Bioactive glasses (BGs) are known for their ability to bond to living bone and cartilage[1]-[3]. In general, they are readily available in powder and monolithic forms, which are not ideal for the optimal filling of bone defects with irregular shapes[4],[5]. In this context, the development of BG-based scaffolds containing flexible fibers is a relevant approach to improve the performance of BGs[6]-[9]. This study is aimed at characterizing a new, highly porous, fibrous glassy scaffold and evaluating its in vitro and in vivo biocompatibility. The developed scaffolds were characterized in terms of porosity, mineralization and morphological features. Additionally, osteoblast cells were seeded in contact with extracts of the scaffolds to assess cell proliferation and genotoxicity after 24, 72 and 144 h. Finally, scaffolds were placed subcutaneously in rats for 15, 30 and 60 days. SEM images indicated that the scaffolds presented interconnected porous structures[10]-[12] (Figure 1).

The evaluation of porosity (via SEM) demonstrated a total porosity of 75 ± 0.7%. In addition, FTIR spectra showed that the precursor bioglass could mineralize a hydroxyapatite (HCA) layer in simulated body fluid (SBF) after only 12 h, being similar to that for Bioglass 45S5, which takes approximately 8 h for an HCA layer formation[3],[13]. Citotoxicity tests using MTT indicated that the biomaterial elicited increased osteoblast cell proliferation (Figure 2). The biomaterial degradation may have created a microenvironment that improved cellular activity and function.
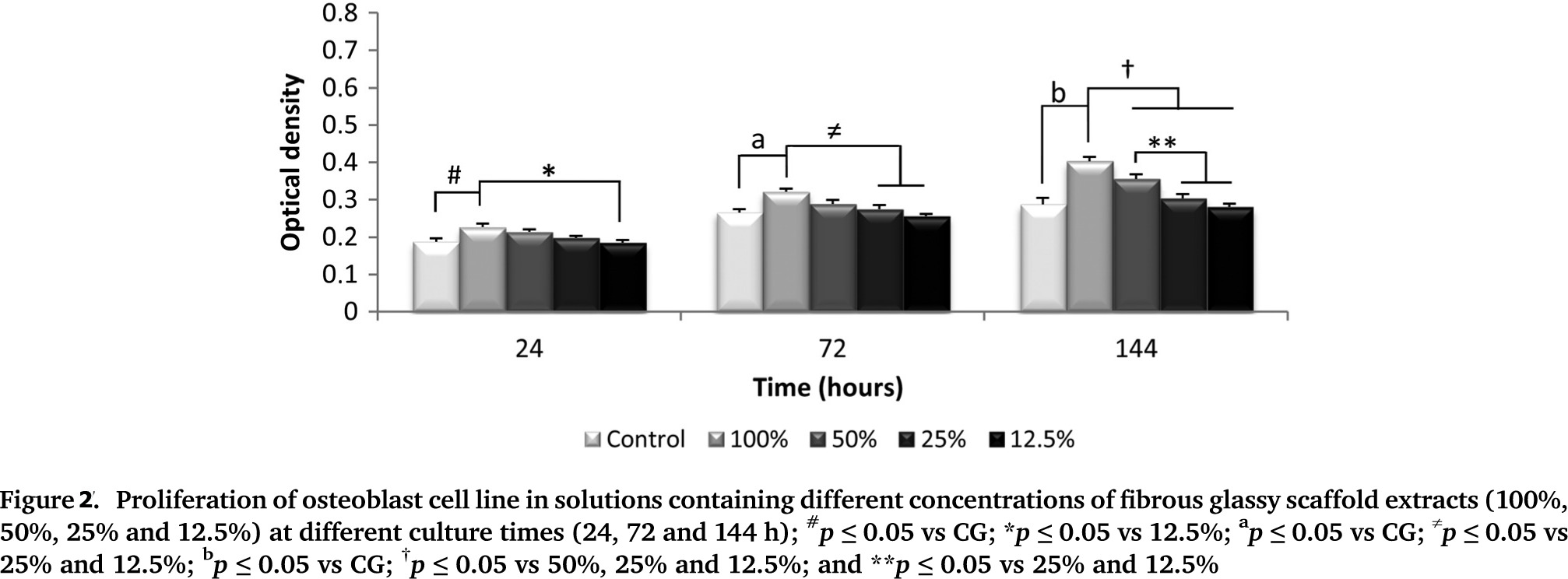
Moreover, no DNA damage was observed. Such data are in agreement with the results of a previous study conducted by Kido et al. (2013)[14], who observed no DNA strand breaks in osteoblasts cultured on Biosilicate scaffolds after 24, 72 and 96 h. Additionally, the in vivo experiment showed degradation of the biomaterial over time, with soft tissue ingrowth into the degraded area and the presence of multinucleated giant cells around the implant. At day 60, the scaffolds were almost completely degraded and an organized granulation tissue filled the area (Figure 3).
