Introduction: Additive manufacturing allows a high degree of freedom in designing implants and a route for patient customized implant treatments. Porous electron beam melted (EBM) implants have shown good bone ingrowth capacity[1]. A majority of the data has been obtained by 2D histological analysis, which is limited to a central ground-section. In bone-implant research, x-ray micro-computed tomography (microCT) allows non-destructive morphometric measurements to be performed in 3D. This work aims to correlate 2D and 3D morphometry obtained by microCT with conventional histological analysis of bone ingrowth in porous metal implants. The study outline (Fig. 1) addresses the accuracy of assessment of bone area and bone-implant contact and the effect of resin embedding.
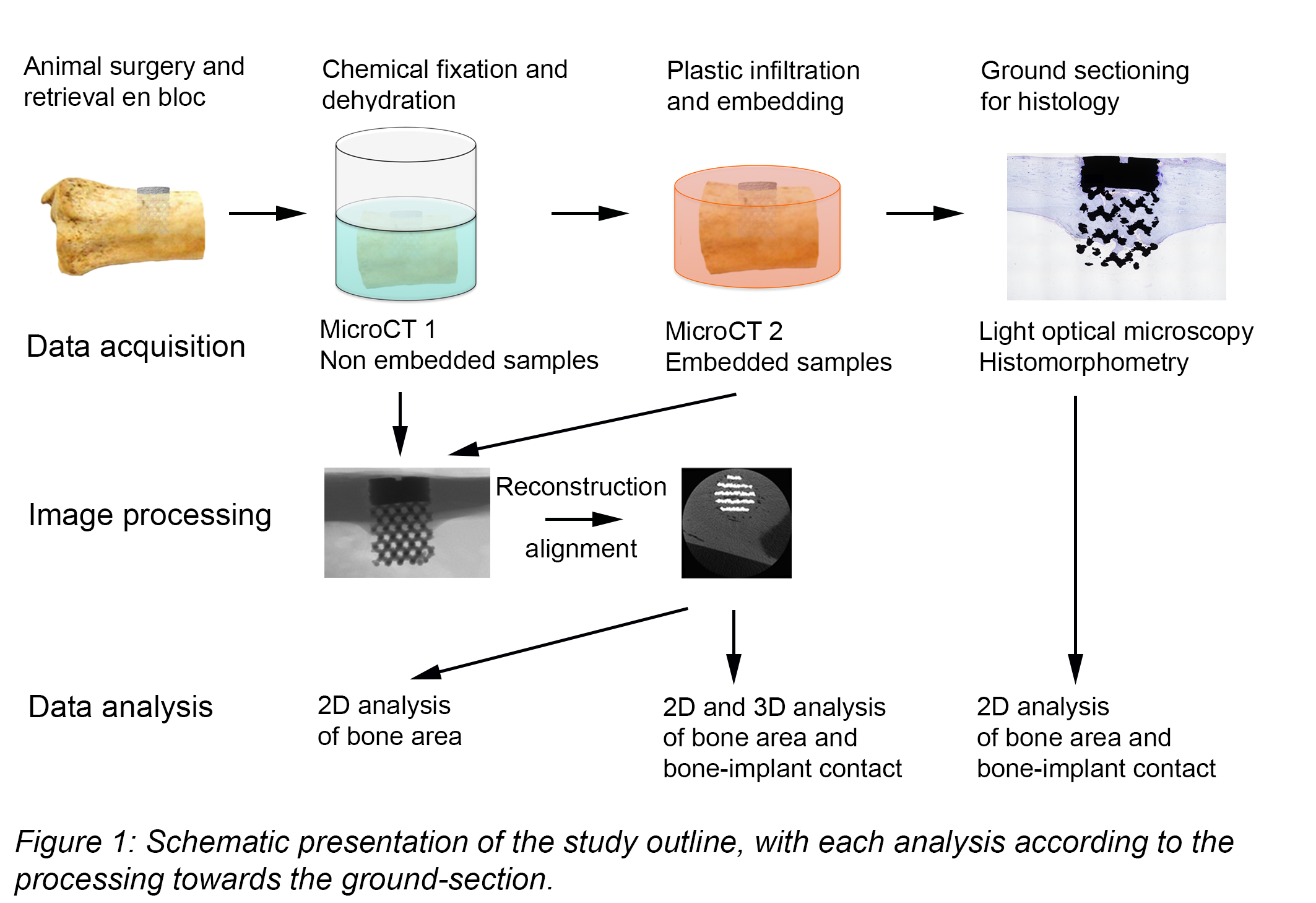
Materials and Methods: 3D printed porous constructs manufactured from Ti6Al4V ELI powder using EBM (Arcam, Mölndal, Sweden) were implanted in adult sheep femora and tibiae. After six months, the implants were retrieved en bloc with surrounding tissue (n=6), fixed in formalin and scanned (in ethanol) by x-ray imaging (Skyscan 1172, Bruker mCT, Kontich, Belgium) at 100 kV with a Cu/Al filter. The specimens were then embedded in LR White resin and scanned again. The resolution level was set to 13.98 µm in all scans with an average scan time of 25 min. Reconstruction and analyses were performed using Skyscan software. A central ground-section (15–20 um thick) was obtained by sawing and stained with toluidine blue for histomorphometric evaluation. MicroCT data sets were aligned: (i) for 3D analysis, transaxial slices were saved along the long-axis, (ii) for 2D analysis, a single coronal section aligned to the corresponding ground-section was saved. The segmentation threshold was set manually by studying the reconstructed images. Different spans were used for the datasets scanned in ethanol and LR white. Bone area and bone-implant contact were measured within and around the porous network (Fig. 2).
The Spearman’s rho (ρ) correlation test was used for statistical analysis (SPSS Statistics 23.0).

