Recently, the accumulation of epigenetics abnormalities in cancer cells has been known to lead the drug resistance, invasion and metastasis. For example, metastasis is induced by epithelial-mesenchymal transition (EMT) through the epigenetics abnormalities. It is expected to improve treatment efficiency of anti-cancer drug and to suppress the invasion and metastasis by controlling histone modification. To control the epigenetics modification, we have established the concept of ‘Epigenetics engineering’[1].
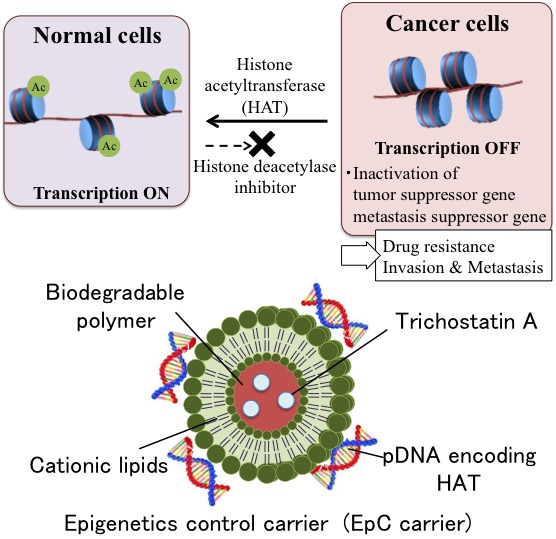
Here, we have prepared the lipid-coated biodegradable nanoparticles, named epigenetics control carrier (EpC carrier) to control histone acetylation for the novel cancer therapy (Figure 1). In particular, the EpC carrier contains the following components; Biodegradable nanoparticles loading histone deacetylase inhibitor (trichostatin A, TSA), cationic lipids to coat the nanoparticles and complex with anionic pDNA. Using this carrier, we have investigated the therapeutic effect of EpC carrier on human lung adenocarcinoma epithelial cell line (A549). The particle size and zeta potential of the EpC carrier was 241nm and +26mV, respectively. EpC carrier showed high gene transfection efficiency. The amount of acetylated histone of the cells treated with EpC carrier was measured by western blotting. EpC carrier led the highest increase of histone acetylation compared with HAT or TSA alone. The resulting cells treated with EpC carrier exhibited the highest increase of tumor suppresser gene (p53). Furthermore, EpC carrier showed the decrease of cell viability and chromosomal fragmentation stem largely from apoptosis. These results suggest that EpC carrier induced apoptosis via reactivation of p53 by histone acetylation.

Next, we have investigated the metastasis suppressive effect of EpC carrier on in vitro EMT model. In vitro EMT model was constructed by adding TGF-beta to A549 cells as previously reported. Epithelial / mesenchymal marker was measured by western blotting. The A549 cells treated with TGF-beta showed the down-regulation of Epithelial marker (E-cadherin) and increase of mesenchymal marker (Vimentin) compared with non-treated cells. On the other hand, the model cells treated with EpC carrier exhibited the recovery of E-cadherin and the suppression of vimentin (Figure 2). From these results, EpC carrier has the potential to suppress the metastasis.
In conclusion, the control of epigenetics modification by EpC carrier has potential role for curative cancer therapy.
References:
[1] Y. Asaba, S. Asayama, H. Kawakami, submitted.