Background: Annually, over 2 million bone graft procedures are performed worldwide – with a significant proportion involving critical-sized bone defects. Such defects present a significant burden to the medical community due to their challenging treatment. However, the Masquelet technique (MT) has significantly improved the prognosis of many segmental bone defects in helping to restore form and function. Although the MT has proven to be clinically effective, the physiology of the healing it induces is not well understood. Briefly, the Masquelet technique firstly involves systematic debridement and placement of a polymethylmethacrylate (PMMA) spacer in the defect; after a period, the spacer is carefully removed and bone graft is placed into the defect. This technique has produced remarkable results in some cases; however, much has yet to be elucidated concerning the mechanism of action. Multiple factors (timing, material, and the induced membrane) have been implicated but not proven as being critical for the success of the MT. In this study we test the hypothesis that the PMMA induced membrane has a physical barrier function rather than a biological action. Experiments were performed with either the traditional MT or a single step surgery wrapping allograft in impermeable PTFE membrane in critical-sized defects in a lapine model.
Methods and Analysis: Twenty-four New Zealand rabbits will be equally divided into three study groups. Based on a well-established critical sized defect model, a defect of 3.5 cm in the ulna will be created in each animal. In the control group the traditional MT will be performed. The second cohort would vary from the traditional technique by the replacement of PMMA with a non-porous PTFE membrane and bone allograft (experiment #1). The third cohort will replace the PMMA with a porous PTFE membrane and bone allograft (experiment #2). This allowed us to confirm whether PTFE is as effective as the induced MT membrane and whether the porosity in membrane impacts bone regeneration. All membrane samples were analyzed pre- and post-implantation. Specifically, Micro-CT distinguished between newly formed bone and soft tissue. Furthermore, the fibrous capsules were examined histologically with stains for alkaline phosphatase (ALP), and Tartrate-resistant acid phosphatase (TRAP) to identify regions of bone formation and remodeling.
Results: Our anticipated results will show that a porous PTFE membrane used in a single-stage technique to mimic the induced membrane of the Masquelet Technique will facilitate greater bone regeneration when compared to the traditional 2-stage Masquelet Technique. Upon visual inspection of the explanted defects, there appears to be integration of the bone graft and formation of new bone. In the PTFE membrane explants (1), visually we can appreciate a more consolidated bone graft in comparison to the traditional Masquelet model explant (2).
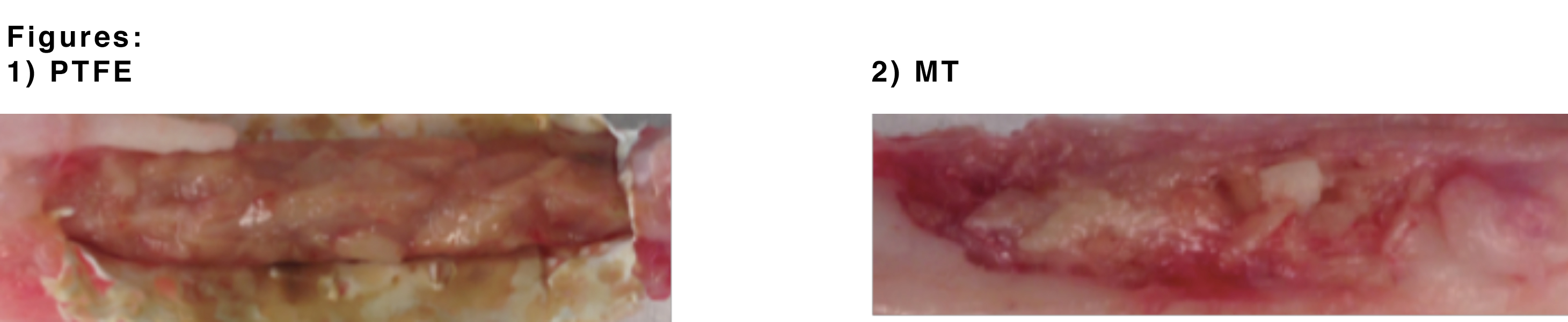
Conclusion and Significance: Based on our expected results, other biomaterials such as PTFE can be integrated into the currently used Masquelet Technique to yield complete bone regeneration in critical sized defects and potentially streamline towards a single-stage technique. This may ultimately expand the clinical application of the MT to treat more complex hard and soft tissue defects.
References:
[1] Masquelet AC, et al. (2000). Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet.