Introduction: Vascular tissue engineering has made significant progress over the past decade towards the creation of functional living tissues that can be used not only for the replacement of damaged arteries but also for the development of in vitro models of vascular tissue[1]. Collagen scaffolds provide biological cues that are essential for cells to produce neo-extracellular matrix[2]. However, these models still lack adequate mechanical properties and tissue organization to better mimic the natural tissues[3].
This work focuses on the combined effects of the static culture and cell remodeling on tissue organization and mechanical properties. Additionally, it gives an insight on the intimate relationship between cells and collagen fibrils reorganization and the anisotropic viscoelastic properties.
Materials and Methods: Collagen gel-based tubular constructs were prepared by mixing collagen solution (4g/L), with buffer solutions and smooth muscle cells (SMCs) at a final concentration of 106 SMCs and 2g of collagen per mL of gel[4],[5]. After 1h of gelation in a tubular mold, this construct was placed in specifically-designed static bioreactor with fresh medium culture (Figure 1) for further static maturation for 1 or 2 weeks.
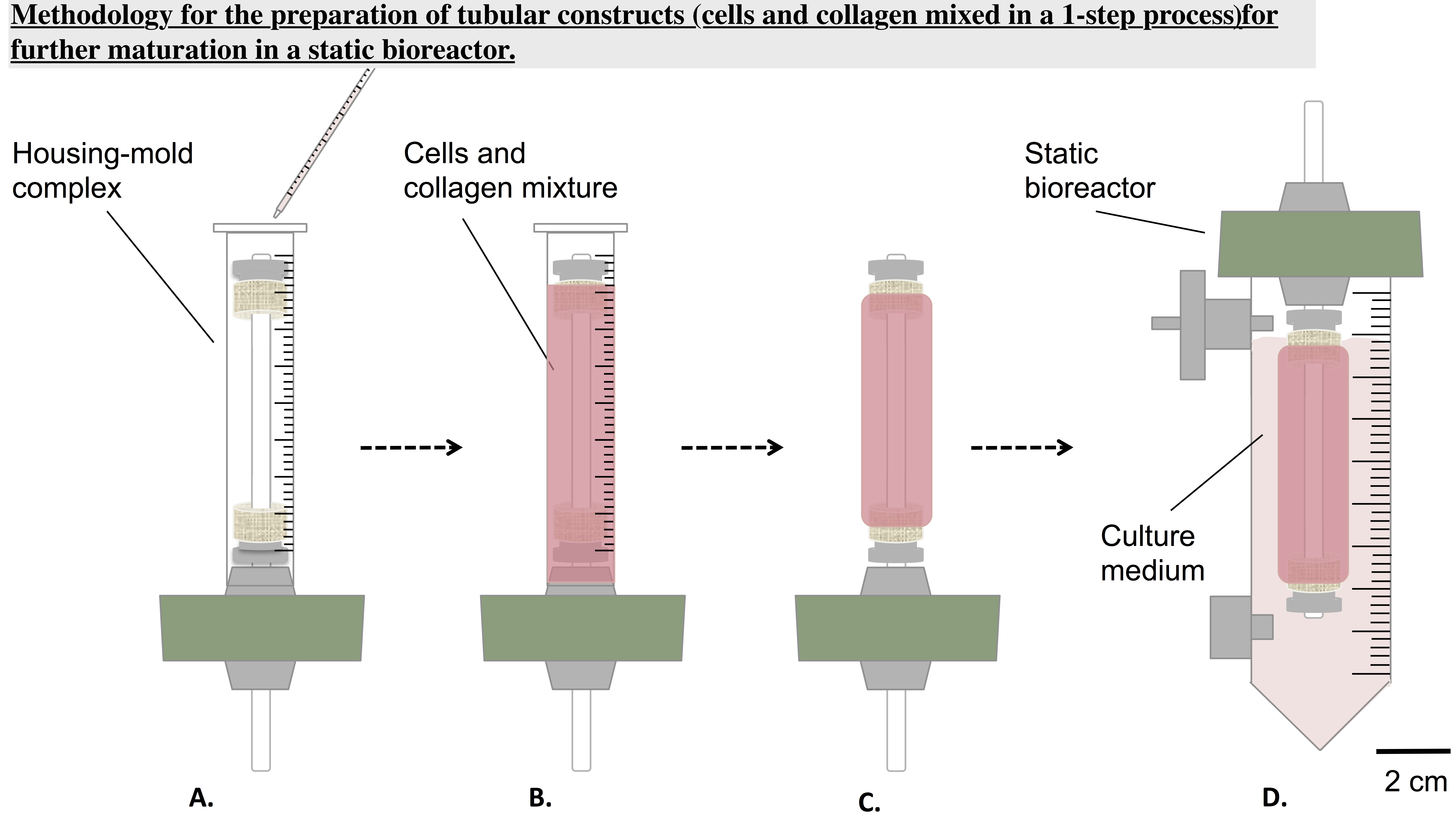
Immunofluorescence (IF) analyses were performed on these samples to observe the organization of cells and collagen fibrils within the constructs. Briefly, after fixation, embedding and mounting of the samples, cell nuclei and actin filaments were stained with Dapi (1:3000) and Rhodamin Phalloïdin (1:200) respectively and observed with an Olympus BX51 fluorescence microscope. To evaluate the anisotropic mechanical and viscoelastic properties of these statically cultured constructs, stress relaxation tests were assessed in two directions: in the longitudinal one or in the circumferential one, respectively directly on the tubular constructs or on rings cut out of them. Samples were mounted on an Electropuls Microtester (Instron Corporation, Norwood, MA, USA), stretched at 10% (5%/s) and maintained constant for 600s while monitoring the decay of the stress. The same procedure was repeated at 20% and 30%.
