Francisella tularensis Susceptibility to Antibiotics: A Comprehensive Review of the Data Obtained In vitro and in Animal Models
- 1Laboratoire de Bactériologie-Hygiène Hospitalière, Département des agents infectieux, Centre National de Référence des Francisella, Institut de Biologie et de Pathologie, Centre Hospitalier Universitaire Grenoble Alpes, Grenoble, France
- 2Université Grenoble Alpes, Centre National de la Recherche Scientifique, TIMC-IMAG, Grenoble, France
The antibiotic classes that are recommended for tularaemia treatment are the aminoglycosides, the fluoroquinolones and the tetracyclines. However, cure rates vary between 60 and 100% depending on the antibiotic used, the time to appropriate antibiotic therapy setup and its duration, and the presence of complications, such as lymph node suppuration. Thus, antibiotic susceptibility testing (AST) of F. tularensis strains remains of primary importance for detection of the emergence of antibiotic resistances to first-line drugs, and to test new therapeutic alternatives. However, the AST methods reported in the literature were poorly standardized between studies and AST data have not been previously evaluated in a comprehensive and comparative way. The aim of the present review was to summarize experimental data on antibiotic susceptibilities of F. tularensis obtained in acellular media, cell models and animal models since the introduction of fluoroquinolones in the treatment of tularaemia in 1989. We compiled MIC data of 33 antibiotics (including aminoglycosides, fluoroquinolones, tetracyclines, macrolides, β-lactams, chloramphenicol, rifampicin, and linezolid) against 900 F. tularensis strains (504 human strains), including 107 subsp. tularensis (type A), 789 subsp. holarctica (type B) and four subsp. mediasiatica strains, using various AST methods. Specific culture media were identified or confirmed as unsuitable for AST of F. tularensis. Overall, MICs were the lowest for ciprofloxacin (≤ 0.002–0.125 mg/L) and levofloxacin, and ranged from ≤ 0.016 to 2 mg/L for gentamicin, and 0.064 to 4 mg/L for doxycycline. No resistant strain to any of these antibiotics was reported. Fluoroquinolones also exhibited a bactericidal activity against intracellular F. tularensis and lower relapse rates in animal models when compared with the bacteriostatic compound doxycycline. As expected, lower MIC values were found for macrolides against type A and biovar I type B strains, compared to biovar II type B strains. The macrolides were more effective against F. tularensis grown in phagocytic cells than in acellular media.
Search Strategy and Selection Criteria
Data on antibiotic susceptibility testing (AST) of Francisella tularensis, both in vitro and in animal models, were collected from the English and French literature in the PubMed database, since the introduction of fluoroquinolones in the treatment of tularaemia in 1989 until December 2016. They were extracted using the key words “tularemia” and “Francisella” in order to achieve a broad screening of the entire body of literature on the subject since 1989. Only studies evaluating more than five F. tularensis strains were selected for analysis.
Francisella tularensis and Tularemia
F. tularensis, the etiological agent of tularaemia, is a Tier 1 biological threat agent according to the classification of the Centers for Disease Control and Prevention (Dennis et al., 2001). It was first isolated from ground squirrels in 1911 in Tulare County, California (USA), and from a human tularaemia case in 1914 in Ohio (USA) (Mccoy and Chapin, 1912; Wherry and Lamb, 1914). The name “Francisella tularensis” was coined in 1959 to honor Dr. Edward Francis, who greatly contributed to improve the knowledge on human tularaemia (Francis et al., 1922; Francis, 1928; Rockwood, 1983). Francisella tularensis is currently divided into three subspecies: subsp. tularensis (type A strains), mainly found in North America; subsp. holarctica (type B strains) found throughout the northern hemisphere; and subsp. mediasiatica found in Central Asia (Olsufiev et al., 1959; Jellison and Owen, 1961; Olsufjev, 1970; Jellison, 1974; Olsufjev and Meshcheryakova, 1983). Debate continues on whether Francisella novicida is a fourth subspecies of F. tularensis or a separate species, but we agree with Johansson et al. in keeping F. novicida in a separate species because of its aquatic reservoir and very low virulence in humans (Busse et al., 2010; Huber et al., 2010; Johansson et al., 2010). Type B strains are also classically differentiated into three biovars (Kudelina and Olsufiev, 1980; Olsufjev and Meshcheryakova, 1982, 1983): biovar I (naturally susceptible to erythromycin) is found in Western Europe and North America; biovar II (naturally resistant to erythromycin) is found in Eastern Europe and Asia; and biovar japonica (susceptible to erythromycin but fermenting glycerol) is mainly found in Japan, although it has recently been described in China and Turkey (Kiliç et al., 2013; Wang et al., 2014). Biovar II strains are found in the Eastern part of Europe (Czech Republic, Finland, Georgia, Russia, Slovakia, Ukraine, Austria, Hungary, and Romania) (Vogler et al., 2009; Svensson et al., 2009b; Chanturia et al., 2011; Gyuranecz et al., 2012) and in Asia and both biovar I and II strains coexist in Germany, Switzerland and Scandinavia (Muller et al., 2013; Origgi et al., 2014; Maurin and Gyuranecz, 2016).
Although the F. tularensis genome displays very low variability, four distinct clades have been identified by pulse-field gel electrophoresis (PFGE) within type A strains in the United States (A.Ia, A.Ib, A.IIa, and A.IIb) with the A.Ib clade being associated with a 24% mortality rate in humans (Kugeler et al., 2009). Type B strains have also been divided into several clades by whole genome sequencing. The four main clades include clade B.4 corresponding to North American strains, clade B.6 to biovar I Western European strains, clade B.12 to biovar II Eastern European strains, and clade B16 to strains belonging to biovar japonica (Fujita et al., 2008; Vogler et al., 2009; Kilic et al., 2015; Karlsson et al., 2016).
F. tularensis is a Gram-negative, facultative intracellular coccobacillus (Broman et al., 2011). It is strictly aerobic, non-motile, non-toxigenic, and non-spore-forming. It is a fastidious bacterium that may be grown in cysteine-enriched media, under 5% CO2 atmosphere. The main virulence factor of F. tularensis corresponds to its ability to multiply within eukaryotic cells, especially in the cytosol of macrophages (Clemens and Horwitz, 2007). Virulence is associated with the presence of a duplicated pathogenicity island in the bacterial genome, encoding a type VI-like secretion system (Nano and Schmerk, 2007). However, the high variations in virulence observed among F. tularensis genotypes remain currently unexplained.
