Introduction: In our previous study, we reported the first use of Spark Plasma Sintering (SPS) to densify 45S5 Bioglass (45S5) and found that SPS could lead to better early-stage cytocompatibility compared with conventional sintering processes[1]. Although SPS has been utilized to fabricate bulk 45S5 and various 45S5-based composites, there is a lack of comprehensive investigation of the in vitro biological properties of such materials [2]-[4]. In this research, cell culture was carried out for 45S5 sintered by SPS at three different temperatures using two established cell lines: L929 and MG63
Materials and Methods: 45S5 powder (XL Sci-Tech Inc., US) was sintered for 3 min by an SPS system (Dr. Sinter 1050, SCM, Japan) at three different temperatures: 500, 550 and 600 °C. X-ray Diffraction (XRD) was utilized to identify the phase composition of the samples. Growth of L929 and MG63 was assessed with a CyQUANT Cell Proliferation Assay Kit (C7026) after culturing them for 3 days in the extractant media of sintered pellets. Alkaline phosphatase (ALP) activity was quantified using the Abcam ALP Assay Kit (ab83369) for MG63 cells cultured on the pellets’ surface for 4 days. In both experiments, the “new” samples were freshly polished and had not been soaked in any medium, while “old” samples had undergone prolonged soaking (~21 days) for other tests before the current study.
Results and Discussion: It can be seen in Fig.1 that 45S5 sintered at 500 °C is amorphous, while pellets sintered at higher temperatures are crystalline with Na2CaSi3O8 as the major phase.
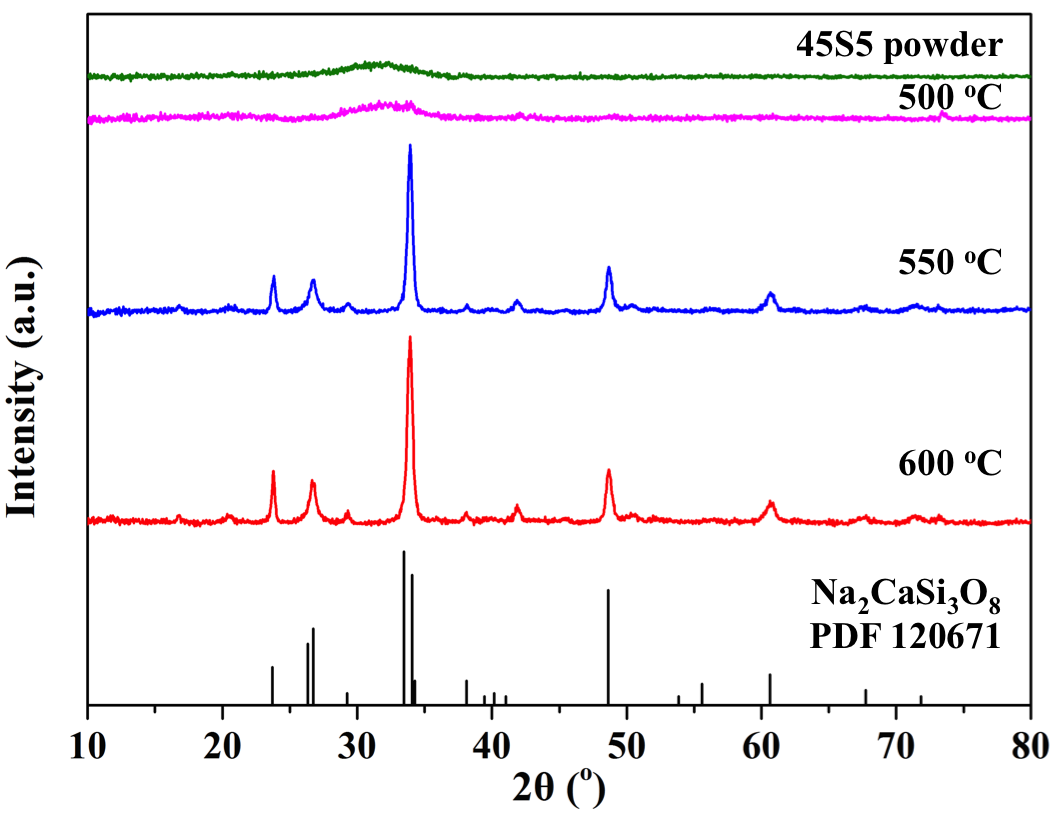
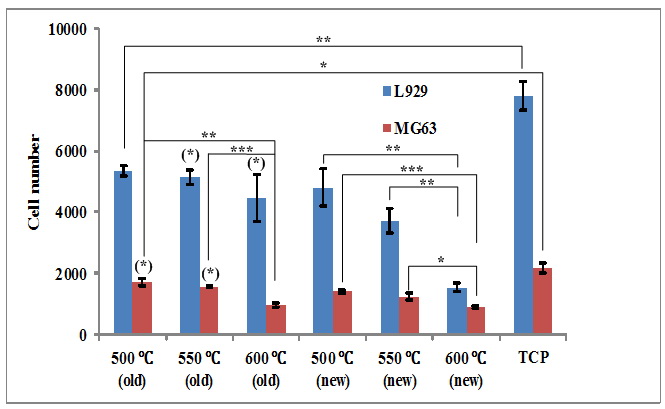
Fig. 2 compares the cell number for different pellets. Statistical difference is represented by * (p≤0.05), ** (p≤0.01) and *** (p≤0.001) in Fig. 2 and Fig. 3, with asterisks in parentheses indicating differences between the old samples and their new counterparts. It is explicitly observed that 45S5 pellets all resulted in slower cell growth than the control (tissue culture plastic, TCP). Furthermore, both a smaller sintering temperature (and hence lower crystallinity) and long-term soaking prior to this research (and thus a smaller amount of ions released into the extractant media) could generally promote cell growth. Both old and new pellets compacted at 600 °C led to the slowest cell growth.
