Potentiating the Activity of Nisin against Escherichia coli
- Department of Molecular Genetics, Groningen Biomolecular Sciences and Biotechnology Institute, University of Groningen, Groningen, Netherlands
Lantibiotics are antimicrobial (methyl)lanthionine-containing peptides produced by various Gram-positive bacteria. The model lantibiotic, nisin, binds lipid II in the cell membrane. Additionally, after binding it can insert into the membrane creating a pore. Nisin can efficiently inhibit the growth of Gram-positive bacteria and resistance is rarely observed. However, the activity of lantibiotics is at least 100-fold lower against certain Gram-negative bacteria. This is caused by the fact that Gram-negative bacteria have an outer membrane that hinders the peptides to reach lipid II, which is located in the inner membrane. Improving the activity of lantibiotics against Gram-negative bacteria could be achieved if the outer membrane traversing efficiency is increased. Here, several anti-Gram-negative peptides (e.g., apidaecin 1b, oncocin), or parts thereof, were fused to the C-terminus of either a truncated version of nisin containing the first three/five rings or full length nisin. The activities of these fusion peptides were tested against Gram-negative pathogens. Our results showed that when an eight amino acids (PRPPHPRL) tail from apidaecin 1b was attached to nisin, the activity of nisin against Escherichia coli CECT101 was increased more than two times. This research presents a new and promising method to increase the anti-Gram-negative activity of lantibiotics.
Introduction
Lantibiotics are ribosomally synthesized and post-translationally modified peptides. After modification, they consist of one or more (methyl)lanthionine rings, dehydroalanines, or dehydrobutyrines. Additionally, some lantibiotics display additional modifications (Willey and van der Donk, 2007). Most lantibiotics inhibit the growth of Gram-positive bacteria using lipid II as a target molecule (Bauer and Dicks, 2005). Lipid II plays an essential role in cell-wall synthesis. Diverse lantibiotics bind to the pyrophosphate group in lipid II, and subsequently form pores in the membrane, which is fatal for the bacteria (Breukink et al., 1999; Hasper et al., 2006). Specific resistance to lantibiotics is therefore rarely found (Breukink and de Kruijff, 2006; Draper et al., 2015). However, Gram-negative bacteria have an outer membrane, which is composed of a phospholipid layer (inside) and an outside layer of lipopolysaccharide (LPS) which contains lipid A and polysaccharide chains (Erridge et al., 2002). The LPS is highly negative-charged and the core oligosaccharide region is ordered by divalent cations (mainly Ca2+ and Mg2+) (Clifton et al., 2015), which can hamper lantibiotics from reaching lipid II in the inner membrane. Thus, enhancing the activity of lantibiotics against Gram-negative pathogens first requires improving the outer membrane penetration capability.
Nisin (Figure 1) produced by Lactococcus lactis is the first identified lantibiotic (Lubelski et al., 2008). The structural gene of nisin encodes a 57 amino acids prepeptide. The first 23 amino acids form the leader part and the last 34 residues constitute the core peptide. The leader peptide guides the core peptide through the modification and transport system, and keeps nisin inactive (Plat et al., 2013). Firstly, NisB dehydrates the serines and threonines to form dehydroalanines (Dha) or dehydrobutyrines (Dhb) (Ortega et al., 2015). Then NisC couples the cysteine to the Dha or Dhb by a sulfhydryl addition reaction (Kuipers et al., 1993; Koponen et al., 2002). The modified peptide is transported to the outside of the cell by NisT (Kuipers et al., 2004). NisP is a protease specifically cutting off the leader peptide liberating active nisin (van der Meer et al., 1993). After modification, the peptide contains five (methyl)lanthionine rings, two Dha residues, and one Dhb. NisB and NisC have a relaxed substrate specificity, and when the core peptide is replaced by other sequences, the modifications can still be performed in most cases (Kluskens et al., 2005; Rink et al., 2007a; Majchrzykiewicz et al., 2010).
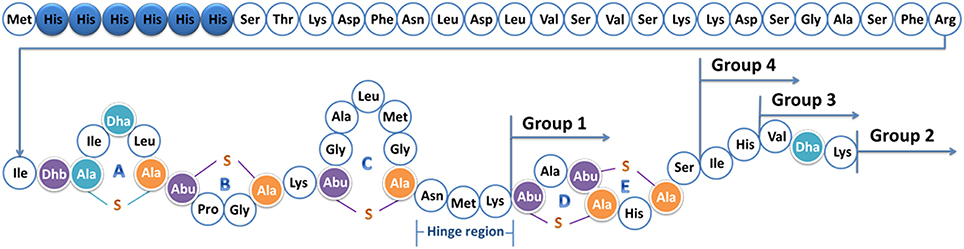
Figure 1. Structure of prenisin with his-tag. The six histidine residues are labeled in blue. Dha, dehydroalanine; Dhb, dehydrobutyrine; Ala-S-Ala, lanthionine; Abu-S-Ala, β-methyllanthionine. The rings ABCDE and the hinge region (Asn- Met-Lys) are marked; the positions of the tails are indicated. The molecular weight is 6640.7 Da.
Nisin can efficiently inhibit the growth of Gram-positive bacteria, with a minimal inhibitory concentration in the nanomolar range. The rings A and B of nisin can bind to the pyrophosphate of lipid II on the membrane of Gram-positive bacteria by forming a pyrophosphate cage (Hsu et al., 2004) and consequently inhibit cell wall synthesis. The N-terminal part is essential for the activity of nisin, and if the C-terminal part is deleted, moderate activity is still observed (Rink et al., 2007b). After binding, the C-terminal part of nisin can translocate across the membrane and form pores by assembling a pore complex in a stoichiometry of 8 nisin and 4 lipid II molecules (Breukink and de Kruijff, 2006).