Human tularaemia is a zoonotic disease usually occurring as sporadic cases or small familial outbreaks (Tärnvik and Berglund, 2003; Bicakci and Parlak, 2008). However, a number of tularaemia outbreaks have been reported, including in the last two decades (Helvaci et al., 2000; Cerný, 2001; Feldman et al., 2001; Pérez-Castrillón et al., 2001; Reintjes et al., 2002; Christova et al., 2004; Payne et al., 2005; Celebi et al., 2006; Kantardjiev et al., 2006; Siret et al., 2006; Petersen et al., 2008; Akalın et al., 2009; Barut and Cetin, 2009; Svensson et al., 2009a; Hauri et al., 2010; Mailles et al., 2010; Larssen et al., 2011, 2014; Wang et al., 2011; Karlsson et al., 2013; Johansson et al., 2014; Mengeloglu et al., 2014). Humans may be infected with F. tularensis through direct contact with infected animals (manipulation of live or dead infected animals, animal bites or scratches), consumption of contaminated food or water, exposure to contaminated environments or through arthropod bites (Keim et al., 2007). F. tularensis can infect a large number of animal species, but lagomorphs and small rodents are considered key hosts for this pathogen (Gyuranecz et al., 2011; Maurin and Gyuranecz, 2016). This bacterium may also persist in water and soil environments for several months, which might be related to an ability to multiply in protozoa, such as amoebae (Abd et al., 2003; Keim et al., 2007; El-Etr et al., 2009; Broman et al., 2011). A terrestrial and an aquatic lifecycle have been described for this bacterium (Maurin and Gyuranecz, 2016). Arthropods, such as ticks and mosquitoes may be contaminated by F. tularensis from the animal or environmental reservoirs (Sjostedt, 2007; Maurin and Gyuranecz, 2016). Large tularaemia outbreaks have occurred, for which multiple sources of contamination and several F. tularensis clones were identified (Akalın et al., 2009; Barut and Cetin, 2009; Celebi et al., 2006; Cerný, 2001; Christova et al., 2004; Feldman et al., 2001; Hauri et al., 2010; Helvaci et al., 2000; Johansson et al., 2014; Kantardjiev et al., 2006; Karlsson et al., 2013; Larssen et al., 2011, 2014; Mailles et al., 2010; Mengeloglu et al., 2014; Payne et al., 2005; Pérez-Castrillón et al., 2001; Petersen et al., 2008; Reintjes et al., 2002; Siret et al., 2006; Svensson et al., 2009a; Wang et al., 2011). Symptoms vary according to the infection route and classically correspond to six different clinical forms: ulceroglandular, glandular, oropharyngeal, oculoglandular, pneumonic and typhoidal (Tarnvik, 2007). Ulceroglandular and glandular forms are consecutive to skin inoculation of bacteria (e.g., through an arthropod bite or contact with infected animals). A regional lymphadenopathy develops with (ulceroglandular form) or without (glandular form) a skin ulcer at the inoculation site. When contamination occurs through the ocular conjunctiva, painful conjunctivitis with regional lymphadenopathy develops, which corresponds to oculoglandular tularaemia. The oropharyngeal form is characterized by pharyngitis with regional lymphadenopathy, usually occurring after consumption of contaminated meat or water. Pneumonic tularaemia results from airborne contamination or hematogenous spread of bacteria to the lungs and is characterized by unspecific symptoms, such as cough, fever, dyspnea and occasionally a mediastinal or hilar lymphadenopathy. The typhoidal form corresponds to systemic disease with neurological symptoms mimicking typhoid, often with F. tularensis bacteraemia, but without detection of any portal of entry of bacteria (especially no skin ulcer) and without symptoms of localized infection (especially no regional lymphadenopathy) (Tärnvik and Chu, 2007). Complications may occur, such as skin eruptions, abscess formation, lymph node suppuration with occasionally skin fistulisation, and secondary infectious locations due to hematogenous spread of bacteria (Tärnvik and Chu, 2007; Meric et al., 2008; Maurin et al., 2011; Erdem et al., 2014). Diagnosis is usually based on compatible clinical and epidemiological features, and positive serological tests. PCR is useful to detect F. tularensis DNA in various clinical samples, especially before specific antibodies can be detected (Tarnvik, 2007). Isolation of a F. tularensis strain from blood or tissue samples is obtained in <20% of patients, which makes antibiotic susceptibility testing (AST) of F. tularensis strains rare (Maurin et al., 2011).
The 2007 WHO guidelines for treatment of tularaemia are based on the 2001 consensus recommendations from Dennis et al. (2001). According to these publications, tularaemia treatment is mainly based on three antibiotic classes: the aminoglycosides, the fluoroquinolones and the tetracyclines, although many other antibiotic classes have been tested against F. tularensis in vitro. Here we have compiled all available experimental data on the evaluation of the activity of antibiotics against F. tularensis in vitro, in acellular and cell models, and in vivo in animal models to provide useful information for clinical microbiologists and physicians.
Antibiotic Susceptibility Testing of F. tularensis
Antibiotic susceptibility testing (AST) of F. tularensis strains is usually not performed on a routine basis because: (1) isolation of the strain involved is only obtained in a few tularaemic patients (Maurin et al., 2011); (2) this procedure is at risk for the laboratory personnel handling cultures of this highly infectious pathogen and requires biosafety level 3 (BSL3) facilities (Tärnvik and Chu, 2007); (3) acquired resistance to antibiotics has never been reported so far in clinical strains of F. tularensis (Tarnvik, 2007) and (4) in vitro data may not be predictive of treatment failure in tularaemia patients. However, reference laboratories have reported AST surveys for collections of human and animal strains of F. tularensis (Scheel et al., 1993; Ikäheimo et al., 2000; Johansson et al., 2000, 2002; García del Blanco et al., 2004; Tomaso et al., 2005; Urich and Petersen, 2008; Valade et al., 2008; Velinov et al., 2011; Yeşilyurt et al., 2011; Georgi et al., 2012; Hotta et al., 2013; Kiliç et al., 2013; Kreizinger et al., 2013; Origgi et al., 2014).
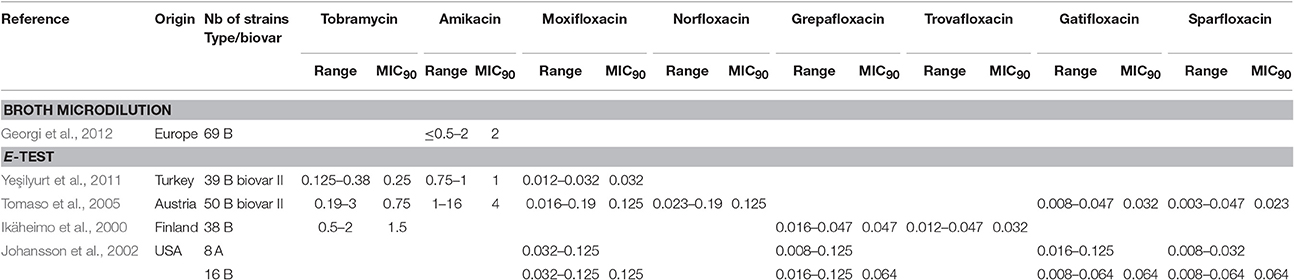
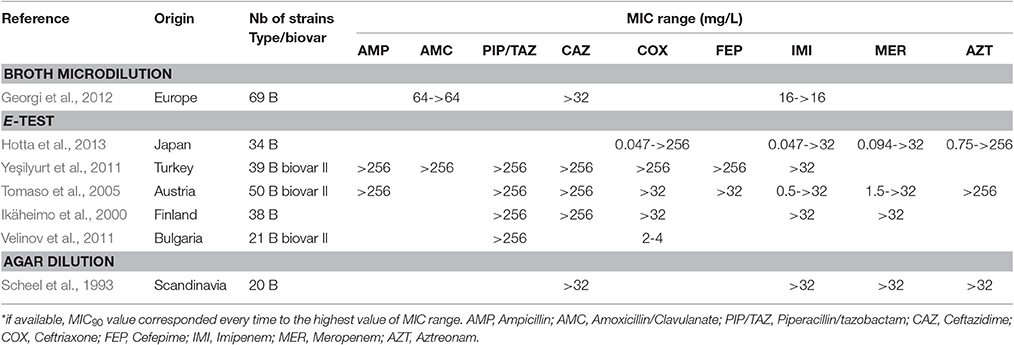
For this review, we collected F. tularensis AST results from all studies of more than five tularaemia cases, published in the literature since 1989. We obtained data from 898 F. tularensis strains isolated from humans (510 strains), animals (200 strains), arthropods (four strains), natural water samples (39 strains) and unknown sources (147). They included 107 type A, 789 type B and four F. tularensis subsp. mediasiatica strains (Table 1 and Table S1) (Scheel et al., 1993; Ikäheimo et al., 2000; Johansson et al., 2000, 2002; García del Blanco et al., 2004; Tomaso et al., 2005; Urich and Petersen, 2008; Valade et al., 2008; Velinov et al., 2011; Yeşilyurt et al., 2011; Georgi et al., 2012; Hotta et al., 2013; Kiliç et al., 2013; Kreizinger et al., 2013; Origgi et al., 2014). AST results were available for 33 antibiotics, although only some of them were tested in all studies (Tables 1–5 and Table S1). These antibiotics included four aminoglycoside compounds (gentamicin, streptomycin, amikacin and tobramycin), nine fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin, norfloxacin, grepafloxacin, sparfloxacin, trovafloxacin and gatifloxacin), three tetracyclines (tetracycline, doxycycline and tigecycline), four macrolides (erythromycin, azithromycin, roxithromycin and clarithromycin), one ketolide (telithromycin), nine β-lactams (ampicillin, amoxicillin + clavulanic acid, ceftazidime, piperacillin + tazobactam, ceftriaxone, cefepime, imipenem, meropenem and aztreonam), chloramphenicol, rifampicin, and linezolid.
Table 1. Characteristics of F. tularensis strains included in this study and MICs for aminoglycosides (gentamicin and streptomycin), the tetracycline compound doxycycline and fluoroquinolones (ciprofloxacin and levofloxacin) according to the culture medium used and to the AST assay used.
MICs: Methods and Results
According to the Clinical and Laboratory Standards Institute (CLSI) and WHO guidelines, AST of F. tularensis strains should be performed using cation-adjusted Mueller-Hinton broth enriched with 2% defined growth supplement, such as Polyvitex® (referred to here as enriched caMHB), in order to fulfill the cystein growth requirement of this bacterium (Tarnvik, 2007; CLSI. M45-A2, 2010). Adjustment of pH to 7.1 ± 0.1 is mandatory after addition of 2% growth supplement because it significantly reduces the pH of caMHB medium. The bacterial inoculum must be calibrated at 5 105 CFU/mL of final concentration. Culture media should be incubated for 48 h in ambient air, but incubation in 5% CO2-enriched atmosphere may be needed for some strains, although it can lead to acidification of the culture medium and therefore overestimation of aminoglycoside and macrolide MICs, or underestimation of tetracyclines MICs (CLSI. M45-A2, 2010).
In the past three decades, three different methods for F. tularensis AST were most frequently reported in the literature: antibiotic agar dilution, broth microdilution and E-test strips. These techniques were poorly standardized between studies, including after CLSI guidelines were available (CLSI. M45-A2, 2010). Most particularly, growth media, incubation conditions (atmosphere and duration) and bacterial inocula used for F. tularensis AST greatly varied between studies. Therefore, the results obtained in these different studies should be compared with caution.
As for the broth microdilution method, a number of authors used Mueller-Hinton II broth supplemented with glucose, Ca2+ and Mg2+ ions, and ferric pyrophosphate (a medium referred to as modified MHII) in spite of the recommended enriched caMHB medium (Baker et al., 1985; García del Blanco et al., 2004; Origgi et al., 2014). The use of MHII resulted in overestimation of the MICs of aminoglycosides and tetracyclines compared to caMHB (Table S1). The use of MHII for F. tularensis AST should be discouraged to avoid erroneous classification of some strains as resistant to aminoglycosides and/or tetracyclines using the CLSI breakpoints for susceptibility (concentration value threshold used for the categorization of a bacterial strain as susceptible, intermediately susceptible or resistant to an antibiotic). To date, antibiotic resistance reported in the literature for natural strains of F. tularensis have never been formally characterized by a reference laboratory. In the following paragraphs, the term resistant is applied to strain whose MICs are not classified as susceptible according to CLSI breakpoints for susceptibility, even though the mechanism of resistance has not been characterized. The MHII medium may also affect the activity of fluoroquinolones since ciprofloxacin MICs up to 0.25 mg/L were reported with this medium (Maurin et al., 2000; García del Blanco et al., 2004), while MICs for this antibiotic range from ≤ 0.002 to 0.125 mg/L (MIC90, ≤ 0.016–0.047 mg/L) for all other studies reported (Table 1 and Table S1).
Several agar media were used for MIC determination using the E-test strip technique (Tables 1 and Table S1). MIC ranges observed with these different solid media were similar to each other and to those obtained with the broth microdilution method using enriched caMHB. No strain was classified as resistant to any of the drugs used for tularaemia treatment and for which CLSI breakpoints have been defined (i.e., gentamicin, streptomycin, ciprofloxacin, levofloxacin, tetracycline, doxycycline and chloramphenicol). Thus, the E-test strip methodology appears to be a convenient alternative for antibiotic susceptibility categorisation of F. tularensis strains compared to the more time-consuming and fastidious broth microdilution method. Moreover, this method is less risky for laboratory personnel because it does not require handling large volumes of liquid suspensions of F. tularensis. However, no study has compared AST results using MIC strips to those of the microdilution reference method using enriched caMHB. Standardization of agar media for F. tularensis AST would be beneficial for comparison of studies conducted in different laboratories.
A few studies have compared F. tularensis antibiotic susceptibilities when using the agar dilution technique with different solid media (Table 1 and Table S1). Blood cysteine agar should be avoided since it identified resistant strains of F. tularensis for gentamicin or streptomycin, which was never confirmed by characterizing the resistance mechanisms involved. For the Thayer Martin agar and enriched Mueller-Hinton agar, results were concordant with those of the broth microdilution and MIC strip assays. One study compared MIC results using the agar dilution method, the MIC strip method or the broth microdilution reference method. Unfortunately, results obtained with the reference method were not reproducible. The correlation of results obtained with either the agar dilution or MIC strip tests, using enriched Mueller Hinton agar, were 87% for doxycycline, 94% for ciprofloxacin, but only 42% for gentamicin. The agar dilution method poorly correlated with the broth microdilution method, with only 72% correlation for doxycycline, 68% for ciprofloxacin and 51% for gentamicin.
According to our previous comments on MIC methods, we excluded MIC values obtained with the broth microdilution method using modified MHII broth and those determined by the agar dilution method using blood cystein agar plates (Table S1) MICs obtained with all other methods were recorded, although analyzed cautiously because of poor methodological standardization, as previously mentioned. The remaining 812 F. tularensis strains were categorized as susceptible to first-line antibiotics for tularaemia, including the aminoglycosides, the fluoroquinolones and the tetracyclines. MIC ranges and CLSI breakpoints for these antibiotics are shown in Table 2.
Table 2. MIC and MIC90 ranges of aminoglycosides, tetracyclines and fluoroquinolones against F. tularensis.
Aminoglycosides, Fluoroquinolones, and Doxycycline
Altogether, among antibiotic classes recommended for first-line treatment of tularaemia, ciprofloxacin displayed the lowest ranges for MICs (≤ 0.002–0.125 mg/L) and MICs90 (≤ 0.016–0.064 mg/L) between studies. Gentamicin MICs ranged from ≤ 0.016 to 2 mg/L, and MICs90 from 0.064 to 1 mg/L. The MIC and MIC90 ranges for doxycycline were 0.064–4 mg/L and 0.25–2 mg/L, respectively (Table 1). Only slight differences in susceptibility to these antibiotics were observed between type A and type B strains of F. tularensis, or between various biovars or clades within type A and type B strains.
Among fluoroquinolones, ciprofloxacin and levofloxacin were the most active compounds in vitro (Table 1). All strains tested displayed MIC levels to these antibiotics at least 4-fold less than the CLSI breakpoint for susceptibility (Table 2). Moxifloxacin, norfloxacin, gatifloxacin, grepafloxacin, trovafloxacin and sparfloxacin were also effective against F. tularensis in vitro (Table 3), but the three latter antibiotics have been withdrawn from the market because of potential severe side effects.
As for the aminoglycosides, tobramycin and amikacin were evaluated in three studies, only against type B strains (Ikäheimo et al., 2000; Tomaso et al., 2005; Yeşilyurt et al., 2011). Tobramycin displayed MIC values (0.125–3 mg/L) close to those of gentamicin and streptomycin. Interestingly, Enderlin et al., reported in 1994 a cure rate with this antibiotic of only 50% but for a limited number of patients (3/6 patients) (Enderlin et al., 1994). Conversely, amikacin was less active in vitro, with MICs up to 16 mg/L (Table 3; Tomaso et al., 2005).
Thus all strains were confirmed as susceptible to the first-line antibiotics recommended for tularemia treatment, with ciprofloxacin and levofloxacin showing the lowest MICs in vitro.
Macrolides
Erythromycin MICs are much higher for biovar II F. tularensis subsp. holarctica strains, than for biovar I strains of the same subspecies and for F. tularensis subsp. tularensis strains. Kudelina et al first described erythromycin-resistant strains of F. tularensis as those able to grow on media containing up to 6400 mg/L of this antibiotic, while susceptible strains could be killed by 25 mg/L of this antibiotic (Kudelina and Olsufiev, 1980). In the literature, type B biovar II strains, which currently correspond to East European strains belonging to the B12 subclade, were all resistant to erythromycin with MIC > 256 mg/L using the E-test strip method (Table 4; Karlsson et al., 2016). Only Tomaso et al. reported one biovar II strain with an erythromycin MIC of only 4 mg/L, but this result can be questioned according to current literature (Tomaso et al., 2005). Using the broth microdilution method, all type B biovar II strains had erythromycin MICs of ≥ 32 mg/L (Table 1). In contrast, all type B biovar I strains displayed erythromycin MICs ≤ 8 mg/L using the broth microdilution method and ≤ 1 mg/L using MIC strips. Among erythromycin-susceptible F. tularensis strains, subtle differences in erythromycin MICs could be found in the literature between the US strains belonging either to type B biovar I (MIC range, 0.125–2 mg/L; MIC90 range, 0.5–1 mg/L) or to type A (MIC range, 0.125–1 mg/L), and the West European type B biovar I strains (MIC range, 1–8 mg/L; MIC90, 4 mg/L). This difference might be correlated with the B6 clade displaying higher MIC levels, or to methodological differences, such as incubation of media in ambient air vs. under 5% CO2 atmosphere, the latter reducing the activity of the macrolides. The Japanese type B strains, including those formally identified as belonging to biovar japonica, displayed erythromycin MICs ranging from 0.094 to 1.5 mg/L.
