Microporous ß-tricalcium phosphate (TCP)
-
1
Albert Ludwig University of Freiburg, Department of Surgery, Clinic of Orthopaedics and Trauma Surgery, Germany
-
2
RMS Foundation, Switzerland
Introduction: Pores in calcium phosphate (CaP) materials are necessary to form bone tissue because they allow the migration and proliferation of osteoblast and osteoblast progenitor cells, as well as vascularisation. Interest continues to focus on macroporous CaP ceramics having pores over 100 µm in diameter. Little attention has been paid to micropores with diameters between 0.5 µm and 10 µm. Only recently has it become clear that microporosity exerts a considerable effect on the osteointegration and neoformation of bone. Some authors found cells inside micropores [1],[2].Sheep studies were made to test the applicability of a microporous cylindrical plug for anterior cruciate ligament reconstructions, tissue engineering of bone, and osteochondral defects. Cells and tissue were found within the ceramic in all anatomical locations at all time-points [3],[4].
Hypothesis: Cells can occupy micropores. The interconnectivity of ceramic scaffold build a network and this is used for nutrient and waste transfer.
Materials and methods: Microporous (5 µm) cylindrical plugs of ß-TCP (diameter, 7 mm; length, 25 mm, porosity 40%, axial failure load 7200 N / cm ²) with interconnecting pores were used. Scaffolds were placed in medial femoral condyles, in the area of origin of the anterior cruciate ligament, or in medial femoral condyles. The healing process was examined by histology, histomorphometry, immunohistochemistry, and electron microscopy. Buffy coats from human donors or ovine blood were used for monocyte isolation. Human primary osteoblasts were isolated from cancellous bone. MSCs were extracted from the bone marrow.
Results: There are four different regions revealing cell type and processes: region I – TCP cylinder before fragmentation, region II - border zone bone and ceramic cylinder, and region III - after fragmentation and incorporation of TCP into bone. Region IV is the original bone. Cell culture experiments showed that human and ovine monocytes (MOMC´s) were able to colonized microspores. Human and ovine osteoblasts as well as human and ovine MSC´s do not colonized micropores.