Nisin displays much lower activity against most Gram-negative bacteria, because the outer membrane can prevent the peptide to reach the periplasm and to exert activity binding lipid II in the inner membrane. When the outer membrane is destabilized using ethylenediaminetetraacetic acid (EDTA) or pyrophosphate, nisin can inhibit the Gram-negative bacteria more efficiently (Boziaris and Adams, 1999; Helander and Mattila-Sandholm, 2000). This indicates that passing the outer membrane is crucial for the activity of nisin against Gram-negative bacteria.
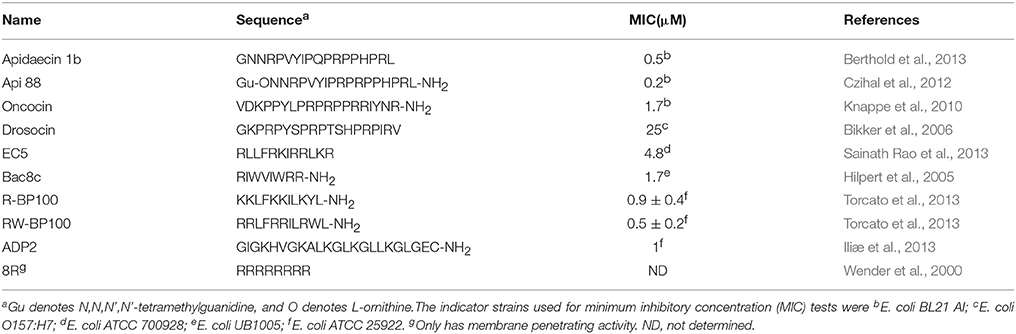
Notably, there are some antimicrobial peptides (AMPs) which can efficiently inhibit the growth of Gram-negative bacteria, such as apidaecin 1b, oncocin, or EC5 (Table 1). These peptides are normally positively charged, some are proline-rich and they all can efficiently traverse the outer membrane. In this paper we aim to design and produce hybrid molecules that combine the lipid II binding capacity of nisin with the ability of these eukaryotic peptides to cross the outer membrane of Gram-negative bacteria. Thus, the anti-Gram negative peptides or the membrane-translocating part of them were attached to nisin or to the N-terminal part of nisin, and the activities of the fusions against Gram-negative bacteria were tested. One fusion was found to have higher activity than nisin, which indicates the potential of this approach. This is the first attempt to potentiate the activity of nisin against Gram-negative microorganisms by adding a tail that could facilitate traversing the outer membrane.
Materials and Methods
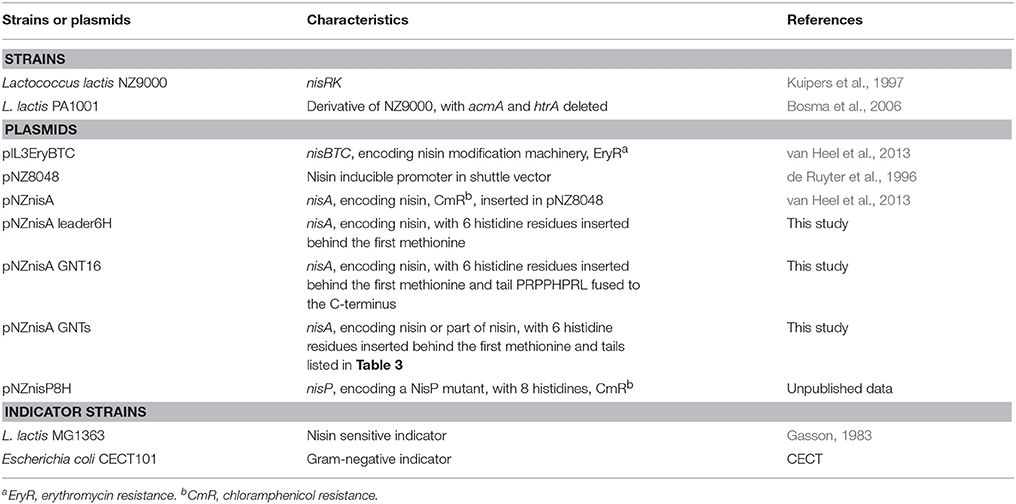
Bacterial Strains and Growth Conditions
The bacterial strains used in this study are listed in Table 2. L. lactis strains were cultured in M17 broth supplemented with 0.5% (w/v) glucose (GM17) for genetic manipulation or in minimal expression medium (MEM) for protein expression at 30°C (Rink et al., 2005). E. coli CECT101 was grown in Luria-Bertani broth aerated by shaking (200 rpm) at 37°C.
Molecular Cloning
A 6 his-tag was added to the leader part of nisin by PCR based on the plasmid pNZnisA as described previously (Zhou et al., 2015). Standard molecular cloning was performed according to Sambrook and Russell (2001). The tails were added to nisin by designing primers containing the sequences of the tail and a selected part of nisin with SacI and HindIII at either end. The primers were annealed according to the protocol on the website of Sigma-Aldrich (1Protocol for Annealing Oligonucleotides). The annealed double strand DNAs were ligated to the pNZnisA leader6H vector cut by SacI and HindIII. Competent cells were prepared and transformed as described previously (Holo and Nes, 1995).