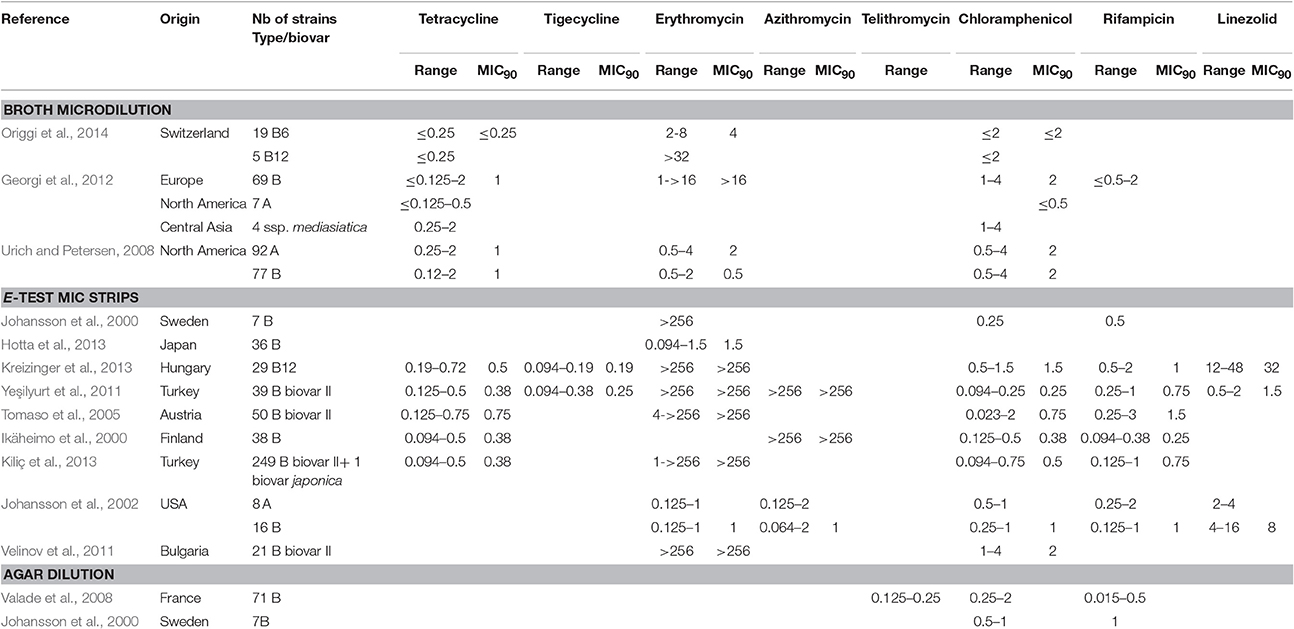
Table 4. MICs of tetracycline, tigecycline, macrolides (erythromycin, azithromycin) telithromycin, chloramphenicol, rifampicin and linezolid against F. tularensis.
According to the above data, a breakpoint for the biovar II definition might be set at erythromycin MIC ≥ 32 mg/L, in agreement with Kudelina's above-mentioned study (Kudelina and Olsufiev, 1980). Moreover, erythromycin resistance in type B biovar II strains has recently been correlated with the presence of a single mutation A2059C in the three copies of the rrl gene, encoding the 23S rRNA (Karlsson et al., 2016). Thus, determination of the rrl gene sequence provides a confirmatory identification of biovar II subtype.
Azithromycin MICs against F. tularensis were also determined in three studies, with the same dichotomy between biovar I and biovar II of type B strains. MIC ranges were similar to those observed for erythromycin (Table 4). Biovar II strains displayed azithromycin MICs > 256 mg/L, whereas MIC ranges were 0.064–2 mg/L for 16 type B and 0.125–2 mg/L for eight type A strains from the USA (Ikäheimo et al., 2000; Johansson et al., 2002; Yeşilyurt et al., 2011). Telithromycin, a ketolide compound, displayed MICs ranging from 0.125 to 0.25 mg/L with the agar dilution method against type B biovar I strains (Valade et al., 2008).
Thus, low MIC values are observed for macrolides and one ketolide against biovar I strains. No susceptibility breakpoints are currently defined by the CLSI for these antibiotics against F. tularensis, although they may represent useful therapeutic alternatives for infection related to biovar I strains, especially if first-line antibiotics are contraindicated. Further experimental data in animal models are needed for the in vivo evaluation of the activity of these compounds against F. tularensis. In contrast, the macrolides and ketolides are ineffective against biovar II strains.
Beta-Lactams
Many β-lactams have been tested in vitro against F. tularensis, although they are considered unreliable for treatment of tularaemia (Cross and Jacobs, 1993; Cross et al., 1995; Tarnvik, 2007; Maurin et al., 2011). In the literature, all the F. tularensis strains tested were resistant to penicillin A, ticarcillin and piperacillin, whether or not associated with a β-lactamase inhibitor, with MICs ≥ 64 mg/L (Table 5). In contrast, the cephalosporins, monobactams and carbapenems displayed larger MIC ranges. Although most F. tularensis strains displayed MICs higher than 32 mg/L to these β-lactams, a few strains displayed MICs lower than 1 mg/L (Table 5). In a study from Hotta et al., 30–60% of 36 Japanese strains displayed MICs lower than 1 mg/L for cefotaxime, ceftriaxone, cefoxitin, aztreonam, imipenem and meropenem (Hotta et al., 2013). Velinov et al. described 21 Bulgarian strains with a MIC range for ceftriaxone between 2 and 4 mg/L (Velinov et al., 2011). To date, two β-lactamase genes (bla1 and bla2) have been identified in the LVS strain (with corresponding homologs in the Schu S4 strain), but the expression of recombinant LVS proteins in Escherichia coli only revealed the bla2LVS gene to encode a functional β-lactamase (Bina et al., 2006). In 2012, Antunes et al. reported a class A β-lactamase (FTU-1) present in 14 strains belonging to all F. tularensis subspecies, including various type B strains from the USA, France, Japan, Russia, and Sweden (Antunes et al., 2012). Actually, the FTU-1 corresponds to the bla2LVS gene, as evidenced by comparison of gene sequences (YP_513599.1 and FTT_0611c, respectively). This class A β-lactamase, which was partially inactivated by β-lactamase inhibitors, induced resistance to penicillin and a 4-fold increase in ceftazidime MIC when cloned and expressed in E. coli. This β-lactamase had no effect on other cephalosporins, monobactams and carbapenems (Bina et al., 2006; Antunes et al., 2012). The presence of the FTU-1/bla2LVS gene is thus compatible with the cephalosporin-susceptibility phenotype observed in Japanese strains, whereas additional resistance mechanisms are probably involved in strains displaying resistance to cephalosporins, monobactams and carbapenems (Table 5). In 2008, Bina et al. characterized an AcrAB RND efflux system in the LVS strain, which conferred increased resistance to all beta-lactams tested in the study (i.e., ampicillin, carbenicillin and cefoperazone) but not to carbapenems (Bina et al., 2008). Thus, β-lactams are not recommended for the treatment of tularemia.
Chloramphenicol
The reported chloramphenicol MICs range from ≤ 0.023 to 4 mg/L, with an MIC90 range of 0.25–2 mg/L (Table 4). Chloramphenicol has been used occasionally (alone or in combination with other antibiotics) to treat patients with tularaemia meningitis, owing to its high distribution in brain tissue and cerebrospinal fluid (Tarnvik, 2007; Hofinger et al., 2009). Considering the MIC breakpoint of 8 mg/L, F. tularensis can be considered susceptible to chloramphenicol, but the clinical use of this antibiotic is currently restricted to meningitis because of potential severe bone marrow toxicity.
Rifampicin
Rifampicin is active in vitro against F. tularensis but with MICs ranging from ≤ 0.094 to 3 mg/L, and a MIC90 range of 0.25–1.5 mg/L (Table 4). However, this antibiotic is not recommended for tularaemia treatment because of potential rapid selection of resistant mutants, as described by Johansson et al. (2000) and Tarnvik (2007). It has been used in combination with ciprofloxacin for treatment of an infected total knee arthroplasty, with resolution of symptoms only after addition of rifampicin and a successful outcome with this antibiotic combination (Cooper et al., 1999).