Discussion: Our data clearly show that cells can migrate into micropores. DAPI and Giemsa staining in Fig. 1 clearly revealed that micropores contained cell nucleii surrounded by cytoplasma.
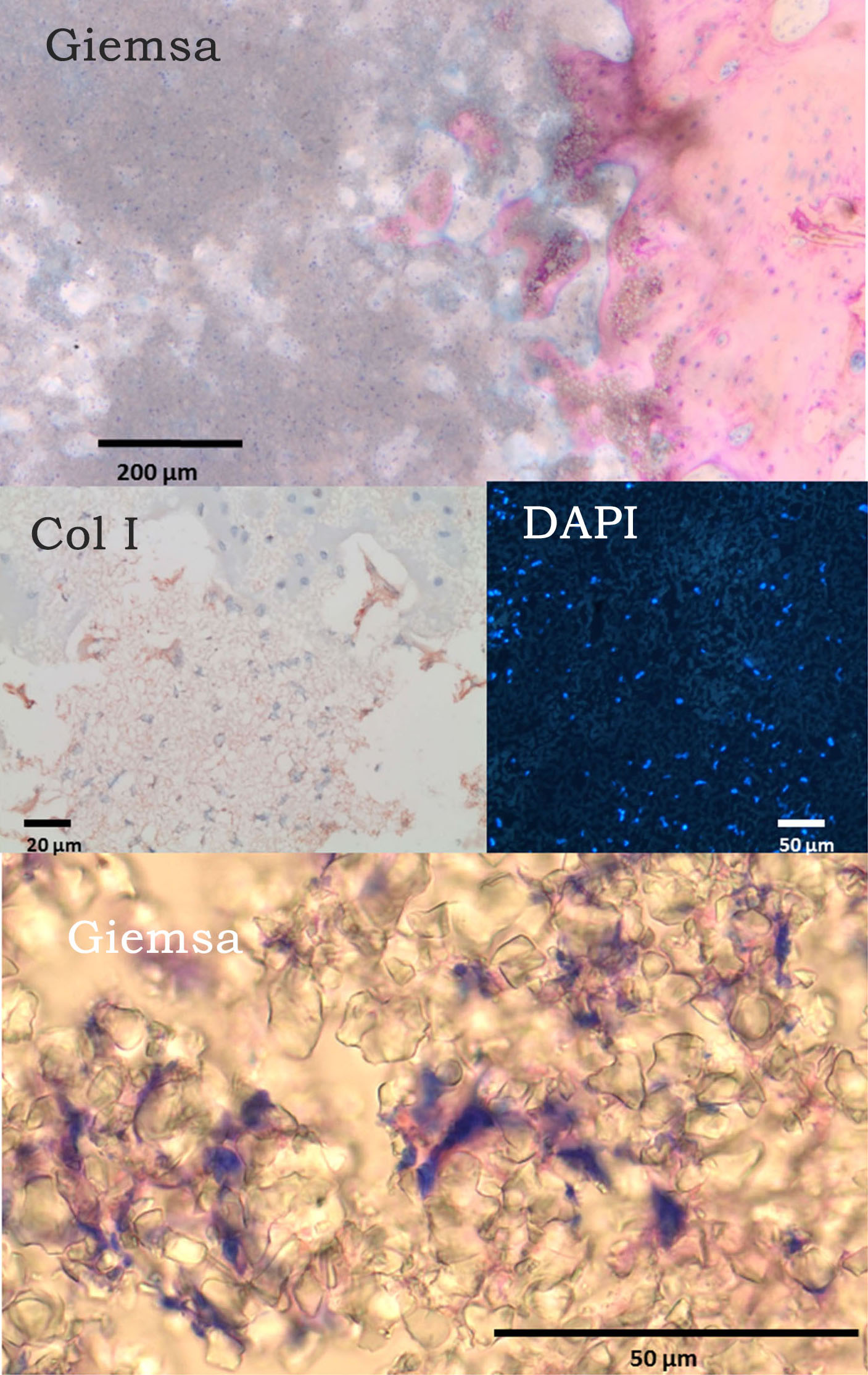
.
The cells present within the micropores expressed markers that display a phenotype similar to CD16+ monocytes. Therefore, it is likely that such cells are monocytes. Monocyte-derived multipotential cells originate from circulating CD14+ monocytes and include primitive cells that can differentiate into cells with the morphologic, phenotypic, and functional features of several distinct mesenchymal cell types. The current results suggest that CD16+ monocytes and MOMCs can occupy micropores and survive there.Monocytes further mature into macrophages and later into osteoclasts in region II. In region III,TCP fragments were surrounded by new bone. MOMPs can differentiate into osteoblasts and later into osteocytes.Cells inside these fragments revealed an osteoblast-like phenotype. Region IV included the original bone.We found there osteoprogenitor cells, osteoblasts, osteocytes, and osteoclasts. In-vitro experiments showed that ovine as well as human cells of monocyte phenotype were able to colonized 5 µm micropores. Further experiments with different subsets of monocytes need to confirm the process of populating micropores with cells.
This study was supported by the RMS Foundation.; The authors would like to thank the members of the Interdisciplinary Center of Materials Science (CMAT), in particular Frank Syrowatka, for Environmental Scanning Electron Microscopy pictures and General Materials Sciences, Martin-Luther-University Halle- Wittenberg for assistance in raster electron microscopy and TEM
References:
[1] Habibovic P et al. Biomaterials 26(17):3565-75. 2005
[2] Lan Levengood SK et al. Biomaterials 31(13):3552-63. 2010
[3] Mayr HO et. al. Acta Biomater. 9(1):4845-55. 2013.
[4] Bernstein A et al. Acta biomaterialia. 2013;9:7490-505.
Keywords:
cell phenotype,
microstructure,
Tissue Regeneration,
Calcium phosphate
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials evaluation in animal models
Citation:
Bernstein
A,
Alsaleh
D,
Bohner
M,
Südkamp
NP and
Mayr
HO
(2016). Microporous ß-tricalcium phosphate (TCP).
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02115
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.