Protein Expression, TCA Precipitation, and Tricine SDS-PAGE
The expression of the peptides was conducted using L. lactis NZ9000 or L. lactis PA1001 containing the plasmids pIL3EryBTC and pNZnisA leader6H harboring the nisin and anti-Gram negative tail fusion. The culture and expression methods were the same as previously described (Zhou et al., 2015). Cells were cultured at 30°C first in GM17 medium with 4 μg/ml chloramphenicol and 4 μg/ml erythromycin until OD (600 nm) reached 0.7, then centrifuged and resuspended in the same amount of MEM medium with 0.5% (w/v) glucose, 3 μg/ml chloramphenicol, 3 μg/ml erythromycin, and 2 nM nisin to induce the expression of the peptides. After 3 h induction, the supernatant was harvested. The supernatant of a small volume of fermentation (< 10 ml) was concentrated by trichloroacetic acid (TCA) precipitation (Sambrook and Russell, 2001) and the concentrated peptides were loaded on a 16% Tricine SDS-PAGE gel (Schägger, 2006). NisP was expressed to cleave off the leader part of the peptides. The strain NZ9000 (pNZnisP8H) was cultured and harvested in the same way as above, but here only chloramphenicol was added.
Purification, Characterization, and Quantification
For large scale purification, 2 L supernatant containing the mature prepeptide and 100 ml supernatant containing NisP were filtered (0.2 μm membrane, Millipore), mixed together and incubated at 30°C for 1 h to cut off the leader peptide. After incubation, the active peptides were first purified by cation-ion exchange chromatography (van Heel et al., 2013). Then, the eluate was loaded on a C18 (Spherical C18, Sigma-Aldrich) column. The peptides were eluted with 30–40% buffer B (Buffer A, miliQ with 0.1% trifluoroacetic acid (TFA); Buffer B, isopropanol: acetonitrile (2:1) with 0.1% TFA). The elutions from the C18 column were freeze dried. The freeze dried peptides were further purified by HPLC (Agilent 1260 Infinity LC) equipped with a semi-preparative C12 column (Phenomenex 250 × 10 mm, 4 μm, Proteo 90Å) as described previously (Zhou et al., 2015). The fractions were collected, tested for activity against L. lactis and analyzed by MALDI-TOF as described previously (van Heel et al., 2013). The active, fully dehydrated, and pure fractions were freeze dried and quantified with HPLC as described previously (Zhou et al., 2015).
Determination of the Minimum Inhibitory Concentration (MIC)
The indicator strains were first cultured until OD (600 nm) reached 0.5. When testing the MIC value, the culture was diluted 1000 times with the appropriate medium. All the tests were performed with a temperature controlled plate reader (Tecan infinite F200, Tecan Group AG) in a 96-well plate (Greiner Bio-one). The peptides were first diluted in gradient with medium and then mixed with diluted indicator strains. The final concentration of the peptides ranged from 0.00625 to 0.8 μM against L. lactis MG1363 and from 0.25 to 32 μM against E. coli CECT101 in 100 μl volume. The plate was incubated at 30°C or 37°C for 18 h depending on the indicator strain being L. lactis or E. coli, respectively. OD (600 nm) was checked every 30 min. For E. coli, 2 min of shaking was performed before every check. When testing the activity of nisin against E. coli in the presence of EDTA, an EDTA solution was prepared and added to a final concentration of 50, 110, or 250 μM. The minimal concentration of peptide causing no observed growth of indicator strains was considered as the MIC value.
Results
The Anti-Gram Negative Tails are Attached to Different Parts of Nisin
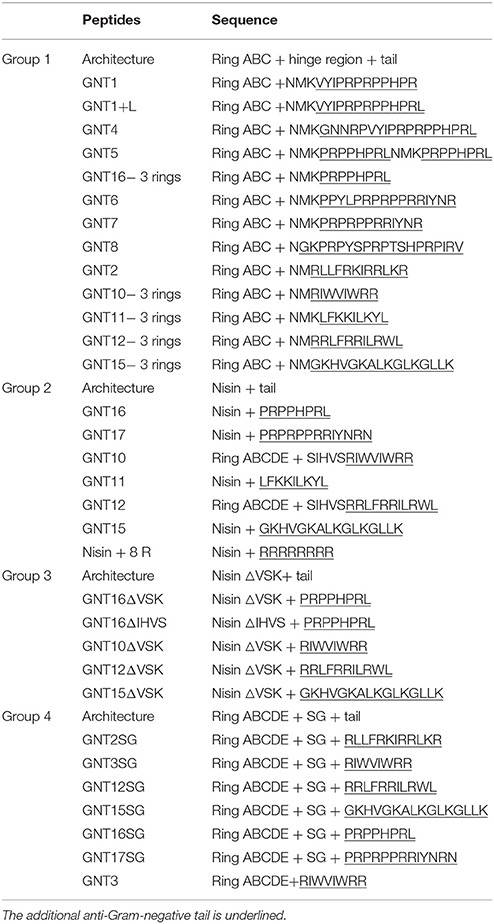
To increase the outer membrane penetration capability of nisin, 10 different anti-Gram-negative peptides were combined with nisin in four different ways (Figure 1, Table 3). More specifically, nisin binds to the lipid II molecule with its first two rings, a process that consequently inhibits the synthesis of the cell wall. Moreover, a mutant of nisin where the amino acids 23–34 were deleted (i.e., rings DE and the C-terminal linear part of nisin) still retains a modest antimicrobial activity (Rink et al., 2007b). We hypothesized that by combining the ABC rings of nisin and an anti-Gram-negative peptide tail, the fusion could gain the potential to traverse the outer-membrane, while maintaining the lipid II binding activity, thereby inhibiting growth. Based on this idea, 13 different peptides were designed (Table 3 Group 1). In the cases of apidaecin 1b and oncocin, different regions of the peptides were linked to the first three rings of nisin. Additionally, the PRPPHPRL tail of apidaecin 1b was added either alone (GNT16-3rings) or as a duplicated motif (GNT5).