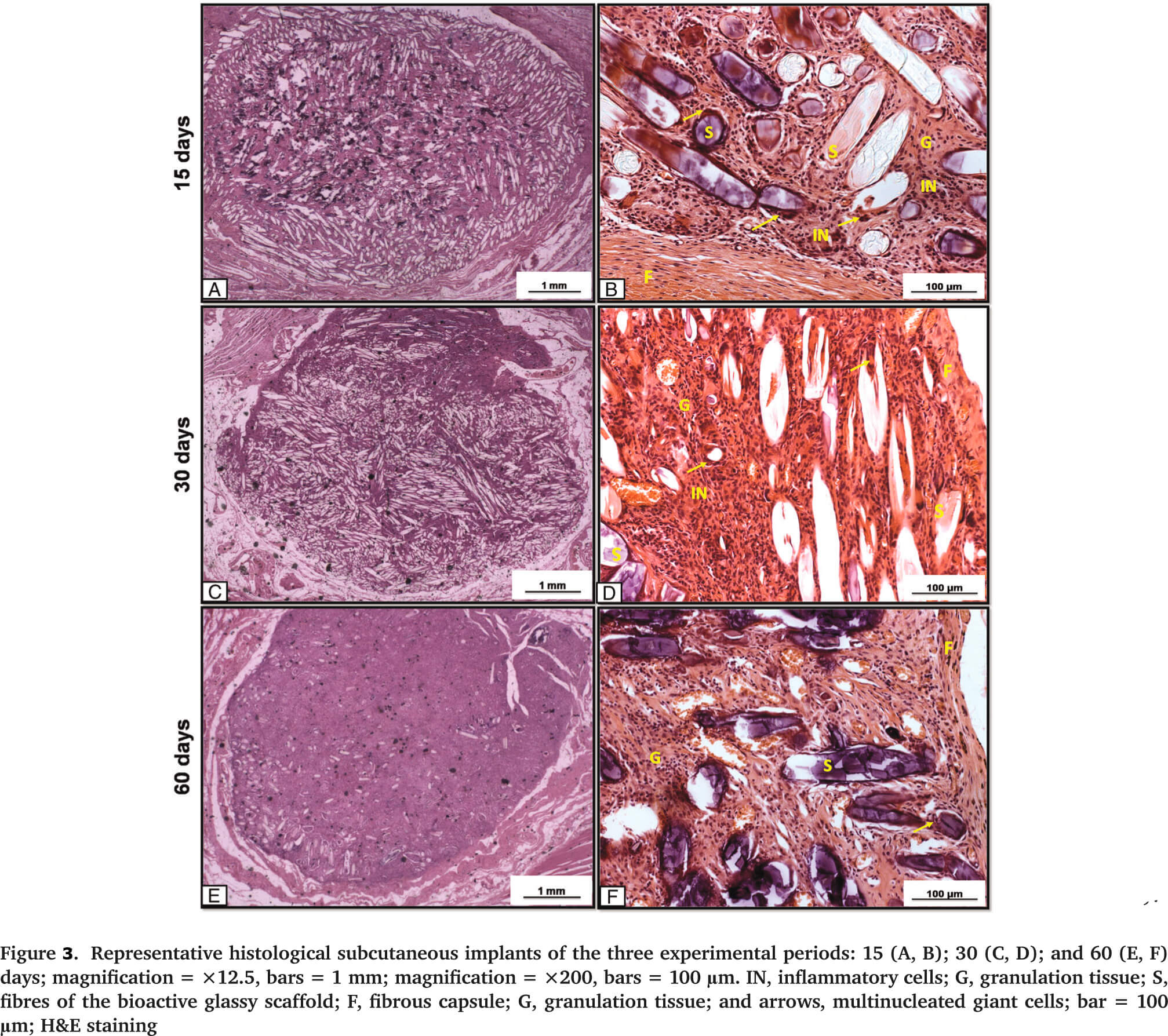
It was likely that the degradation products of the material did not cause a severe tissue irritation; instead, the degraded material was bioabsorped[15],[16] and, consequently, the injured tissue gradually reorganized over time. The results highlight the potential of this fibrous, glassy material for bone regeneration, due to its bioactive properties, non-cytotoxicity and biocompatibility. However, additional long-term studies are required to fully investigate the behavior of this new biomaterial for potential ortothopic in vivo applications.
