Introduction: Cardiovascular disease is the leading cause of morbidity and mortality in the United States. Development of safe and effective cardiovascular drugs is currently hampered by the poor predictive power of existing preclinical animal models. Therefore, new technologies that can quickly and reliably predict drug safety and efficacy in preclinical studies are greatly important. The traditional tissue culture systems used for preliminary drug screening do not allow drugs to be tested in the context of human tissues that consider the high complexity of the native extracellular matrix (ECM) microenviroments. Meanwhile, it has been recognized that the altered ECM composition and tissue stiffness are correlated with atherosclerosis progression, but whether these changes contribute to atherosclerosis development and affect the drug treatment is obscure. To resolve this question and circumvent the limitations of traditional tissue culture system, we developed a high content screening platform using 3D nanofibrous hydrogel matrices to present an array of combinatorial ECM compositions, which recapitulates the mechanical, topographical and biochemical features of the native healthy and diseased vascular ECM.
Methods: First, poly(ethylene glycol) dimethacrylate (PEGDM) fibrous matrix was fabricated by electrospinning on glass slide and then was stabilized with polymerization under UV exposure. Second, various protein combinations in healthy ECM (Collagen IV (Col IV), Laminin (LN), Fibronectin (FN)) and diseased ECM (Col I, Col III, Osteopontin (OPN), Biglycan (BGN)) were deposited on the PEGDM fibrous matrix using Aushon 2470 arrayer. Finally, the human umbilical vein endothelial cells (HUVECs) were seeded onto the protein microarray slides. The morphology and phenotype of HUVECs on protein arrays were evaluated by immunostaining.
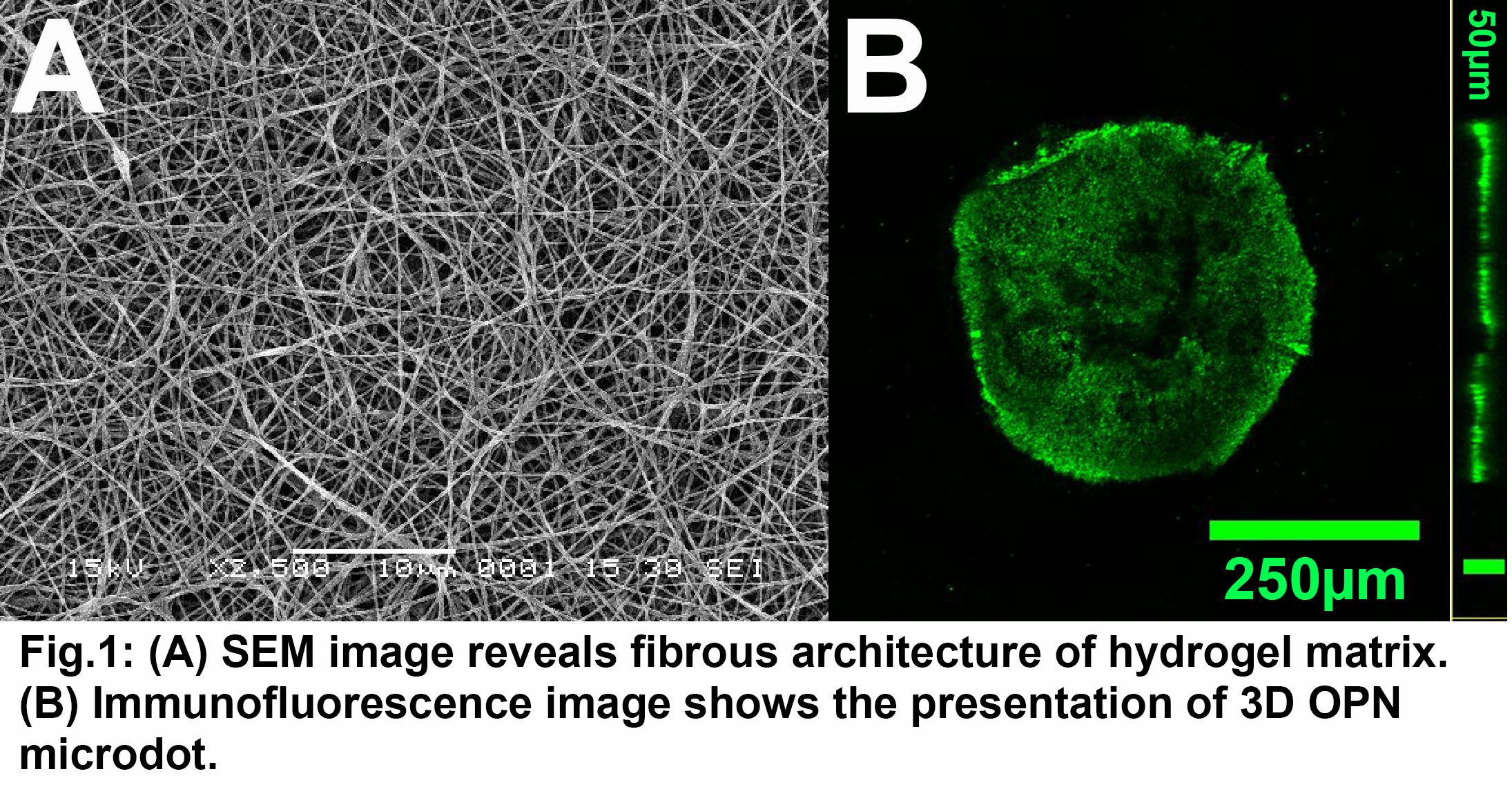
Results: The scanning electron microscopy (SEM) image revealed that nanofibrous architecture was formed by electrospun PEGDM hydrogel that mimicked the topography of native ECM (Fig. 1A). The mechanical properties were evaluated by rheology measurements (data not shown). The two different elastic moduli were prepared by tuning the UV exposure time: ~2 kPa and ~30 kPa, which recapitulated the elasticity of native healthy and diseased vascular tissues. The deposition and retention of protein microdots was confirmed by immunostaining (Fig. 1B).
