Simulation of drug release and mobility in healthy and osteoporotic bone
-
1
Justus-Liebig University Giessen, Physical Chemistry, Germany
-
2
University Hospital Giessen-Marburg GmbH, Trauma Surgery, Germany
-
3
Justus-Liebig University Giessen, Laboratory for experimental trauma surgery, Germany
Introduction: Drug functionalization of biomaterials is an innovative and popular approach in biomaterials research. Amongst others this concept is used for the functionalization of bone implants to stimulate locally the osseointegration. Due to practical and technical reasons it is almost impossible to track the drug release kinetics, drug distribution and the degradation of the implant material in vivo. Due to low drug concentrations in bone it is also not easy to image the drug distribution by analytical methods in post mortem examinations. Based on the results of various analytical experiments we developed a two-phase model for the release and mobility of the pharmaceutical agents Sr2+ and Bortezomib. Sr2+ is used as drug in osteoporosis therapy to stimulate bone growth and was released locally by a strontium doped cement. Bortezomib is an anti-cancer drug administered in therapy of multiple myeloma and was released by a collagen scaffold. The presented simulation experiments allow the determination of appropriate functionalized biomaterials and might help to reduce the number of animal experiments in the future.
Materials and Methods:The time and spatially resolved drug movement was calculated by a finite element calculation for the femur of healthy and osteoporotic rats. Therefore scanning electron microscopy (SEM) images were taken of cross sections of healthy and osteoporotic rat femur. The backscattering electron detector (BSE) was used to obtain materials contrast in the image. The dark regions of the images belong to mineralised areas and were defined as area 1. The bright areas correspond to bone marrow and were defined as area 2. By using the finite element software Comsol 5.1 the time dependent drug mobility was calculated in a 2D model. For the Sr2+ release from the cements we assumed a first order kinetics and defined two different diffusion coefficients for area 1 and 2 with D1 < D2. For the second model (Bortezomib mobility) we assumed a finite source with immediate drug availability and D1 = D2. The obtained results are compared to mass images that were obtained from sections of in vivo experiments by Time of Flight Secondary Ion Mass Spectrometry (ToF-SIMS).
Results and Discussion: In contrast to healthy rat bone (see Fig.1), the osteoporotic bone shows a reduced trabecular network and the trabeculae itself are thinner as can be seen from Fig. 2. The first model focuses on the Sr2+ release from the doped calcium phosphate cement. For the simulation a virtual drill hole of 2 mm diameter, which is filled with the cement, is placed in the bone area. A fast distribution of Sr2+ in the bone marrow is calculated due to the reduced trabecular network in the osteoporotic bone. In contrast, in to healthy bone the single trabeculae act as diffusion barrier for the drug. The simulated distribution image fits quite well to the experimentally obtained results from ToF-SIMS imaging.
