Introduction: Liver diseases affect over 600 million people worldwide. Currently, liver transplantation is the only therapy to prevent mortality of end-stage liver diseases. However, the situation is deteriorating given the severe shortage of donor livers/hepatocytes. Human induced pluripotent stem cells (iPSCs) are promising cell source to provide sufficient hepatocytes for transplantation. To generate hepatocytes from human iPSCs, various approaches have been energetically pursued in the past decade. Although hepatocyte-like cells were derived to some extent, their functions were similar to fetal rather than adult human hepatocytes. Moreover, previous transplantations were performed in immunocompromised animals without immunoisolation of hepatocyte-like cells; whether these cells can engraft, mature and function in immunocompetent animals and eventually humans remains unknown. In this study, we combined the 2D monolayer culture and differentiation with the 3D microwell co-culture with stromal cells (SCs) to enhance the maturation and function of human iPSCs-derived hepatocytes (iPS-H) in vitro. Furthermore, we encapsulated the iPS-H/SCs aggregates in recently developed, non-fibrotic alginate capsules[1] and evaluated the in vivo function of iPS-H in an immunocompetent mouse model.
Materials and Methods: PDMS microwells were fabricated using standard soft lithography. Undifferentiated iPSCs were maintained and differentiated into iPS-H on 2D monolayer culture. To enhance the maturation and function of iPS-H, 3D co-aggregates of iPS-H/SCs (Cell ratio: iPS-H:SCs=2:1) were formed in PDMS microwells. Cell aggregates of iPS-H/SCs in PDMS microwells were analyzed for albumin secretion and cytochrome P450 activity. Cell aggregates of iPS-H/SCs were encapsulated in recently developed, non-fibrotic alginate capsules[1] and transplanted into the intraperitoneal cavity of immunocompetent C57BL/6 mice. Mouse blood was collected and human albumin in mouse serum was measured for 24 days.
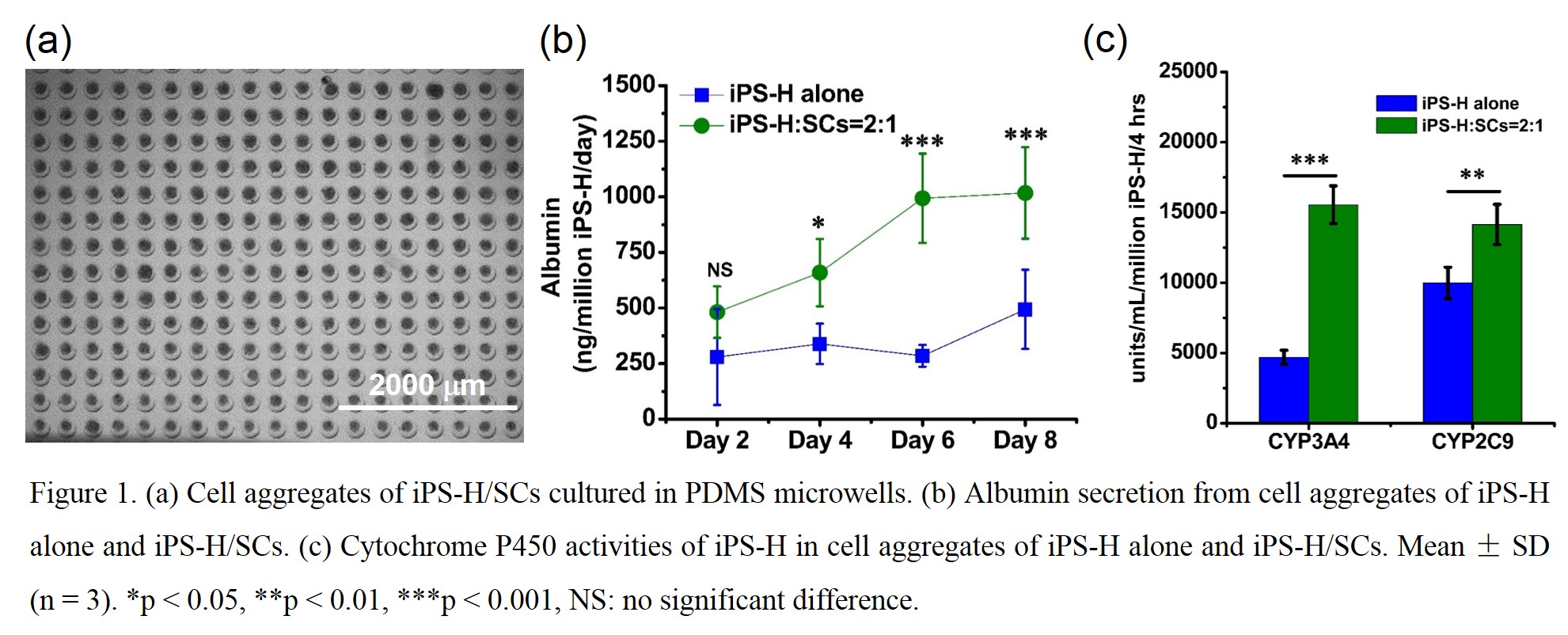
Results and Discussion: iPS-H were differentiated from iPSCs on 2D monolayer and then placed into PDMS microwells with SCs in 3D to recapitulate the vital cell-cell and cell-matrix interactions in vivo. Numerous size-controllable iPS-H/SCs aggregates were formed in microwells (Fig 1a). Compared with cell aggregates of iPS-H alone, iPS-H/SCs aggregates generated more mature and functional hepatocytes as indicated by enhanced albumin secretion (Fig 1b) and cytochrome P450 activities (Fig 1c). Cell aggregates of iPS-H alone consistently secreted albumin at low level. In contrast, the amount of secreted albumin gradually increased as culture time prolonged in iPS-H/SCs aggregates. It is well-known that cytochrome P450 enzymes are of great importance in phase I xenobiotic biotransformation. CYP3A4 and CYP2C9 activities of iPS-H/SCs aggregates increased approximately 1.4 ~ 3.0 folds when compared to cell aggregates of iPS-H alone. The physical contacts, deposited matrix proteins, and paracrine signaling from SCs in 3D may play important roles in formation of cell aggregates as well as maturation and function of iPS-H.
