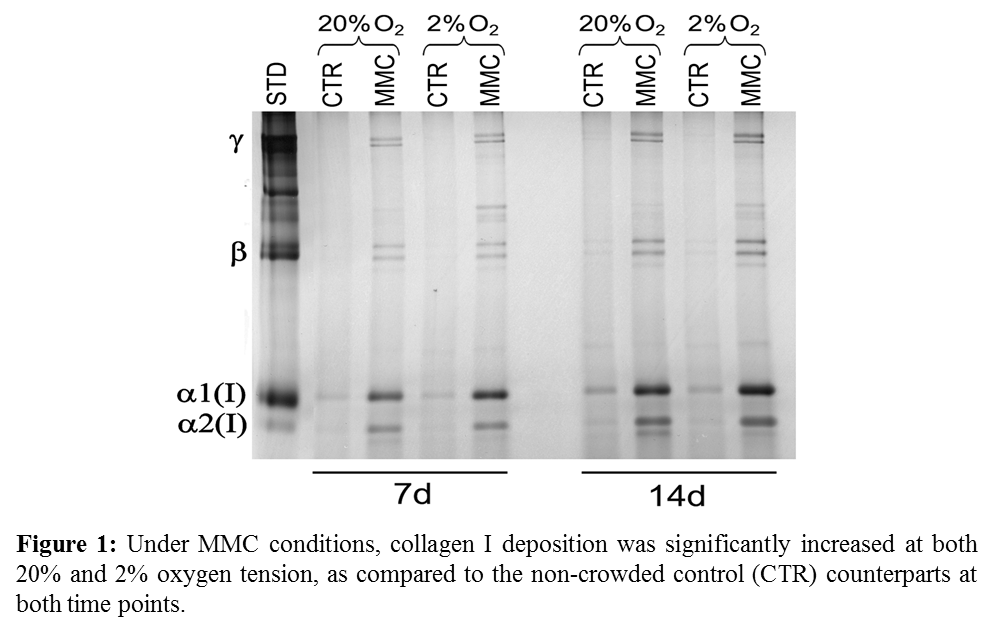
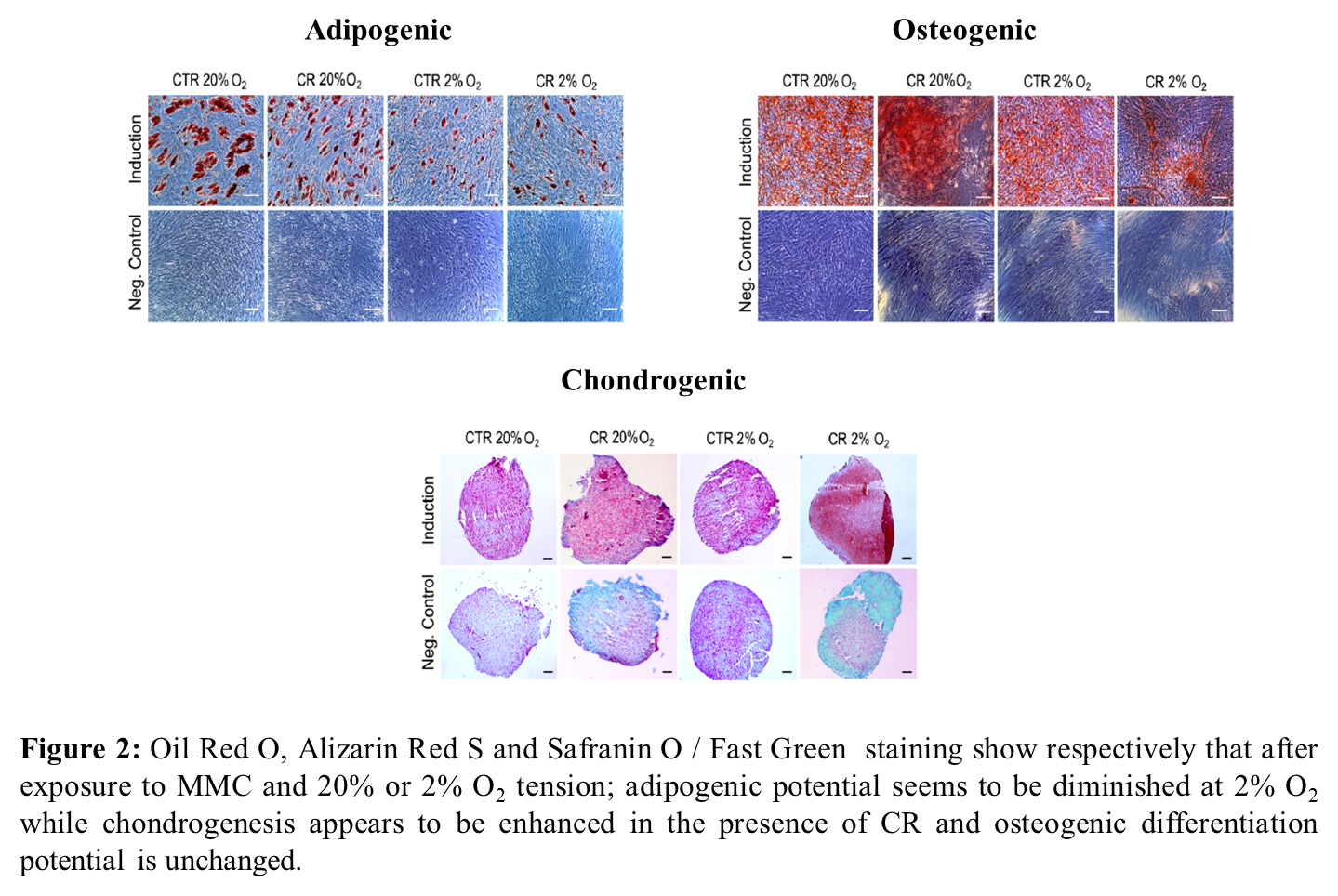
Conclusions: MMC and low oxygen tension can be used effectively to enhance extracellular matrix deposition in human MSC culture, without detrimental effect on cell phenotype and function. This multifactorial approach paves the way for the development of scaffold-free cell therapies for various clinical targets.
Irish Research Council; Health Research Board
References:
[1] Satyam A. et al, Adv Mat, 26:3024-3034, 2014
[2] Chen C. et al, Adv Dr Del Rev 63: 277-290, 2011
[3] Mohyeldin A. et al, Cell Stem Cell, 7:150-161, 2010