Introduction: Systemic administration of chemotherapeutic agents results in indiscriminate drug distribution and severe toxicity. Until now, encapsulation and targeting of drugs have typically relied on synthetic vehicles, which cannot minimize clearance by the renal system and may also increase the risk of chemical side effects. Cell membrane capsules (CMCs) provide a generic and far more natural approach to the challenges of drug encapsulation and delivery in vivo[1],[2].
Results and Discussion: Here aptamer AS1411, which can recognize and bind over-expressed nucleolin on cancer cell membrane, was chemically conjugated onto CMCs. As a result, AS1411 modified CMCs showed enhanced ingestion in certain cancer cells in vitro and accumulation in mouse cancer xenografts in vivo. Chemotherapeutics and contrast agents with therapeutically significant concentrations can be packaged into CMCs by reversible permeating their plasma membranes. The systematic administration of cancer targeting CMCs loaded with doxorubicin hydrochloride can significantly inhibit tumor growth in mouse xenografts, with significantly reduced toxicity compare to free drug. These findings suggest that cancer targeting CMCs may have considerable benefits in drug delivery and cancer treatment.
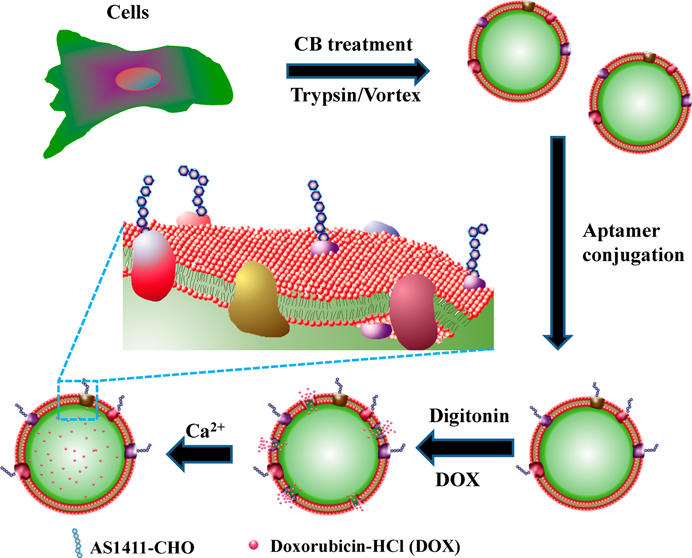
Conclusion: In this study, biocompatible cell membrane capsules were prepared from human endothelial cells with preserved membrane structure and functions. Cancer cell targeting aptamer AS1411 was conjugated onto the CMCs, resulted in enhanced ingestion of CMCs in certain cancer cells and accumulation of CMCs in mouse tumor xenografts. Chemotherapeutics and contrast agents can be packaged into the CMCs by reversible permeating their plasma membranes. The systematic administration of cancer targeting CMCs loaded with doxorubicin can inhibit tumor growth in mouse xenografts, with significantly reduced toxicity compare to free drug. These findings suggest that targeting CMCs may have considerable benefits in drug delivery and cancer treatment.
Natural Science Foundation of China (51120135001); Key Science Technology Innovation Team of Zhejiang Province (2013TD02)
References:
[1] Z. Mao, et al. Cells as Factories for Humanized Encapsulation. Nano Letters, 2011, 11, 2152-2156.
[2] L. Peng et al. ACS Applied Materials & Interfaces, 2015, http://dx.doi.org/10.1021/acsami.5b05065.