The full length nisin can additionally form pores in the cytoplasmic membrane after binding to lipid II, which increases its potency as compared to lipid II sequestering by the first three rings (Breukink and de Kruijff, 2006). Therefore, in this research the anti-Gram negative tails were also fused behind full length nisin (Table 3 Group 2). In those cases where the anti-Gram-negative tail was starting with a positively charged amino acid, Lys34 of nisin was deleted (i.e., GNT10 and GNT12).
As some of the Group 2 peptides (e.g., GNT15) tend to be truncated behind Val32 (data not shown), part of the C-terminal sequence of nisin was deleted in some of the variants (Table 3 Group 3). Following a similar reasoning, the C-terminus of nisin behind ring E was deleted and instead a Ser-Gly linker was added as a flexible linker in front of the tail (Table 3 Group 4). An exception in Group 4 is GNT3, because in this case the anti-Gram-negative tail is directly pasted behind ring E with no linker in between.
The Fusions Show Strongly Varying Production Levels
The nisin and anti-Gram-negative tail fusions were produced by the nisin inducible production system previously described, which consists of NZ9000(pIL3EryBTC, pNZ8048-nisin derivative) (Rink et al., 2005; van Heel et al., 2013). The production levels were monitored by TCA precipitations of the supernatants analyzed by tricine SDS-PAGE (Figure 2A). The production levels of the fusion peptides vary greatly, mainly depending on the types of the tail, e.g., the variants containing the tail from apidaecin 1b (GNT1, GNT16 and GNT16-3rings), oncocin (GNT6 and GNT7) and drosocin (GNT8) showed good production levels. The fusions containing other kinds of tails showed low production levels. Additionally, the MALDI-TOF analysis showed that some variants were partly degraded (data not shown). Furthermore, with the same kind of tail, the design rules also affect the production level, e.g., GNT16-3rings showed generally higher expression levels than GNT1 and GNT16.
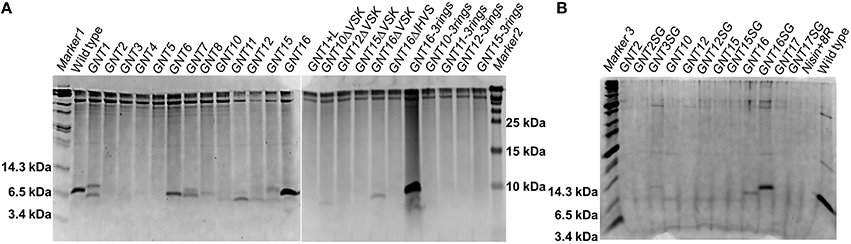
Figure 2. Coomassie-blue stained tricine SDS-PAGE gel. The fusion peptides were produced with NZ9000 (A) or with PA1001 (B). Each well contains TCA precipitated peptides from 600 μl supernatant. All the samples are prepeptides with the leader and his tag part still attached. The experiment was performed several times showing similar results.
To reduce the amount of extracellular proteases and obtain more intact peptides, the deletion strain PA1001 (ΔacmAΔhtrA) (Bosma et al., 2006) was tested to express some of the fusions (Figure 2B). In this system, wild type nisin showed almost the same production level as in the NZ9000 system, but the amount of contaminant proteins was much less. In this case, the GNT3SG (containing Bac8c) was expressed in high amounts. The mutants GNT16 and GNT16SG also showed good production and the production level of GNT16SG was even higher.
The Nisin and Anti-Gram Negative Tail Fusions were Characterized by MS
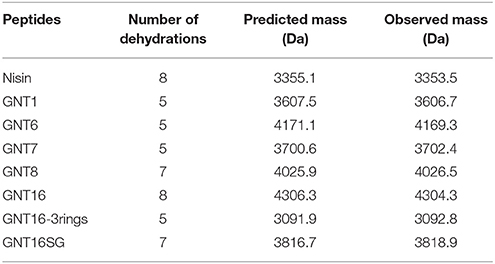
The fusions with relatively higher production levels (GNT1, GNT6, GNT7, GNT8, GNT16, GNT16 -3 rings, and GNT16SG) were further purified and characterized by MS after leader peptide cleavage. After purification, the variants GNT1, GNT16, GNT16 -3rings, and GNT16SG showed almost pure peaks, while the GNT6, GNT7, and GNT8 contained degradation products (Supplementary Figure 1). The mass of the degraded peptides (Supplementary Table 1) indicates that the peptides GNT6, GNT7, and GNT8 tend to be degraded at the C-terminus, with the C-terminal R, NR, YNR, IYNR, or IRV deleted.
The dehydration extent of the intact peptides was analyzed, and the results (Table 4) showed that the GNT1, GNT6, GNT7, GNT16, GNT16 -3 rings, and GNT16SG were fully dehydrated. The GNT8 fusion contains 2 serines and 1 threonine in the tail, and the full peptide was dehydrated seven times, thus five times in the nisin part and two in the tail.
Effect of EDTA on the Activity of Nisin Against E. coli
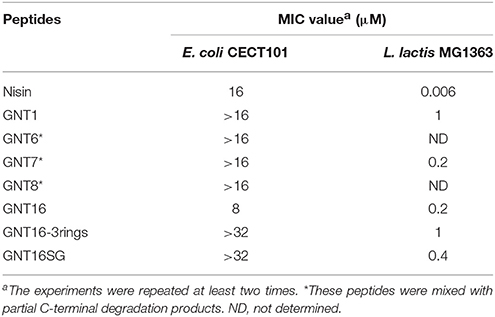
Nisin normally shows low activity against Gram-negative organisms. In this research, we determined a MIC value of nisin of 16 μM against E. coli CECT101 (Table 5). As described before, nisin can inhibit the growth of Gram-negative bacteria when a sufficient amount of EDTA(>100 μM) was added (Helander and Mattila-Sandholm, 2000). In this research, different concentrations of EDTA were added together with nisin. The results show that as the concentration of EDTA went up, less nisin was needed to exhibit full inhibition.