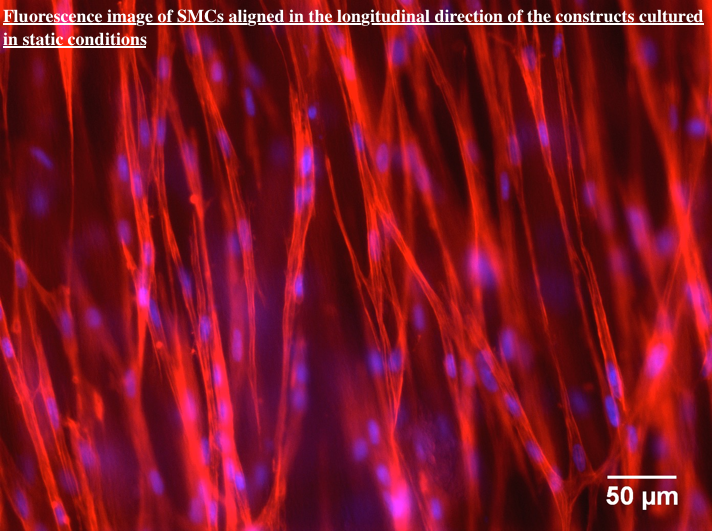
Results and Discussion: The method described here resulted in a homogenous mixture of SMCs-collagen in tubular shape in a one-step process. IF analyses showed that SMCs were uniformly distributed and longitudinally oriented within the constructs after static culture (Figure 2).
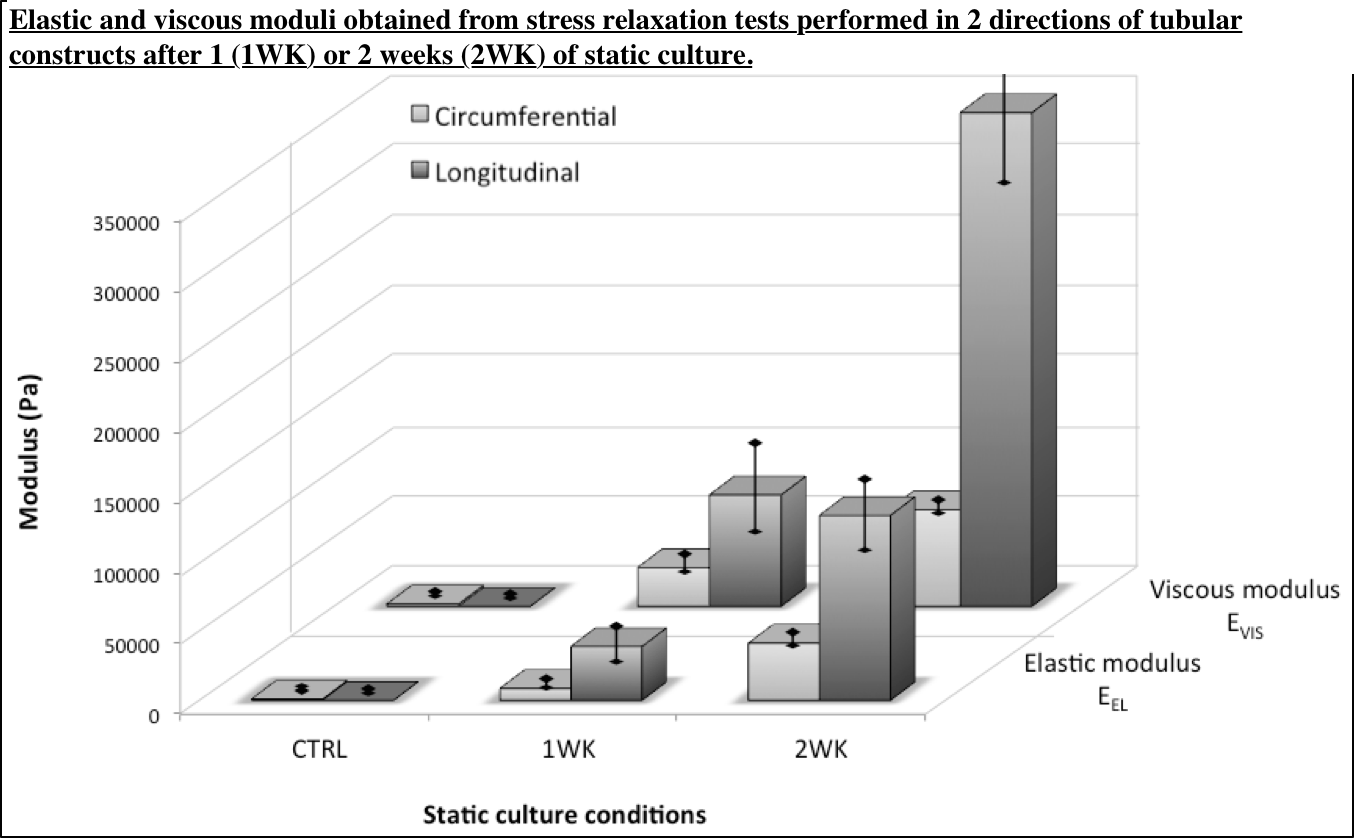
This cell-driven reorganization had a great impact on the overall viscoelastic behavior of the constructs. Indeed, important mechanical reinforcement occurred during the 2 weeks of static culture since the elastic modulus in the circumferential direction increased by a factor 35. Moreover, cell remodeling during this period resulted in anisotropic elastic properties since the longitudinal elastic modulus was 3 times higher than the circumferential one (similar trend in the viscous behavior) (Figure 3).
Conclusions: Cell-driven remodeling of the constructs during static culture resulted in an important reorganization of cells and collagen matrix and overall increase in the mechanical properties. Hence, through an original and simple method, static culture of engineered tissues appears to be crucial step leading to tubular constructs that can be easily manipulated for further mechanical stimulation. Moreover, this work pointed out the intimate relationship between tissue reorganization and anisotropic mechanical properties. Therefore, this rapid easy-to-process method allows to produce and culture viable or pathological arterial models, with more physiological organization and mechanical properties, in the potential of being used for diagnosis purposes, drug screening or therapeutic.