New Antibiotics
Among more recently developed antibiotics, tigecycline, a new glycylcycline, has been evaluated in two studies using the E-test method, with reported MICs (0.094–0.38 mg/L) and MIC90 (0.19–0.25 mg/L) ranges lower than those determined in the same studies for tetracycline and doxycycline (Tables 1, 4) (Yeşilyurt et al., 2011; Kreizinger et al., 2013). No breakpoints are currently defined for F. tularensis susceptibility to tigecycline, but all tested strains displayed MICs ≤ 0.5 mg/L. Further studies are needed to evaluate tigecycline MICs using the microdilution reference method. Importantly, fresh medium (<24 h) must be used to prevent overestimation of tigecycline MICs (Bradford et al., 2005). Tigecycline might be a suitable alternative to doxycycline for treatment of tularaemia, but its much broader antibacterial spectrum is a disadvantage due to a greater deleterious effect on the gut commensal flora.
Conflicting results were observed for linezolid, an oxazolidinone compound. The activity of this antibiotic is considered mainly restricted to Gram-positive bacteria, with a CLSI susceptibility breakpoint for the corresponding species set at ≤ 2 mg/L. However, MICs of 4–8 mg/L have been found for Gram-negative bacteria of the genera Bacteroides, Moraxella and Pasteurella, owing to the absence or low efficacy of efflux systems, or a high affinity of their ribosomes for linezolid (Livermore, 2003). As for F. tularensis, linezolid displayed MICs of 0.5–2 mg/L for 39 biovar II type B strains from Turkey, 2–4 mg/L for eight type A strains from the USA, and 4–16 mg/L for 16 type B strains from the USA, when using the E-test strip procedure. In contrast, MICs ranged from 12 to 48 mg/L for 29 Hungarian biovar II type B strains using the same technique, although such differences could be related to the use of different solid media for AST (Tables 1, 4). The relative susceptibility of F. tularensis to linezolid may be related to its small genome with a limited number of efflux systems, while MIC variations between strains may reflect variable expression of such efflux systems. This hypothesis needs to be further assessed on a larger panel of F. tularensis strains.
Conclusion
In conclusion, MIC data confirm that among the antibiotics recommended as first-line treatment of tularaemia, ciprofloxacin and levofloxacin display the lowest MIC ranges against F. tularensis. Gentamicin ranks second, while doxycycline has the highest MIC range with some strains displaying an MIC at 4 mg/L, which is the breakpoint for susceptibility (4 mg/L). However, no resistant strains to any of these antibiotics have been characterized so far. Moreover, chloramphenicol is active and may be used in combination for rare meningitis due to F. tularensis. Azithromycin and telithromycin may be useful alternatives for infection related to biovar I strains of F. tularensis subsp. holarctica, when all first line antibiotics are contraindicated, but still require confirmation of their efficacy in animal models. Tigecycline and rifampicin are active in vitro and should be also further evaluated in animal models. Rifampicin may be an interesting antibiotic in association to fluoroquinolones for rare bone and joint infections.
MBCs and Other Bactericidal Assays
A few in vitro studies have reported either MBC or time-kill kinetic experiments for F. tularensis. MBC testing is performed similarly to MIC testing using a broth microdilution method, but an aliquot of each well with no visible growth after 48 h incubation is plated on enriched chocolate agar media for CFU count determination. After 48 h incubation of the chocolate agar plates at 37°C, under 5% CO2 atmosphere, CFU counting can determine the MBC, which is the lowest antibiotic concentration allowing 3-log or greater reduction of the primary bacterial inoculum.
Time-kill kinetic experiments determine CFU counts over time in antibiotics containing cultures, incubated for 48 h at 37°C, under 5% CO2 atmosphere. Experiments are conducted using enriched caMHB medium, containing two, four or eight times the MIC of the tested antibiotic compound (Maurin et al., 2000; Caspar et al., 2014).
The MBCs of several antibiotics (ceftriaxone, streptomycin, amikacin, gentamicin, thiamphenicol, telithromycin, erythromycin, clarithromycin, rifampicin, ofloxacin, ciprofloxacin, pefloxacin, doxycycline, and cotrimoxazole) were determined against a single human strain of F. tularensis subsp. holarctica biovar I, using the modified MHII as the experimental medium (Maurin et al., 2000). MBCs were equal to MICs for ofloxacin and ciprofloxacin; 2-fold higher for the aminoglycosides (streptomycin, amikacin, and gentamicin), thiamphenicol, telithromycin, rifampicin, pefloxacin, and doxycycline; 4-fold higher for erythromycin; 16 times higher for cotrimoxazole; and 32 times higher for clarithromycin. Despite a MIC of 2 mg/L, a bactericidal effect was never observed with ceftriaxone against F. tularensis, although β-lactams are classically considered bactericidal drugs against most other bacteria susceptible to these compounds. The latter results reinforce prior statements that β-lactams and cotrimoxazole are not reliable for tularaemia treatment (Tarnvik, 2007). According to this work, most antibiotics tested could be considered as bactericidal against F. tularensis. However, it should be mentioned that the use of modified MH2 in these experiments may have influenced both the bacteriostatic and the bactericidal activity of antibiotics compared to caMHB, as previously discussed. Indeed, in that study, the MIC value for doxycycline was 8 mg/L, which is higher than usually found in other studies.
We recently evaluated MBCs for gentamicin and doxycycline against the LVS strain and two human strains of F. tularensis subsp. holarctica biovar I from France, using caMHB medium (Caspar et al., 2014). The MBC was 8-fold higher than the MIC for gentamicin (respectively, 2 and 0.25 mg/L) and could not be determined for doxycycline, which was mainly bacteriostatic (MIC = 0.25 mg/L) (Caspar et al., 2014). These data are more in agreement with the known bactericidal and bacteriostatic nature of these two antibiotics, respectively. Such a difference may participate to the lower relapse rates reported with gentamicin (Enderlin et al., 1994).
Finally, a third study measured the MBCs of ciprofloxacin, gentamicin and doxycycline against the LVS strain, using enriched caMHB medium (Aloni-Grinstein et al., 2015). The MBC/MIC ratios were measured at 1–2 for gentamicin and ≤ 4 for ciprofloxacin, confirming the bactericidal nature of these antibiotics against F. tularensis. The MBC/MIC ratio was equal to 4 for chloramphenicol. In contrast, the MBC/MIC ratio was ≥ 64 for doxycycline, demonstrating a bacteriostatic activity (Aloni-Grinstein et al., 2015).
In conclusion, MBC data investigated with suitable media for F. tularensis AST revealed a bactericidal activity for the aminoglycosides and the fluoroquinolones, but a bacteriostatic activity for doxycycline. These data should be further confirmed using a larger sample of F. tularensis strains.
Cell Systems
The activity of antibiotics against F. tularensis has rarely been evaluated in cell models. These models used either of the following cell systems: murine macrophage-like cell lines J774.1 or P388D1; kidney epithelial Vero cells from African green monkey; human cell lines, including lung epithelial A549 cells, pulmonary fibroblastic MRC5 cells, non-phagocytic kidney epithelial HEK 293 and THP-1 human monocytes; or human monocytes purified from buffy coat (Maurin et al., 2000; Golovliov et al., 2003; Madrid et al., 2013; Schmitt et al., 2013; Sutera et al., 2014). In these models, the multiplicity of infection (MOI, bacteria/eukaryotic cell ratios used for cell infection) varied between 10 and 3000 according to the F. tularensis strain used (i.e., F. tularensis subsp. tularensis SchuS4 strain, F. tularensis subsp. holarctica FSC200 strain, or LVS) and the nature of the eukaryotic cells. Cell infection usually occurs after 2–3 h of bacteria–cell contact. The penetration of bacteria within eukaryotic cells may be enhanced by centrifugation of infected cell monolayers (Maurin et al., 2000; Madrid et al., 2013; Schmitt et al., 2013; Sutera et al., 2014; Aloni-Grinstein et al., 2015). Then non-phagocytised bacteria are removed by adding gentamicin to the extracellular medium for 1–4 h, which is referred to as the gentamicin protection assay. The cell monolayers are then washed and incubated at 37°C in 5% CO2 atmosphere, either in drug-free medium (growth control) or in the presence of the tested antibiotics. At various incubation times, cell monolayers are washed and lysed with detergents, and CFU counts in cell lysates are determined. Antibiotic activity is deduced from the ratio of CFU counts in antibiotic-containing cultures compared to the drug-free growth control.