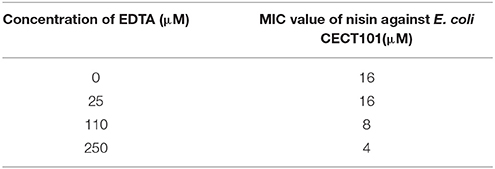
Table 5. Minimum inhibition concentration (MIC) of nisin against E.coli CECT101 in the presence of different concentration of EDTA.
The Fusions Displayed Lower Net Activity than Nisin Against L. lactis
Nisin inhibits the growth of L. lactis MG1363 at the nanomolar range (6 nM Table 6). However, when equipped with a tail, the MIC values against L. lactis increased and ranged between 200 nM (GNT16 and GNT7) and 1000 nM (GNT1 and GNT16-3rings). The activity of GNT16SG against L. lactis was two times lower than GNT16, but 2.5 times higher than GNT16-3rings.
Activity of the Fusions Against Gram-Negative Bacteria
Table 6 shows the activities of nisin and the fusions against E. coli CECT101. The peptides GNT1, GNT6, GNT7, GNT8, GNT16 -3rings, and GNT16SG showed lower net activity than nisin against E. coli, while GNT16 displayed two times higher activity than nisin (Table 6). In addition, the mutants GNT6, GNT7, and GNT8 were a mix of intact and partly degraded peptide (Supplementary Figure 1). Figure 3 shows that 4 μM GNT16 displayed better inhibitory activity than 8 μM nisin, which means that the activity of GNT16 was more than two times higher than that of nisin. GNT16 showed a significant improvement comparable to the concentration of nisin needed in the presence of 110 μM EDTA. Furthermore, GNT16 also showed an equal or slightly better inhibitory activity than nisin against E. coli CIP and Enterobacter aerogenes CECT684, which exhibited lower growth after prolonged incubation (Supplementary Figure 2).
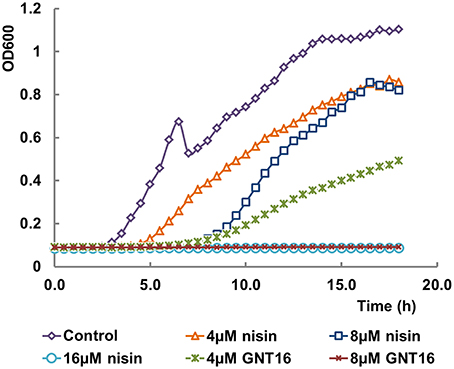
Figure 3. Inhibitory effects of nisin and GNT16 against E. coli CECT101. Either 16 μM nisin or 8 μM GNT16 could fully inhibit the growth of bacteria within 18 h. The experiment was repeated three times.
Discussion
The concomitant use of nisin with other compounds to increase the inhibition effects against Gram-negatives has been described. For instance, the combination with polymyxin E (Naghmouchi et al., 2013) or with chelating agents such as EDTA (Boziaris and Adams, 1999) gave a better inhibition. There are also genetic engineering products which have improved activity. Mutating the hinge region of nisin Z and thereby increasing the positive charge was found to make nisin slightly more active against several species of Gram-negative bacteria, such as Shigella, Pseudomonas and Salmonella but not E. coli (Yuan et al., 2004). In the study of Field et al. (2012), S29 of nisin was mutated into G, A, or E, which resulted for all the mutants in a two-fold increased activity against E. coli 0157-H7 and Salmonella enterica serovar Typhimurium. In our research, the outer membrane-passing capacity of nisin was aided by attaching some highly positive charged (at least four positive charges) antimicrobial peptides as a tail. These AMPs are usually rich in proline, arginine, lysine, and hydrophobic amino acids. In contrast to polymyxin E and EDTA, which disrupt the stability of the outer membrane (Naghmouchi et al., 2013; Clifton et al., 2015), the AMPs go into the cells without breaking the outer membrane. They tend to form a helical structure when interacting with the negatively charged outer membrane and at the same time transit the membrane (Torcato et al., 2013). With this kind of tail as a sort of Trojan horse, the trans-outer membrane efficiency of nisin probably could be increased.
The peptide GNT16 contains a full length nisin and a tail from apidaecin 1b. Apidaecin 1b is a kind of proline-rich AMP, which inhibits the growth of Gram-negative bacteria by translocation into the cytoplasm and binding the chaperone DnaK. Api88 is a derivative of apidaecin 1b with increased positive charges and an amidated C-terminus, which displays higher activity. The crystal structure of DnaK and Api88 showed that residues 5–11(PVYIPRP) of Api88 bind to DnaK. The tail (PRPPHPRL), which does not display DnaK binding activity, probably has a good outer membrane and inner membrane-passing capability (Czihal et al., 2012). In this research, the nisin-PRPPHPRL fusion showed increased activity compared to nisin against E. coli, which is probably because the tail has improved the trans-outer membrane capability of the peptide. Furthermore, the fusion GNT16 showed 32 times decreased activity against L. lactis MG1363, which means that attaching the tail is detrimental for the original inhibition activity of nisin probably due to the elimination of the pore forming ability of nisin (Rink et al., 2007b).
Interestingly, the part of nisin that is retained in the fusion affects the activity of the fusion peptide. The GNT16SG displayed two times lower activity than GNT16 against L. lactis, and more than four times lower activity against E. coli. The GNT16-3 rings displayed even more reduced activity than GNT16SG against both strains. This indicates that the intertwined rings and the last six amino acids of nisin are helpful for the activity. Normally, a helical conformation is formed during the AMPs passing the outer membrane. The C-terminus of nisin has a helical structure (van de Ven et al., 1991) as well, which may be helpful for the outer membrane passing capacity. Alternatively, a longer linker could be beneficial for the tail from apidaecin 1b to form a PP II helix (polyproline helical type II) (Li et al., 2006), and traverse the outer membrane.