This work was partially funded by NSERC-Canada, CIHR-Canada, FRQ-NT-Quebec, CFI-Canada. CL and DS were awarded of a doctoral scholarship from NSERC Create Program in Regenerative Medicine (www.ncprm.ulaval.ca)
References:
[1] Catto, V., Farè, S., Freddi, G. & Tanzi, M. C. Vascular Tissue Engineering: Recent Advances in Small Diameter Blood Vessel Regeneration. ISRN Vascular Medicine 2014, 1–27, doi:10.1155/2014/923030 (2014)
[2] L’Heureux, N., Germain, L., Labbe, R. & Auger, F. A. In vitro construction of a human blood vessel from cultured vascular cells: A morphologic study. Journal of Vascular Surgery 17 (3), 499–509, doi:10.1067/mva.1993.38251 (1993)
[3] Meghezi, S., Couet, F., Chevallier, P. & Mantovani, D. Effects of a pseudophysiological environment on the elastic and viscoelastic properties of collagen gels. International Journal of Biomaterials 2012, 319290, doi:10.1155/2012/319290 (2012)
[4] Rajan, N., Habermehl, J., Coté, M.-F., Doillon, C. J. & Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nature protocols 1 (6), 2753–8, doi:10.1038/nprot.2006.430 (2006).
[5] Meghezi, S., Seifu, D. G., Bono, N., Unsworth, L., Mequanint, K. & Mantovani, D. Engineering 3D Cellularized Collagen Gels for Vascular Tissue Regeneration. Journal of Visualized Experiments (100), doi:10.3791/52812 (2015)