Introduction: A critical challenge in the development of organ-on-chip devices is the need to read-out relevant biological information in real-time. Quantifying the mechanical forces exerted during tissue remodelling is critical to understand biological processes such as development, wound healing, and disease progression. Current approaches to measure cell-generated forces are severely limited in their utility; 3D traction force microscopy relies on foreknowledge of matrix mechanical properties, which change during remodelling[1], and monitoring the deformation of incompressible oil droplets in situ cannot be used to quantify isotropic forces[2]. To address these issues, we developed soft, compressible, and fluorescent hydrogel microdroplets that deform under cell-generated forces, enabling real-time and absolute readouts of force during tissue morphogenesis (Fig. 1A).
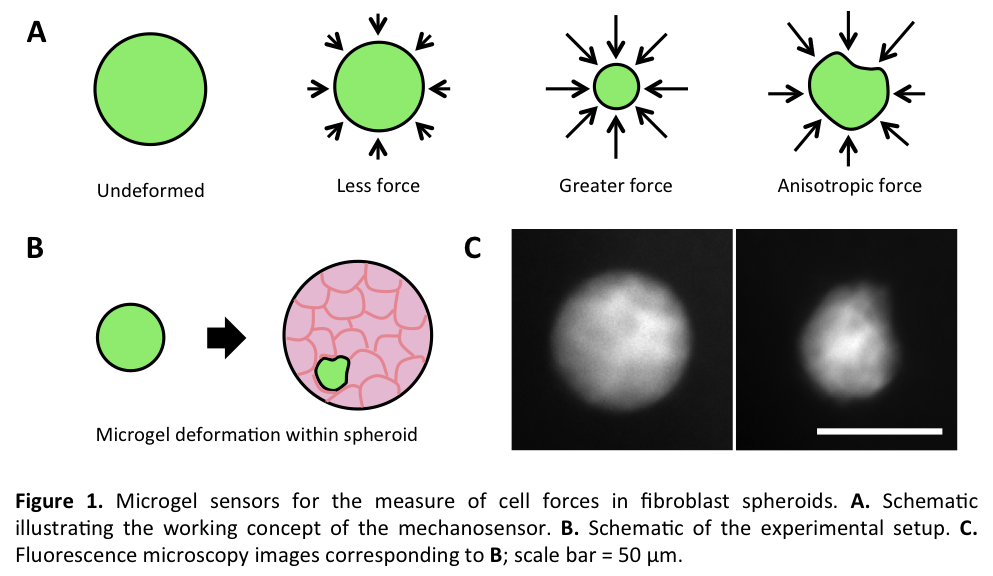
Materials and Methods: Fluorescent polyacrylamide (PA) hydrogel microdroplets with constant mechanical properties were fabricated by dispersing picolitre volumes of PA prepolymer in an immiscible oil bath via mechanical agitation or a microfluidic droplet generator. The elastic modulus of the material was determined using bulk shear rheometry. Microgels were embedded into 3D engineered tissues including fibroblast spheroids (Fig. 1B) and fibroblast-laden contractile collagen gels (Fig. 2A). Microgel shape was monitored by fluorescent microscopy over several days during tissue morphogenesis, with candidate growth factors (TGF-β1), mitogens (lysophosphatidic acid), and cytoskeletal inhibitors (blebbistatin). A finite element model was used to extract tissue forces based on the measured deformations.
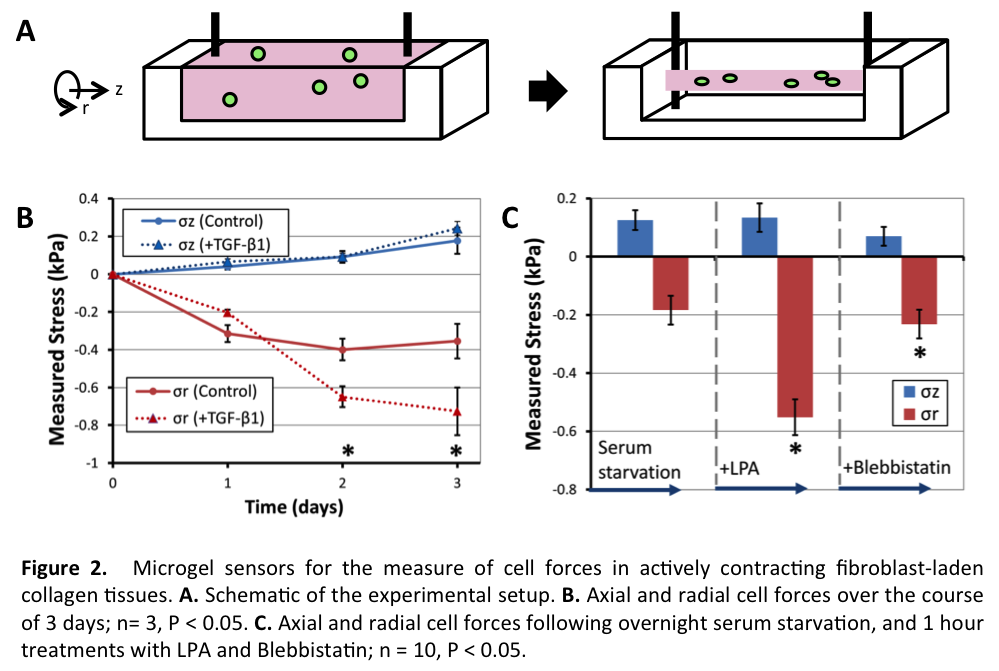
Results and Discussion: Changes in microgel (G~0.6 kPa) shape were observed when gels were cultured in fibroblast spheroids (Fig. 1C). Spherical microgels changed in size and shape, demonstrating the ability to measure absolute cell forces in dense tissues. Microgels located closer to the periphery of the spheroid showed less deformation, indicating that spatial mechanical forces vary considerably within spheroid cultures. When the microgels (G~0.2 kPa) were embedded within cylindrical fibroblast-laden contractile collagen matrices that contract anisotropically (Fig. 2A), significant radial compressive forces were measured, compared to axial tensile forces over 3 days of compaction (Fig. 2B); TGF-β1 increased radial stress significantly. Finally, serum-starvation, activation, and inhibition of cellular contractile forces were sequentially applied (Fig. 2C), demonstrating the real-time readout potential of the sensors.
Conclusion: The mechanosensor microgels fabricated in this study allow for the measure of real-time, local, internal forces in soft 3D cell cultures. These sensors may hence be used to monitor biological forces in situ, and thus aid in real-time analysis of physiologically realistic organ-on-a-chip platforms.
This work was supported by a Discovery grant from the Natural Sciences and Engineering Research Council of Canada, and a new investigator award from the Fonds de recherché Nature et Technologies.
References:
[1] Legant, W. R. et al. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nature Methods 7, 969-971 (2010).
[2] Campas, O. et al. Quantifying cell-generated mechanical forces within living embryonic tissues. Nature Methods 11, 183-189 (2014).