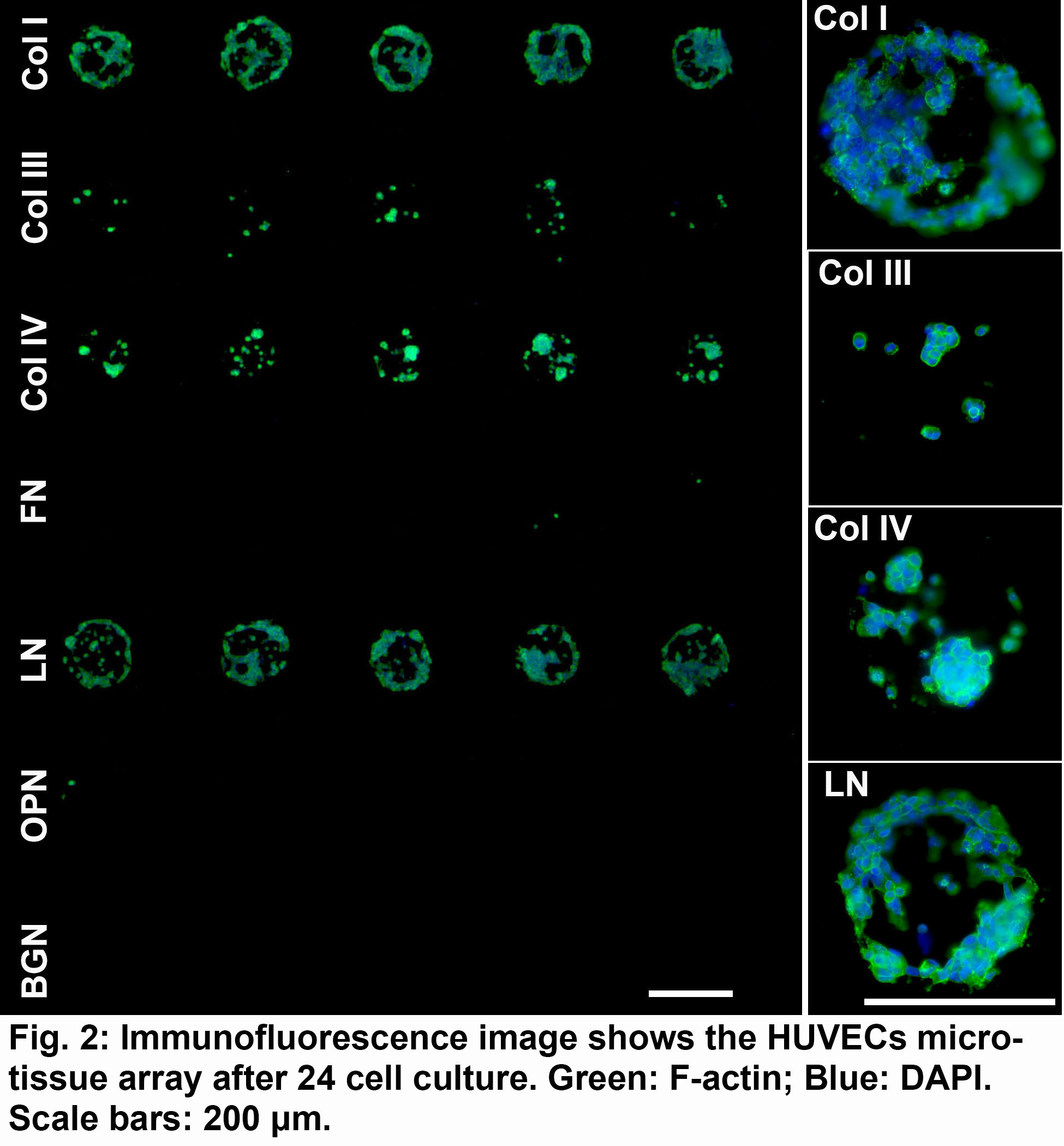
The HUVEC growth is supported and well confined by the protein dots on the PEGDM matrix. The distinct cellular islands form a 3D microtissue array within specialized matrix microenvironments. Fig. 2 demonstrates the strong dependence of HUVEC attachment and morphology on protein environments. For instance, the Col IV and LN as two major healthy ECM proteins strongly promote cell attachment and growth, while Col III, FN, OPN and BGN display much less cell attachment.
Discussion: The platform developed in this study accurately and efficiently captured the high complexity of the native ECM microenvironments by integrating 3D fiber architecture, tuneable elasticity, and combinatorial ECM protein library upon the substrates. Moreover, the 3D microtissue array of HUVECs with diverse phenotypes and functions were successfully established on this platform, which can be future employed for high-throughput drug screening.
Conclusion: We engineered a 3D microtissue array platform for study HUVECs that recapitulates aspects of the native healthy and diseased endothelium. The platform can be used to direct cell attachment, growth, phenotype and function by tuning the elasticity and protein compositions on the 3D nanofibrous matrix. Work in progress includes investigating how the adhesion molecule expression, monocyte adhesion, and platelet adhesion as well as the vascular drug efficacy regulated by the matrix elasticity and protein environments.
This work is finically supported by NHLBI 119371.