Evaluation for the testosterone undecanoate loaded nanostructured lipid carriers
-
1
Beijing Institute of Pharmacology and Toxicology, Department of Pharmaceutics, China
Aim: the purpose of this study was to preprare a nanostructured lipid carriers (NLC) nanosuspension loading a novel insoluble drug TU(testosterone undecanoate) by heat high pressure homogenization with Tween 80 as stabilizer to improve its solubility, oral bioavailability in vivo.
Method: the TU NLC was preprared by heat high pressure homogenization.Briefly, the solid lipid was melting at 75℃, and then admixing the liquid lipid, dissolving the TU. Dropping the blend into water solution containing Tween 80 at the identical temperature. homogenizing the crude pre-emulsion at 100~600 bar and 1~3cycles.The mean particle size and polydispersity index of TU nanostructured lipid carriers were determined by Nano ZS90. The ratios of drug/lipid and solutes/water solution were screened.Different concentrations of samples were prepared in order to improve the nanostructured lipid carrier suspension drug loading.The vivo experiment was carried out comparing TU nanostructured lipid carriers with commercial product Andriol Testocaps® in beagle dogs. After absorption TU is fast metabolised into T, so the “original” molecule testosterone was investigated.
Results: TU nanostructured lipid carriers was successfully prepared by heat high pressure homogenization.The more solutes were added,the larger sizes would be prepared.10%~20% adding ingredients were allowed to raise the content of the drug.the sizes were between 165.4nm and 240nm [Fig1]. Nevertheless,in the same drug loading, With the increase of the ratio of drug/lipid, particle sizes decreased, but the Zeta potential and the stability reduced.we successfully prepared 30 .60 and 80mg/ml concentration NLC nanosuspension which all presented a uniform and stable appearances [Tab1]. In vivo experiment, The TU nanostructured lipid carriers nanosuspension possessed higher cmaxand shorter tmax,which was well described in the literature as a general feature of orally administered NLC.The NLC/ Andriol Testocaps® ratios of Cmax and the AUC values were 1.43-fold and1.26-fold, respectively [Fig2].
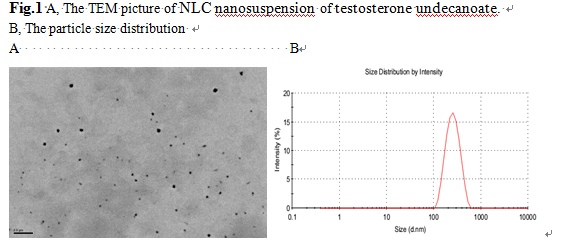

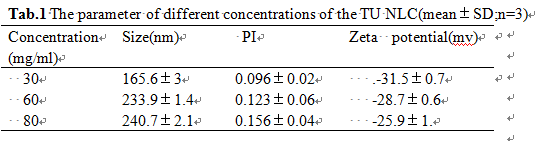
Conclusion: NLC can effectively improve oral bioavailability of testosterone undecanoate than the soft capsule.But the suspension can’t storage for a long time. Aqueous suspensions will be transferred to a tablet or a spray-dried product anyway, with increasing stability and not affecting the efficacy.This research is under way.
Keywords:
nanoparticle,
Bioactivity,
Biocompatibility
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials for therapeutic delivery
Citation:
Zheng
A,
Sun
J and
Cheng
Z
(2016). Evaluation for the testosterone undecanoate loaded nanostructured lipid carriers.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01312
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Aiping Zheng, Beijing Institute of Pharmacology and Toxicology, Department of Pharmaceutics, Beijing, China, Email1
Dr. Jianxu Sun, Beijing Institute of Pharmacology and Toxicology, Department of Pharmaceutics, Beijing, China, Sunjx@163.com