The fusion GNT7 consists of the first three rings of nisin and a PRPRPPRRIYNR tail from oncocin. The partly purified GNT7 displayed equal activity as GNT16 against L. lactis and higher activity than GNT16-3rings and GNT16SG, which means that the GNT7 possibly displays relatively better pore formation activity.
We have seen that all the peptides in this research are active against L. lactis (data in Supplementary Figure 3). This is in accordance with the literature, which indicates that the first three rings of nisin retain activity (Rink et al., 2007b). However, as most of the peptides were expressed at very low levels or were degraded after expression, full length fusions are difficult to obtain. To overcome this problem, some in vitro synthesis methods (e.g., solid-phase peptide synthesis) might be employed to increase the success rate of this novel design (Montalbán-López et al., 2012). Some kinds of AMPs (e.g., Api 88, oncocin, Bac8c, R-BP100, RW-BP100, and ADP2) contain a C-terminal amide, which can increase the activity. If the fusion peptides could be amidated as well, probably the activities against Gram-negatives could be enhanced. However, to the best of our knowledge there are no in vivo amidation strategies available in L. lactis. The stability of the tails is very important for the production level of the fusions. The seven fusions obtained in this research contain tails from apidaecin 1b, oncocin, and drosocin, which indicates that proline-rich AMPs are more stable and easier to be expressed in L. lactis. The production level of GNT16-3rings > GNT16SG > GNT16> GNT16ΔVSK > GNT16ΔIHVS, which indicates that a shorter part of nisin (first three or five rings) can render a higher production level but also that the last five residues of nisin form a weak region.
Compared to apidaecin 1b which can inhibit the Gram-negative organisms at 0.5 μM (Berthold et al., 2013), the best fusion peptide showed still a 16-fold higher MIC value, although the experimental setup is different. To increase the activity of nisin against Gram-negative bacteria, both the inhibition activity and trans-outer membrane activity are important. When the first three rings of nisin are intact, the lipid II binding activity is stable. Retaining the pore formation activity is crucial. By further variation of the linker between the nisin part and the tail or searching for more efficient tails, higher pore formation activity and trans-outer membrane efficiency can undoubtedly be obtained. Moreover, after the fusion peptides enter the periplasm, particular periplasmic proteases might be used to liberate both compounds. In this way, both nisin and the anti-Gram-negative peptides could perform their inhibitory activity more efficiently although this point need further study. The periplasmic HtrA protease might be a good candidate to cleave the fusions (Lipinska et al., 1990). Our data combining in a single nisin derivative the lipid II binding activity of nisin with the penetrating activity of eukaryotic antimicrobial peptides indicate that a rational design can improve the activity of lantibiotics against Gram-negative bacteria.
Author Contributions
OPK contributed to the conception and design of the work, analysis, and interpretation of data, and revising the work critically for important intellectual content. LZ performed the acquisition, analysis, and interpretation of data for the work, and drafted the work. AJvH and MML participated the design of the work, analysis, and interpretation of the data, and revised the manuscript critically for important intellectual content. All the authors show final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
LZ is supported by the Chinese Scholarship Council (CSC) and State Key Laboratory of Microbial Technology, School of Life Sciences, Shandong University, Jinan, P. R. China. MML was funded by the NWO project SynMod (855-01-162) and the FP7 project Synpeptide.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fcell.2016.00007
Footnotes
1. ^Protocol for Annealing Oligonucleotides Sigma-Aldrich. Available online at: http://www.sigmaaldrich.com/technical-documents/protocols/biology/annealing-oligos.html (Accessed April 8, 2015).
References
Bauer, R., and Dicks, L. M. T. (2005). Mode of action of lipid II-targeting lantibiotics. Int. J. Food Microbiol. 101, 201–216. doi: 10.1016/j.ijfoodmicro.2004.11.007
Berthold, N., Czihal, P., Fritsche, S., Sauer, U., Schiffer, G., Knappe, D., et al. (2013). Novel apidaecin 1b analogs with superior serum stabilities for treatment of infections by gram-negative pathogens. Antimicrob. Agents Chemother. 57, 402–409. doi: 10.1128/AAC.01923-12
Bikker, F. J., Kaman-van Zanten, W. E., de Vries-van de Ruit, A.-M. B. C., Voskamp-Visser, I., van Hooft, P. A. V., Mars-Groenendijk, R. H., et al. (2006). Evaluation of the antibacterial spectrum of drosocin analogues. Chem. Biol. Drug Des. 68, 148–153. doi: 10.1111/j.1747-0285.2006.00424.x
Bosma, T., Kanninga, R., Neef, J., Audouy, S. A. L., van Roosmalen, M. L., Steen, A., et al. (2006). Novel surface display system for proteins on non-genetically modified gram-positive bacteria. Appl. Environ. Microbiol. 72, 880–889. doi: 10.1128/AEM.72.1.880-889.2006
Boziaris, I. S., and Adams, M. R. (1999). Effect of chelators and nisin produced in situ on inhibition and inactivation of Gram negatives. Int. J. Food Microbiol. 53, 105–113. doi: 10.1016/S0168-1605(99)00139-7
Breukink, E., and de Kruijff, B. (2006). Lipid II as a target for antibiotics. Nat. Rev. Drug Discov. 5, 321–332. doi: 10.1038/nrd2004
Breukink, E., Wiedemann, I., van Kraaij, C., Kuipers, O. P., Sahl, H. G., and de Kruijff, B. (1999). Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286, 2361–2364.