Alternative methods for the evaluation of antibiotic activity against intracellular F. tularensis have been proposed. A dye-uptake assay based on the capacity of eukaryotic cells to internalize a vital dye has recently been reported. In this model, activity of antibiotics is deduced from their ability to inhibit bacterial growth within eukaryotic cells, preventing lysis of the cell monolayers. The term “minimal inhibitory extracellular concentration” (MIEC) was coined to define the minimal extracellular antibiotic concentration capable of preventing cytotoxic effects of F. tularensis multiplication (Sutera et al., 2014). The turnaround time of the dye uptake assay was 2 days compared to 4–5 days for the CFU count-based model. Cell mortality could also be evaluated by measuring lactate dehydrogenase activity in cell supernatants (Madrid et al., 2013). More recently, a qPCR assay was used to determine F. tularensis growth in Vero cells (Aloni-Grinstein et al., 2015). MIECs were determined after 24 h infection, with results comparable to those obtained with the CFU method (Aloni-Grinstein et al., 2015). However, qPCR may overestimate viable bacterial loads since it quantifies DNA from both viable and non-viable bacteria.
Fluoroquinolones
The intracellular activity of antibiotics against a French type B biovar I strain of F. tularensis (erythromycin MIC of 4 mg/L) was evaluated in a P388D1 murine macrophage-like cell model, using the CFU count methodology (Maurin et al., 2000). The results showed that ciprofloxacin and ofloxacin at 1 mg/L were the most potent compounds, with more than a 4- and 3-log reduction of bacterial inocula after only 48 h incubation of cultures, respectively (Maurin et al., 2000). In a systematic screening of FDA-approved drugs to identify compounds that may inhibit multiplication of biological threat agents, including F. tularensis, norfloxacin (50 μM, i.e., 16 mg/L) exhibited 83% protection of cells against cytotoxicity of the SchuS4 strain in J774.1 murine macrophages, as determined by the supernatant's lactate dehydrogenase activity (Madrid et al., 2013). In a dye-uptake assay using MRC5 cells, MIECs and MICs were equal for ciprofloxacin, levofloxacin and moxifloxacin, suggesting good penetration of these antibiotics within F. tularensis-infected cells (Sutera et al., 2014).
Tetracyclines
In the P388D1 murine macrophage-like model, doxycycline at 4 mg/L only induced a 2-log reduction of bacterial inocula after 72 h incubation (Maurin et al., 2000). In the systematic screening assay of FDA-approved drugs, tetracycline completely protected infected cells from cytotoxicity of the SchuS4 strain at 50 μM (22 mg/L), and minocycline induced 92% survival of infected cells at 23 mg/L (Madrid et al., 2013). In the dye uptake assay, MIECs were also equal to MIC for doxycycline, also demonstrating good penetration within infected cells. However, MIEC values were eight times higher for doxycycline (0.5 mg/L) compared to ciprofloxacin (0.064 mg/L) against the two biovar I type B strains evaluated in the study. In another study, the same ciprofloxacin MIEC was found for the LVS strain in Vero cells, as determined by both qPCR and CFU count methods, but a lower MIEC (0.125–0.5 mg/L) was found for doxcycline (Aloni-Grinstein et al., 2015).
Aminoglycosides
In the P388D1 murine macrophage-like cell model, gentamicin at 3 mg/L was not bactericidal after 48 h incubation of cultures and only induced a 1-log reduction of the bacterial inoculum after 72 h incubation. When tested at 10 mg/L, a 2-log reduction of bacterial counts was observed after 48 h incubation and more than 3-log reduction after 72 h (Maurin et al., 2000). In another study using the attenuated LVS strain and the Vero cell line, gentamicin at 20 mg/L did not show any intracellular activity after 32 h incubation (Aloni-Grinstein et al., 2015). In the dye-uptake assay using MRC5 cells, the gentamicin MIEC after 48 h incubation of cultures was 2 mg/L against two French type B biovar I strains of F. tularensis, while the MICs of this antibiotic were, respectively, 0.25 and 0.5 mg/L for the same strains (Sutera et al., 2014). Delayed activity of gentamicin correlated with the slow penetration of this antibiotic within eukaryotic cells, usually detectable only after 48–72 h of antibiotic–cell contact.
The other aminoglycosides also penetrate slowly within eukaryotic cells. Amikacin and streptomycin, at 10 mg/L, only decreased F. tularensis inoculum by 1 log after 48 h, and 2 logs after 72 h (Maurin et al., 2000). In contrast, netilmicin did not prevent cell lysis in J774.1 cells infected with the SchuS4 strain (Madrid et al., 2013).
Macrolides/Lincosamides/Ketolides
Conflicting results have been reported between studies testing the intracellular form of F. tularensis, which may be due to the use of different cell types and bacterial strains. In P388D1 murine macrophage-like cells, erythromycin and clarithromycin at 4 mg/L exhibited no intracellular activity against a type B biovar I strain of F. tularensis displaying MICs of 4 and 8 mg/L in acellular media for these antibiotics, respectively. In contrast, telithromycin at 4 mg/L (MIC at 0.5 mg/L in acellular medium) was able to reduce the bacterial inoculum between two to three log10 after 72 h (Maurin et al., 2000). Unlike telithromycin, the intracellular concentration of erythromycin and clarithromycin was probably not sufficient in this experiment to kill bacteria, as the extracellular concentration used was equal or superior to their MIC. In another study, a high concentration of erythromycin (50 μM, 37 mg/L) conferred full protection of J774.1 cell viability after infection with the SchuS4 type A strain (Madrid et al., 2013). In the same assay, clindamycin (50 μM, 21 mg/L) exhibited 83% protection of cells against the cytotoxic effect of F. tularensis proliferation (Madrid et al., 2013). In the dye uptake assay reported by Sutera et al., erythromycin MIECs were four times lower than MICs (respectively, 1–2 mg/L and 4–16 mg/L) for two French biovar I type B strains in MRC5 cells (Sutera et al., 2014). Altogether, these experiments show that erythromycin may be effective in preventing intracellular replication of type A and biovar I type B strains.
In another experiment reported by Ahmad et al., azithromycin (a C15 macrolide) showed complete killing of the LVS strain at 5 mg/L in murine macrophage J774.1 cells, although this antibiotic displays an MIC of 25 mg/L against this biovar II type B strain (Ahmad et al., 2010). This result suggests a high bactericidal concentration of azithromycin within F. tularensis-infected J774.1 macrophages. This may not be true for all cell types since a previous experiment from Ahmad et al., demonstrated that azithromycin was fully bactericidal at only 25 mg/L against the LVS strain grown in human lung epithelial A549 cells (Ahmad et al., 2010).
Beta-Lactams
In the P388D1 murine macrophage-like model, β-lactams at 10 mg/L (penicillin G, amoxicillin, ceftriaxone) displayed no activity against intracellular F. tularensis (Maurin et al., 2000). Meropenem was ineffective against both intracellular and extracellular F. tularensis in the dye uptake assay (Sutera et al., 2014).
Other Antibiotics
In the P388D1 murine macrophage-like model, thiamphenicol at 4 mg/L displayed no intracellular activity. In contrast, rifampicin at 4 mg/L induced a 2-log reduction of bacterial inocula after 72 h incubation (Maurin et al., 2000). In the dye uptake assay using MRC5 cells, MIECs were close to MICs for rifampicin, also suggesting good penetration of this compound within F. tularensis-infected cells. However, MIEC values were 16 times higher than those of ciprofloxacin (1 vs. 0.064 mg/L, respectively) against the two biovar I type B strains tested for F. tularensis (Sutera et al., 2014). Interestingly, linezolid exhibited higher activity against intracellular than extracellular F. tularensis, suggesting its accumulation within MRC5 cells, with a MIEC of 1 mg/L and an MIC of 8 mg/L (Sutera et al., 2014). Finally, daptomycin was ineffective both intracellularly and extracellularly (Sutera et al., 2014).