Results: Histology and microCT showed high amounts of bone ingrowth into the porous implants. Variance was observed across the animals and at different locations on each implant. A different bone ingrowth pattern was noted for femur and tibia (Fig. 3A). Lower amounts of new bone were found in the inner than the outer part of the porous network and outside the implant. A high correlation was found between embedded and non-embedded microCT scans (Fig. 3B). Exemplified by histological section and the corresponding segmented sections from 2D and 3D (Fig. 3C) significant correlation in bone area was found between 2D and histology (Fig. 3D). Histology, 2D and 3D analyses showed comparative values for the individual implants (Fig. 3F). In contrast, and despite similar mean values, the bone-implant contact showed no correlation between 2D and histology (Fig. 3E).

Discussion and Conclusions: The correlation between microCT and histology have only been performed for solid screw-shaped implants[2],[3]. Metal-related artefacts, e.g., scattering and beam hardening, make resolving the zone close to implant surfaces challenging[4]. While bone-implant contact is difficult to assess due to technical limitations, this work demonstrates that bone area (or volume) is possible to accurately assess by microCT. Further, resin embedding does not affect quantitative analysis of bone area within porous metal implants.
The financial support from the Swedish Research Council (grant K2015-52X-09495-28-4), the BIOMATCELL VINN Excellence Center of Biomaterials and Cell Therapy, the Region Västra Götaland, the IngaBritt and Arne Lundberg Foundation, Hjalmar Svensson Foundation, Adlerbertska Foundation and the Swedish Government Strategic Funding of Materials Science Area of Advance is acknowledged.
References:
[1] Palmquist A, Snis A, Emanuelsson L, Browne M, Thomsen P. Long-term biocompatibility and osseointegration of electron beam melted, free-form-fabricated solid and porous titanium alloy: Experimental studies in sheep. J Biomater Appl. 2013;27:1003-16.
[2] Liu S, Broucek J, Virdi AS, Sumner DR. Limitations of using micro-computed tomography to predict bone-implant contact and mechanical fixation. J Microsc. 2012;245:34-42.
[3] Arvidsson A, Sarve H, Johansson CB. Comparing and visualizing titanium implant integration in rat bone using 2D and 3D techniques. J Biomed Mater Res B Appl Biomater. 2015;103:12-20.
[4] Li JY, Pow EH, Zheng LW, Ma L, Kwong DL, Cheung LK. Quantitative analysis of titanium-induced artifacts and correlated factors during micro-CT scanning. Clin Oral Implants Res. 2014;25:506-10.