Clifton, L. A., Skoda, M. W. A., Le Brun, A. P., Ciesielski, F., Kuzmenko, I., Holt, S. A., et al. (2015). Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir ACS J. Surf. Colloids 31, 404–412. doi: 10.1021/la504407v
Czihal, P., Knappe, D., Fritsche, S., Zahn, M., Berthold, N., Piantavigna, S., et al. (2012). Api88 is a novel antibacterial designer peptide to treat systemic infections with multidrug-resistant Gram-negative pathogens. ACS Chem. Biol. 7, 1281–1291. doi: 10.1021/cb300063v
de Ruyter, P. G., Kuipers, O. P., and de Vos, W. M. (1996). Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62, 3662–3667.
Draper, L. A., Cotter, P. D., Hill, C., and Ross, R. P. (2015). Lantibiotic resistance. Microbiol. Mol. Biol. Rev. 79, 171–191. doi: 10.1128/MMBR.00051-14
Erridge, C., Bennett-Guerrero, E., and Poxton, I. R. (2002). Structure and function of lipopolysaccharides. Microbes Infect. 4, 837–851. doi: 10.1016/S1286-4579(02)01604-0
Field, D., Begley, M., O'Connor, P. M., Daly, K. M., Hugenholtz, F., Cotter, P. D., et al. (2012). Bioengineered nisin A derivatives with enhanced activity against both Gram positive and Gram negative pathogens. PLoS ONE 7:e46884. doi: 10.1371/journal.pone.0046884
Gasson, M. J. (1983). Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154, 1–9.
Hasper, H. E., Kramer, N. E., Smith, J. L., Hillman, J. D., Zachariah, C., Kuipers, O. P., et al. (2006). An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313, 1636–1637. doi: 10.1126/science.1129818
Helander, I. M., and Mattila-Sandholm, T. (2000). Permeability barrier of the Gram-negative bacterial outer membrane with special reference to nisin. Int. J. Food Microbiol. 60, 153–161. doi: 10.1016/S0168-1605(00)00307-X
Hilpert, K., Volkmer-Engert, R., Walter, T., and Hancock, R. E. W. (2005). High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 23, 1008–1012. doi: 10.1038/nbt1113
Holo, H., and Nes, I. F. (1995). Transformation of Lactococcus by electroporation. Methods Mol. Biol. 47, 195–199. doi: 10.1385/0-89603-310-4:195
Hsu, S.-T. D., Breukink, E., Tischenko, E., Lutters, M. A. G., de Kruijff, B., Kaptein, R., et al. (2004). The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 11, 963–967. doi: 10.1038/nsmb830
Iliæ, N., Novkoviæ, M., Guida, F., Xhindoli, D., Benincasa, M., Tossi, A., et al. (2013). Selective antimicrobial activity and mode of action of adepantins, glycine-rich peptide antibiotics based on anuran antimicrobial peptide sequences. Biochim. Biophys. Acta 1828, 1004–1012. doi: 10.1016/j.bbamem.2012.11.017
Kluskens, L. D., Kuipers, A., Rink, R., de Boef, E., Fekken, S., Driessen, A. J. M., et al. (2005). Post-translational modification of therapeutic peptides by NisB, the dehydratase of the lantibiotic nisin. Biochemistry 44, 12827–12834. doi: 10.1021/bi050805p
Knappe, D., Piantavigna, S., Hansen, A., Mechler, A., Binas, A., Nolte, O., et al. (2010). Oncocin (VDKPPYLPRPRPPRRIYNR-NH2): a novel antibacterial peptide optimized against gram-negative human pathogens. J. Med. Chem. 53, 5240–5247. doi: 10.1021/jm100378b
Koponen, O., Tolonen, M., Qiao, M., Wahlström, G., Helin, J., and Saris, P. E. J. (2002). NisB is required for the dehydration and NisC for the lanthionine formation in the post-translational modification of nisin. Microbiol. Read. Engl. 148, 3561–3568. doi: 10.1099/00221287-148-11-3561
Kuipers, A., de Boef, E., Rink, R., Fekken, S., Kluskens, L. D., Driessen, A. J. M., et al. (2004). NisT, the transporter of the lantibiotic nisin, can transport fully modified, dehydrated, and unmodified prenisin and fusions of the leader peptide with non-lantibiotic peptides. J. Biol. Chem. 279, 22176–22182. doi: 10.1074/jbc.M312789200
Kuipers, O. P., Beerthuyzen, M. M., Siezen, R. J., and de Vos, W. M. (1993). Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216, 281–291.
Kuipers, O. P., de Ruyter, P. G., Kleerebezem, M., and de Vos, W. M. (1997). Controlled overproduction of proteins by lactic acid bacteria. Trends Biotechnol. 15, 135–140. doi: 10.1016/S0167-7799(97)01029-9
Li, W.-F., Ma, G.-X., and Zhou, X.-X. (2006). Apidaecin-type peptides: biodiversity, structure-function relationships and mode of action. Peptides 27, 2350–2359. doi: 10.1016/j.peptides.2006.03.016
Lipinska, B., Zylicz, M., and Georgopoulos, C. (1990). The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J. Bacteriol. 172, 1791–1797.