The iPS-H/SCs aggregates were collected from microwells (Fig 2a) and then encapsulated into alginate capsules (Fig 2b). The capsules containing cell aggregates were transplanted into immunocompetent mice to evaluate the engraftment and function of iPS-H in vivo. The beneficial effect of alginate encapsulation was obvious as human albumin secreted from iPS-H/SCs aggregates was detected in mouse sera for 24 days in vivo (Fig. 2c). The level of secretion was generally comparable to Hum-H/SCs aggregates and previous reports using immunocompromised mice[2].
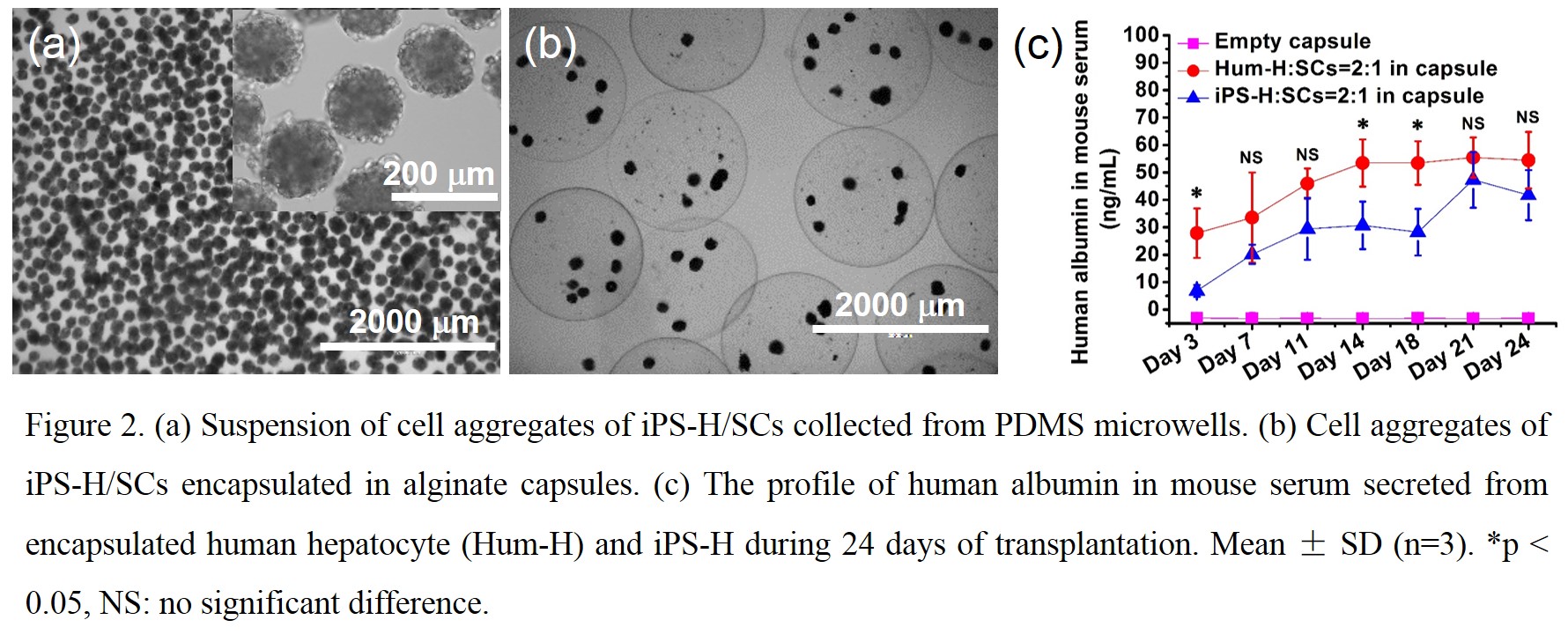
Conclusion: The in vitro hepatic functions of iPS-H were enhanced by 3D co-aggregation of iPS-H and SCs in microwells. Engraftment of iPS-H in immunocompetent mice was demonstrated through encapsulation of iPS-H/SCs aggregates in biocompatible alginate capsules. Human albumin in mouse sera secreted by encapsulated iPS-H/SCs aggregates reached a level comparable to the primary human hepatocyte control. This proof-of-concept study provides a simple yet robust approach to improve the engraftment of iPS-H and may be applicable to other stem cell-based therapies.
Dr. Robert E. Schwartz, Yen-Chun Lu, Angela Frankel, and Duo An
References:
[1] Veiseh O., et al. Nat. Mater. 14: 643-51 (2015).
[2] Liu H., et al. Sci. Transl. Med. 3, 82ra39 (2011).