Conclusion of Intracellular Activity of Antibiotics against Tularaemia
To summarize the data from the intracellular models, the fluoroquinolones ciprofloxacin, levofloxacin, moxifloxacin and ofloxacin displayed the lowest MIECs and the fastest bactericidal activity against intracellular F. tularensis, suggesting rapid and efficient concentration of these compounds within infected eukaryotic cells. The tetracyclines were less effective and mainly bacteriostatic against intracellular F. tularensis. The aminoglycosides displayed a bactericidal activity, but only after 48–72 h of antibiotic exposure of infected cells, which correlated with the slow intracellular accumulation of these basic compounds (Maurin and Raoult, 2001). Thus, the aminoglycosides are probably much more effective against the extracellular form of F. tularensis at the early stage of antibiotic treatment, while these antibiotics may also be active against intracellular F. tularensis after several days of treatment. Streptomycin and amikacin were slightly less effective than gentamicin at the same concentration, and netilmicin was ineffective. The beta-lactams and daptomycin were not effective in cell systems. In contrast, the activity of linezolid against intracellular F. tularensis should be further investigated both in vitro and in vivo.
Interestingly, in two studies, the macrolides erythromycin and azithromycin were found to be active against the intracellular form of type A and biovar I type B F. tularensis strains, which are naturally susceptible to these antibiotics. Azithromycin was also effective against the intracellular form of biovar II type B LVS strain, although naturally more resistant to macrolides, but only in phagocytic and fibroblastic cells. Indeed, the intracellular accumulation of the macrolides varies according to the eukaryotic cell type considered, with a lower accumulation in epithelial cells compared to phagocytic and fibroblast cells (Ahmad et al., 2010; Sutera et al., 2014). PK/PD studies have demonstrated the accumulation of azithromycin within human phagocytic and fibroblast cells, and in many human tissues, such as lungs, soft tissues, prostate and tonsils (McDonald and Pruul, 1991; Matzneller et al., 2013). The intracellular/extracellular azithromycin ratio in tissues is generally between 10 and 100, and can be >200 in polymorphonuclear (PMN) leukocytes, with probable large amounts of this antibiotic at the site of infection (Hand and Hand, 2001; Hall et al., 2002; Matzneller et al., 2013). The intracellular accumulation of erythromycin is lower, with intracellular/extracellular ratios generally between 1 and 10 in tissues and PMNs (McDonald and Pruul, 1991; Hand and Hand, 2001). The activity of azithromycin against intracellular F. tularensis warrants further evaluation of its activity in animal models. These experiments should be conducted using type A and type B strains, especially the type B biovar II strains.
Animal Models
Although several animal models have been developed to study the pathogenesis of F. tularensis infection and vaccine efficacy, few of these models have evaluated in vivo antibiotic efficacy (Rick Lyons and Wu, 2007). This may be related to the absence of an optimal animal model mimicking human infection. In these models, antibiotic activity is usually deduced from the death rate and survival time of infected animals and the bacterial load in their organs (especially in the spleen) at the time of death or sacrifice, when treated with various antibiotic regimens compared to infected and untreated controls. BALB/c and C57BL/6 mice and guinea pigs are highly susceptible hosts to F. tularensis infection, but also to the LVS strain of F. tularensis subsp. holarctica and F. novicida strains, which have often been used as surrogates of F. tularensis, although they are much less virulent in humans (Stundick et al., 2013; Kingry and Petersen, 2014). Rabbits and Fisher 344 rats are less susceptible to F. tularensis infection and may better mimic human infection. However, variable immune responses to F. tularensis infection also exist between rat strains, with Sprague-Dawley being much more resistant than Fisher 344 rats (Raymond and Conlan, 2009). Results may also vary according to the route of infection, i.e., the intraperitoneal, intradermal, subcutaneous or intranasal routes for Fischer 344 rats (Stundick et al., 2013). Moreover, the “Animal Rule” states that experimental animal models should be developed using the true etiologic agent causing human disease. Thus, evaluation of antibiotic activity in Fisher 344 rats infected with virulent type A or type B strains would be more predictive of the clinical situation.
Few experiments have evaluated the activity of antibiotics in F. tularensis-infected animal models. However, interesting data have been obtained regarding the in vivo efficacy of antibiotics, and relapse rates according to delay in antibiotic therapy and treatment duration. Unfortunately, these models are highly heterogeneous with respect to the route of infection, the route of antibiotic administration, the antibiotic dose administrated and the F. tularensis strain tested.
Fluoroquinolones and Doxycycline
In 1998, Russel et al evaluated the effect of subcutaneous injection of doxycycline or ciprofloxacin on the median lethal dose (MLD) of the Schu S4 strain injected intraperitoneally to Porton outbred mice (Russell et al., 1998). The animals either received a 2-day antibiotic prophylaxis before the bacterial challenge, or were treated 48 h post-infection with 20 or 40 mg/kg twice a day, of doxycycline or ciprofloxacin for 10 days, and then were monitored for 24 days. Although the MLD was close to 1 CFU of the Schu S4 strain in untreated mice, full protection at the highest bacterial inoculum tested (8.8 × 106 CFU) was achieved by a 2-day prophylaxis with ciprofloxacin or doxycycline, and by 10 days of post-infection treatment with either of these antibiotics at 40 mg/kg twice a day. At this dosage, the serum concentrations were higher than 4 × MIC for at least 12 h for ciprofloxacin, and 3 × MIC for 9 h for doxycycline (Russell et al., 1998).
The in vivo efficacy of fluoroquinolones was confirmed in 2005, by Piercy et al. (2005). BALB/c mice were infected subcutaneously with 106 CFU of the Schu S4 strain and treated orally with 100 mg/kg of ciprofloxacin, gatifloxacin or moxifloxacin, from 6 h to 14 days post-infection. Survival rates at day 42 post-infection were 94, 100, and 100%, respectively, for these three antibiotics (Piercy et al., 2005).
In 2008, Klimpel et al. showed that a 13-day course of levofloxacin (5 mg/kg/day, intraperitoneally), starting 24 h following an intranasal challenge with 3 LD50 of F. tularensis Schu S4 strain, prevented death of all Balb/c mice (Klimpel et al., 2008). No bacteria were detectable in the spleen of the animals after 10 days of treatment, while very few were detected in the lungs at this time (Klimpel et al., 2008).
In 2012, Rotem et al. compared the efficacy of ciprofloxacin and doxycycline in Balb/c mice infected by inhalation of a 100-LD50 dose of F. tularensis LVS (105 CFU) or Schu S4 (102 CFU) strains (Rotem et al., 2012). Untreated controls died 5–7 days post-infection, whereas all LVS-infected mice were cured by intraperitoneal injection of ciprofloxacin (50 mg/kg bid) for 7 days or doxycycline (40 mg/kg bid) for 14 days, whether the antibiotic treatment was started at 24, 48, or 72 h post-infection (Rotem et al., 2012). F. tularensis LVS strain was undetectable by culture in the lungs, liver and spleen after completion of any of these treatments. In contrast, for animals infected with the Schu S4 strain, no death occurred when ciprofloxacin was administered 24 or 48 h post infection, while doxycycline only cured 90% of the animals even when administrated 24 h post-infection (Rotem et al., 2012).
Treatment Delay
In the study from Russel et al., a 48-h prophylaxis with ciprofloxacin or doxycycline protected mice from a 3.7 × 106 CFU and 6.0 × 106 CFU challenge with the Schu S4 strain, respectively (Russell et al., 1998). In contrast, when antibiotic treatment was started 24 h post-infection, complete protection was only obtained against an intraperitoneal challenge with 880 CFU for ciprofloxacin and 100 CFU for doxycycline. Thus, antibiotic efficacy was dramatically reduced after only 24 h infection (Russell et al., 1998). In the study by Rotem et al., in which mice were infected via Schu S4 strain aerosols, no death occurred when ciprofloxacin was administered 24–48 h post infection, while a 30% mortality rate was observed when this antibiotic was administered 3 days post-infection (Rotem et al., 2012). Doxycycline only cured 90, 30, and 0% of the mice when started at 24, 48, and 72 h post-infection, respectively (Rotem et al., 2012). In the study reported by Piercy et al., the survival rates decreased dramatically (94, 67, and 0%) when ciprofloxacin was administrated 6, 24, or 48 h post-infection, respectively (Piercy et al., 2005). In contrast, the survival rates were 100, 96, and 84% for gatifloxacin, and 100, 100, and 62% for moxifloxacin, respectively, showing the better therapeutic efficacy of these drugs compared to ciprofloxacin. In this study, the infectious dose was much higher than in that from Rotem et al (106 CFU intraperitoneally vs. 102 CFU intranasally). Finally, in the Klimpel et al. study, full protection against the SchuS4 strain (99 CFU intraperitoneally) was observed for levofloxacin at 40 mg/kg/day, as long as the treatment delay did not exceed 72 h (Klimpel et al., 2008). If started at 72 h, bacterial load decreased approximately from 106 to 10 CFU per organ after 1 week of treatment. Delaying the treatment of more than 120 h resulted in the death of all infected mice (Klimpel et al., 2008).
