Introduction: Collagen-based materials, used in soft tissue repair, are naturally degraded in the body by the complementary action of proteolytic enzymes[1], primarily MMP-1 and MMP-8, which are commonly encountered during physiological wound healing[2]. However, customarily in vitro degradation assays, not only do not take into consideration the composition of the device, but also fail to closely imitate the host’s microenvironment, frequently resulting in overestimation of the device self-life. Herein, we aim to develop a collagen-based devices specific in vitro assay that would most closely imitate the in vivo degradation.
Experimental Methods: Commercially available tissue grafts (fig.1) derived from porcine skin [Permacol™ (Covidien), non-cross-linked Permacol™ (Covidien) and Strattice™ (Life Cell)] were subjected to enzymatic degradation using MMP-1 (Sigma C0130) and MMP-8(Invitrogen 17101015). The degradation assays were performed under three concentrations of each enzyme (50, 100 and 200 U/ml) and two pH values (5.5 and 7.4) at 3, 6, 9, 12 and 24 hours. The extent of degradation was assessed by visual inspection (fig 2); weight loss (table 1); hydroxyproline assay; and differential scanning calorimetry (DSC)
Results and Discussion:

Fig 1. Environmental scanning electron microscope ESEM images of PermacolTM non-cross-linked PermacolTM and StratticeTM surfaces before degradation.
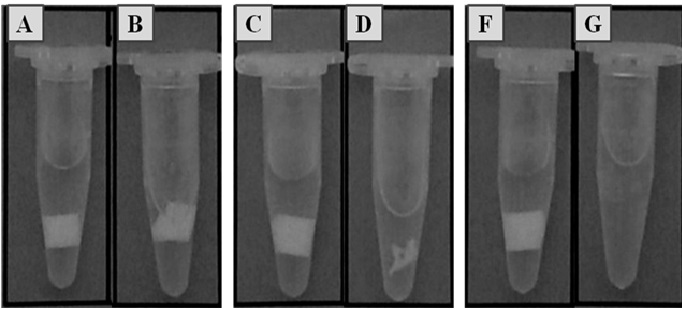
Fig 2.Images of Permacol™ (A: not degraded, B:24h of degradation), non-cross-linked Permacol™ (C: not degraded, D:24h) and Strattice™ (F: not degraded, G:24h).
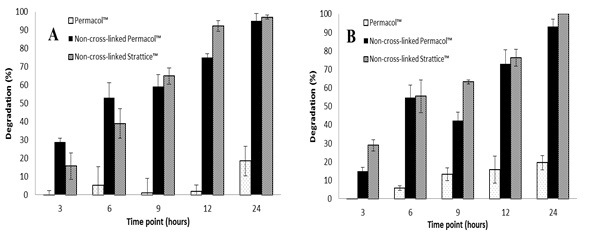
Table 1. Degradation with MMP-1(A) and MMP-8 (B) of the materials to different time points, 200 U/ml and pH 7.4 using weight loss assays.
The results showed an increase in the degradation with the time and with the concentration of the enzyme at pH 7.4. Also, the degradation of non-cross-linked materials is higher that the cross-linked after 24 hours. The degradation temperature of the collagen-based meshes, measured with DSC, decrease after enzymatic degradation.
Conclusion: The natural degradation of collagen-based meshes can be recreated in vitro in order to predict future results after implantation.
The authors would like to acknowledge Covidien for providing financial support to this project.
References:
[1] Zeugolis, D. Compr. Biomater. 261–278, 2011
[2] Badylak, S. et al., Acta Biomater. 5, 1–13, 2009