CAPES; CNPq (Grant No. 303662/2012-3); FAPESP, the São Paulo Research Funding Agency, the Centre for Research Technology (Grant No. 2013/07793-6)
References:
[1] Hubbell, J.A., Synthetic biodegradable polymers for tissue engineering and drug delivery. Curr Opin Solid State Mater Sci, 1998. 3: p. 4.
[2] Hench, L.L. and J.M. Polak, Third-generation biomedical materials. Science, 2002. 295(5557): p. 1014-7.
[3] Hench, L.L., The story of Bioglass. J Mater Sci Mater Med, 2006. 17(11): p. 967-78.
[4] Day, R.M., et al., In vitro and in vivo analysis of macroporous biodegradable poly(D,L-lactide-co-glycolide) scaffolds containing bioactive glass. J Biomed Mater Res A, 2005. 75(4): p. 778-87.
[5] Vallet-Regi, M., Revisiting ceramics for medical applications. Dalton Trans, 2006(44): p. 5211-20.
[6] Chen, Q.Z., I.D. Thompson, and A.R. Boccaccini, 45S5 Bioglass-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials, 2006. 27(11): p. 2414-25.
[7] Rahaman, M.N., et al., Bioactive glass in tissue engineering. Acta Biomater, 2011. 7(6): p. 2355-73.
[8] Jones, J.R.,Review of bioactive glass: from Hench to hybrids. Acta Biomater, 2013. 9(1): p. 4457-86.
[9] Lacroix, J., E. Jallot, and J. Lao, Gelatin-bioactive glass composites scaffolds with controlled macroporosity. Chemical Engineering Journal, 2014. 256(0): p. 9-13.
[10] Bretcanu, O., et al., Novel resorbable glass-ceramic scaffolds for hard tissue engineering: from the parent phosphate glass to its bone-like macroporous derivatives. J Biomater Appl, 2014. 28(9): p. 1287-303.
[11] Franca, R., et al., Nanoscale surface characterization of biphasic calcium phosphate, with comparisons to calcium hydroxyapatite and beta-tricalcium phosphate bioceramics. J Colloid Interface Sci, 2014. 420: p. 182-8.
[12] Yang, J.-Z., et al., Novel Layered Hydroxyapatite/Tri-Calcium Phosphate–Zirconia Scaffold Composite with High Bending Strength for Load-Bearing Bone Implant Application. International Journal of Applied Ceramic Technology, 2014. 11(1): p. 22-30.
[13] Peitl, O., et al., Compositional and microstructural design of highly bioactive P2O5-Na2O-CaO-SiO2 glass-ceramics. Acta Biomater, 2012. 8(1): p. 321-32.
[14] Kido, H.W., et al., Histopathological, cytotoxicity and genotoxicity evaluation of Biosilicate(R) glass-ceramic scaffolds. J Biomed Mater Res A, 2013. 101(3): p. 667-73.
[15] Akazawa, T., et al., Biodegradation and bioabsorption innovation of the functionally graded bovine bone-originated apatite with blood permeability. J Biomed Mater Res A, 2006. 76(1): p. 44-51.
[16] Murata, M., et al., Blood permeability of a novel ceramic scaffold for bone morphogenetic protein-2. J Biomed Mater Res B Appl Biomater, 2007. 81(2): p. 469-75.