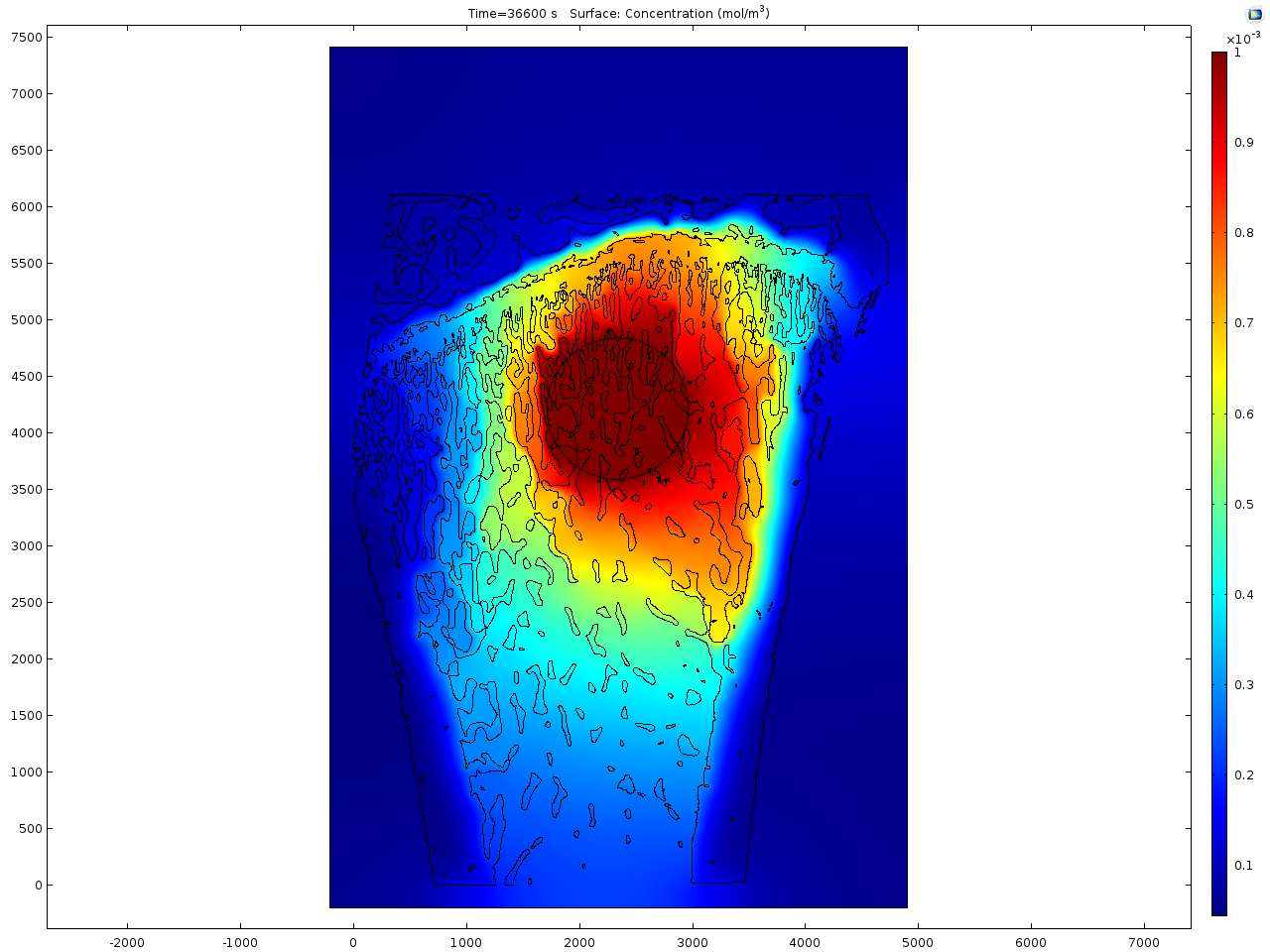
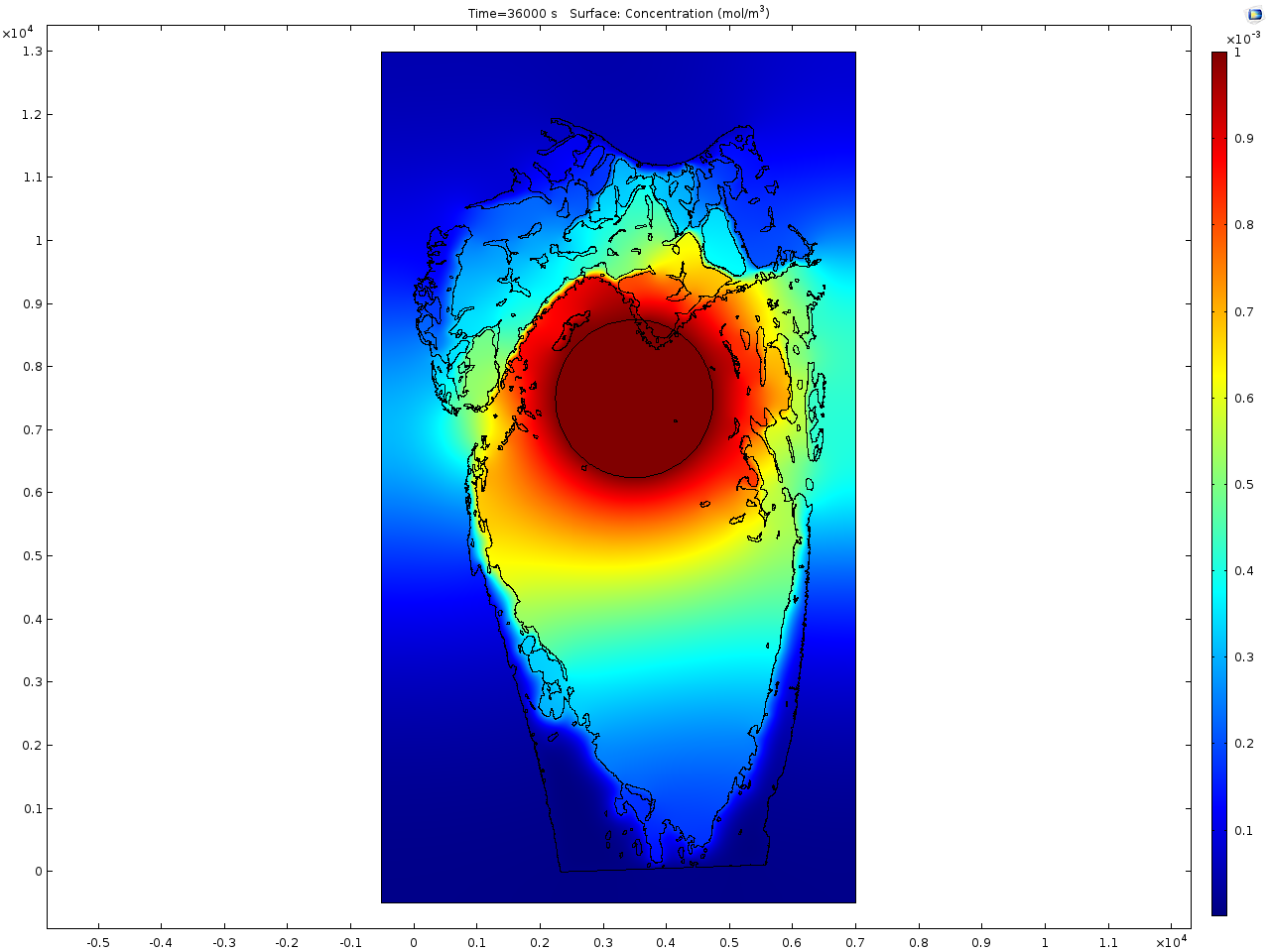
In the second model the distribution of an organic drug molecule is simulated. In contrast to Sr2+ the mobility is definitely higher and it seems to be the same mobility in the bone marrow as in the mineralised regions. Here no significant difference between the healthy and osteoporotic bone is calculated that is again in line with the obtained results from the ToF-SIMS measurements.
Conclusions: The presented simple models for drug release and mobility in bone fit quite well to the obtained experimental data. It appears that drug removal via the vascular system is negligible. This is a good basis for predictions of drug mobility in bone and might help to reduce the number of animal studies. Certainly, it is necessary to determine more precise diffusion coefficients for the drug in cortical, trabecular bone and bone marrow in the future.
Keywords:
Mechanism,
Drug delivery
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Drug-eluting devices
Citation:
Rohnke
M,
Mogwitz
B,
Henss
A,
Ray
S,
Alt
V and
Janek
J
(2016). Simulation of drug release and mobility in healthy and osteoporotic bone.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01592
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.