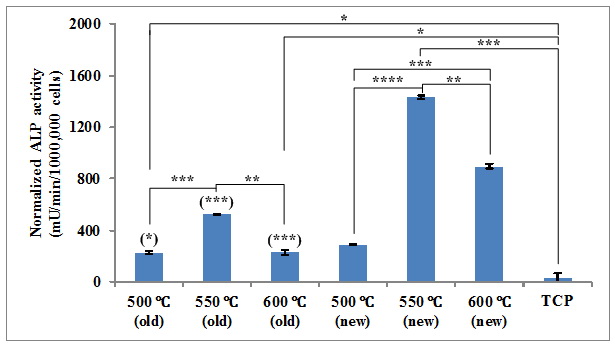
It is interesting to find in Fig. 3 that 45S5 pellets all brought about remarkably higher ALP activity in MG63 than TCP, which is an important feature of the osteoblast phenotype. In addition, new samples could all result in significantly higher ALP activity than their old counterparts, indicating the presence of more bioactive substances which could facilitate cell differentiation and bone formation. Interestingly, 45S5 sintered at 550 °C led to much higher ALP activity in MG63 than those fabricated at higher or lower temperatures. This phenomenon suggests that neither fully amorphous nor highly crystalline 45S5 favors the differentiation of MG63 and bone formation [1].
Conclusions: This study investigated the in vitro biological properties of 45S5 pellets fabricated by SPS and reported for the first time the difference in ALP activity of MG63 cells cultured on 45S5 caused by different SPS temperatures. It was found that relatively low SPS temperatures (<600 °C) could bring about faster cell proliferation, and a moderate temperature of 550 °C could result in highest ALP activity. Therefore, it can be concluded that 550 °C should be used for sintering 45S5 used as orthopaedic implants.
Z. LI thanks NTU for the University Research Scholarship
References:
[1] Chen, Q.Z., et al., Spark plasma sintering of sol–gel derived 45S5 Bioglass®-ceramics: Mechanical properties and biocompatibility evaluation. Materials Science and Engineering: C, 2011. 23(3): p. 494-502.
[2] Jia, Z., et al., Preparation and characterization of mechanical properties of carbon nanotube/45S5Bioglass composites for biologic applications. Materials Science and Engineering: A, 2011. 528(3): p. 1553-1557.
[3] Grasso, S., et al., Low temperature spark plasma sintering of 45S5 Bioglass®. Journal of Non-Crystalline Solids, 2013. 362(0): p. 25-29.
[4] Porwal, H., et al., Processing and bioactivity of 45S5 Bioglass®-graphene nanoplatelets composites. Journal of Materials Science: Materials in Medicine, 2014. 25(6): p. 1403-1413