Lubelski, J., Rink, R., Khusainov, R., Moll, G. N., and Kuipers, O. P. (2008). Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin. Cell. Mol. Life Sci. 65, 455–476. doi: 10.1007/s00018-007-7171-2
Majchrzykiewicz, J. A., Lubelski, J., Moll, G. N., Kuipers, A., Bijlsma, J. J. E., Kuipers, O. P., et al. (2010). Production of a class II two-component lantibiotic of Streptococcus pneumoniae using the Class I nisin synthetic machinery and leader sequence. Antimicrob. Agents Chemother. 54, 1498–1505. doi: 10.1128/AAC.00883-09
Montalbán-López, M., Zhou, L., Buivydas, A., van Heel, A. J., and Kuipers, O. P. (2012). Increasing the success rate of lantibiotic drug discovery by Synthetic Biology. Expert Opin. Drug Discov. 7, 695–709. doi: 10.1517/17460441.2012.693476
Naghmouchi, K., Baah, J., Hober, D., Jouy, E., Rubrecht, C., Sané, F., et al. (2013). Synergistic effect between colistin and bacteriocins in controlling Gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimicrob. Agents Chemother. 57, 2719–2725. doi: 10.1128/AAC.02328-12
Ortega, M. A., Hao, Y., Zhang, Q., Walker, M. C., van der Donk, W. A., and Nair, S. K. (2015). Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature 517, 509–512. doi: 10.1038/nature13888
Plat, A., Kuipers, A., Rink, R., and Moll, G. N. (2013). Mechanistic aspects of lanthipeptide leaders. Curr. Protein Pept. Sci. 14, 85–96. doi: 10.2174/1389203711314020001
Rink, R., Kluskens, L. D., Kuipers, A., Driessen, A. J. M., Kuipers, O. P., and Moll, G. N. (2007a). NisC, the cyclase of the lantibiotic nisin, can catalyze cyclization of designed nonlantibiotic peptides. Biochemistry 46, 13179–13189. doi: 10.1021/bi700106z
Rink, R., Kuipers, A., de Boef, E., Leenhouts, K. J., Driessen, A. J. M., Moll, G. N., et al. (2005). Lantibiotic structures as guidelines for the design of peptides that can be modified by lantibiotic enzymes. Biochemistry 44, 8873–8882. doi: 10.1021/bi050081h
Rink, R., Wierenga, J., Kuipers, A., Kluskens, L. D., Driessen, A. J. M., Kuipers, O. P., et al. (2007b). Dissection and modulation of the four distinct activities of nisin by mutagenesis of rings A and B and by C-terminal truncation. Appl. Environ. Microbiol. 73, 5809–5816. doi: 10.1128/AEM.01104-07
Sainath Rao, S., Mohan, K. V. K., and Atreya, C. D. (2013). A peptide derived from phage display library exhibits antibacterial activity against E. coli and Pseudomonas aeruginosa. PLoS ONE 8:e56081. doi: 10.1371/journal.pone.0056081
Sambrook, J., and Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual, 3rd Edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Torcato, I. M., Huang, Y.-H., Franquelim, H. G., Gaspar, D., Craik, D. J., Castanho, M. A., et al. (2013). Design and characterization of novel antimicrobial peptides, R-BP100 and RW-BP100, with activity against Gram-negative and Gram-positive bacteria. Biochim. Biophys. Acta 1828, 944–955. doi: 10.1016/j.bbamem.2012.12.002
van der Meer, J. R., Polman, J., Beerthuyzen, M. M., Siezen, R. J., Kuipers, O. P., and de Vos, W. M. (1993). Characterization of the Lactococcus lactis nisin A operon genes nisP, encoding a subtilisin-like serine protease involved in precursor processing, and nisR, encoding a regulatory protein involved in nisin biosynthesis. J. Bacteriol. 175, 2578–2588.
van de Ven, F. J., van den Hooven, H. W., Konings, R. N., and Hilbers, C. W. (1991). NMR studies of lantibiotics. The structure of nisin in aqueous solution. Eur. J. Biochem. FEBS 202, 1181–1188.
van Heel, A. J., Mu, D., Montalbán-López, M., Hendriks, D., and Kuipers, O. P. (2013). Designing and producing modified, new-to-nature peptides with antimicrobial activity by use of a combination of various lantibiotic modification enzymes. ACS Synth. Biol. 2, 397–404. doi: 10.1021/sb3001084
Wender, P. A., Mitchell, D. J., Pattabiraman, K., Pelkey, E. T., Steinman, L., and Rothbard, J. B. (2000). The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. U.S.A. 97, 13003–13008. doi: 10.1073/pnas.97.24.13003
Willey, J. M., and van der Donk, W. A. (2007). Lantibiotics: peptides of diverse structure and function. Annu. Rev. Microbiol. 61, 477–501. doi: 10.1146/annurev.micro.61.080706.093501
Yuan, J., Zhang, Z.-Z., Chen, X.-Z., Yang, W., and Huan, L.-D. (2004). Site-directed mutagenesis of the hinge region of nisinZ and properties of nisinZ mutants. Appl. Microbiol. Biotechnol. 64, 806–815. doi: 10.1007/s00253-004-1599-1
Keywords: lantibiotic, nisin, fusion, Gram-negative, outer membrane
Citation: Zhou L, van Heel AJ, Montalban-Lopez M and Kuipers OP (2016) Potentiating the Activity of Nisin against Escherichia coli. Front. Cell Dev. Biol. 4:7. doi: 10.3389/fcell.2016.00007
Received: 21 November 2015; Accepted: 18 January 2016;
Published: 08 February 2016.
Edited by:
Michaela Wenzel, University of Amsterdam, NetherlandsReviewed by:
Stefano Donadio, Naicons, ItalySander Hj Smits, Heinrich-Heine-Universität Düsseldorf, Germany
Copyright © 2016 Zhou, van Heel, Montalban-Lopez and Kuipers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oscar P. Kuipers, o.p.kuipers@rug.nl
 Liang Zhou
Liang Zhou Auke J. van Heel
Auke J. van Heel Manuel Montalban-Lopez
Manuel Montalban-Lopez Oscar P. Kuipers
Oscar P. Kuipers