Bactericidal Activity and Treatment Duration
In the Russel et al. study, when treatment was administrated for 5 days rather than 10 days post-infection, the MLD was reduced from > 8.8 × 106 CFU to 3.7 × 106 CFU for ciprofloxacin and 6.0 × 106 CFU for doxycycline (Russell et al., 1998). The protective effect of the antibiotic therapy also decreased when animals were treated with a lower dose (20 mg/kg bd rather than 40 mg/kg bd) of ciprofloxacin or doxycycline. Moreover, the authors reported that death occurred in 90% of animals after antibiotic treatment withdrawal, demonstrating that a 5-day course of antibiotic treatment was not sufficient to eradicate F. tularensis (Russell et al., 1998). Thus, in this study, the dose and length of the antibiotic therapy administered highly influenced the outcome of F. tularensis infection in animals (Russell et al., 1998).
Significant insight into relapses came from the study reported by Piercy et al. (2005). Among the nine groups tested (ciprofloxacin, moxifloxacin or gatifloxacin, initiated 6, 24, or 48 h post-infection), these authors showed that the administration of 7 days of dexamethasone to surviving mice in order to abolish their immune system resulted in 17–73% relapse rates depending on the treatment group. These findings suggested that quiescent bacteria controlled by the immune system persisted in mice despite 14 days of fluoroquinolone therapy. Fluoroquinolones were thus not fully bactericidal when a high inoculum of F. tularensis was injected to the mice (e.g., subcutaneous injection of 106 CFU of the Schu S4 strain). Suppression of the immune system enabled latent bacteria to multiply and kill mice, even in the 6-h post-exposure treatment group (respectively, 36 and 42% mortality rates in the ciprofloxacin- and moxifloxacin-treated groups; Piercy et al., 2005).
Data from the study by Rotem et al. corroborated this hypothesis (Rotem et al., 2012). In their experiments with the virulent Schu S4 strain, no death occurred when ciprofloxacin was administered 24 or 48 h post-infection, while a 30% mortality rate was observed when this antibiotic was administered 3 days post-infection. In the latter case, bacteria were undetectable in organs at the time of antibiotic treatment withdrawal, while cultures were positive 3 days later for three out of 10 mice that died after antibiotic withdrawal. The extension of ciprofloxacin treatment duration from 7 to 10 days cured all animals, even when the antibiotic was administrated 72 h post-infection. In contrast, when doxycycline was started 72 h post-infection, all animals had approximately 100 CFU/g of tissue (lungs, liver or spleen) at the end of treatment, after which the bacterial loads in organs increased in all mice until death. Extension of treatment from 14 to 21 days resulted in no cultivable bacteria in lungs, liver or spleen at the end of treatment, but all mice relapsed after 2 days of doxycycline withdrawal (Rotem et al., 2012). A different picture was observed with the LVS strain, which was below detectable level by culture in the lungs, liver and spleen after completion of any of the treatment regimens (ciprofloxacin for 7 days or doxycycline for 14 days, started 24, 48, and 72 h post-infection) in the mice sacrificed at the end of the treatment. However, 2 days after doxycycline treatment withdrawal, the LVS strain was detected in all organs, and bacterial loads gradually decreased to an undetectable level in the following 7 days and resulted in no death. Thus, although undetectable after doxycycline treatment, the attenuated LVS strain was not fully eradicated and regrowth of the bacteria was observed as soon as doxycycline was stopped, although it did not kill the mice. In contrast, re-emergence of bacteria did not occur for the ciprofloxacin group of LVS-infected mice after ciprofloxacin withdrawal.
Conclusions Drawn from Animal Model Data
Altogether, these data indicate that both ciprofloxacin and doxycycline are able to prevent tularaemia in mice when treatment is started 48 h before a challenge with F. tularensis SchuS4 strain. These antibiotics are also able to cure the disease and eradicate F. tularensis when administrated post-infection at a concentration of 40 mg/kg or higher, for 5–10 days. Efficacy of the antibiotic treatment is highly correlated with the antibiotic dose administered, which is probably related to the duration over which serum concentrations of antibiotics are above the MIC values of the infecting strain.
However, when treatment was started 24–72 h post-infection, ciprofloxacin was superior to doxycycline: (1) in the study conducted by Rotem et al., doxycycline was not able to cure all mice when infected with 102 CFU of the SchuS4 strain via the aerosol route, even when started at 24 h post-infection, while ciprofloxacin cured all mice when started either 24 or 48 h post-infection (Rotem et al., 2012); (2) relapse rates were higher for doxycycline as it was unable to fully eradicate the Schu S4 or LVS strains from the lungs, liver and spleen, even when administrated for 14–21 days; (3) the time until antibiotic treatment initiation reduced doxycycline efficacy much more than for ciprofloxacin.
F. tularensis multiplies within the slightly acidic cytosol of eukaryotic cells, and a low pH may alter the activity of the basophilic antibiotics ciprofloxacin and doxycycline (Russell et al., 1998). Data obtained in animal models indicate that persistent bacteria may develop in hosts infected with F. tularensis when the antibiotic therapy administered has poor bactericidal activity or is too short in duration. These data suggest that long antibiotic treatment duration should be used in immunocompromised patients infected with F. tularensis. This could also be true for patients suffering from suppurated lymphadenopathy, who often experience relapses despite administration of ciprofloxacin for 14 days or doxycycline for 21 days.
Treatment must be initiated as soon as possible since antibiotic efficacy decreased significantly when treatment was delayed by 24–48 h post-infection. It should be noted that, because of diagnosis delays, antibiotics are often given several days to weeks after infection in patients suffering from tularaemia. Importantly, moxifloxacin efficacy was less impacted by treatment delay than ciprofloxacin and thus may represent an advantageous therapeutic option in case of late diagnosis. MIC values are close for moxifloxacin and ciprofloxacin.
General Conclusion and Perspectives
Since the introduction of streptomycin in the mid-1950s, antibiotic treatment of human tularaemia has remained challenging. In most tularaemia endemic countries, human infections are now rare, and F. tularensis isolation is even rarer. Thus, AST data for isolated strains of F. tularensis may not reflect the true situation of current antibiotic resistance in this pathogen. Importantly, the analysis of AST methodological variations between studies shows an urgent need for international standardization. Following the CLSI guidelines, including using the appropriate experimental medium, bacterial inoculum and incubation time are mandatory, although some F. tularensis strains grow better or exclusively under 5% CO2 atmosphere. The broth microdilution technique using enriched caMHB medium should be considered the reference method. As recommended by the CLSI, a final inoculum calibrated at 5 105 CFU/mL should be used, as a 10-fold higher or lower inoculum is associated to interpretation errors (Georgi et al., 2012). However, the modified Mueller-Hinton II liquid medium should not be used for F. tularensis AST to avoid reporting MICs that could categorize F. tularensis strains as resistant to aminoglycosides or doxycycline whereas such resistances have never been characterized so far in this pathogen. From the data currently available, use of the CLSI broth microdilution method can be recommended to test large collections of F. tularensis strains in reference laboratories, while the E-test method would be more convenient for testing one or a few strains. However, the E-test MIC strip method, although more convenient to perform, still requires comparative studies with the reference method.
Altogether, available AST data indicate that the fluoroquinolones display the lowest MICs, are strongly and rapidly bactericidal in cell models and are highly effective in curing F. tularensis infection in infected mice when administered for at least 10 days. Among the fluoroquinolones, ciprofloxacin and levofloxacin have the lowest MICs. In contrast, doxycycline MICs are closer to the CLSI susceptibility breakpoint and this antibiotic is only bacteriostatic in cell models. In the mouse model, a 14-day course of doxycycline did not eradicate F. tularensis when treatment was started 72 h post-infection (Rotem et al., 2012). A 21-day course of treatment was still not fully effective to eradicate bacteria, whereas 10 days of ciprofloxacin treatment was effective. Therefore, doxycycline should be considered less effective than fluoroquinolones for treatment of tularaemia, which is consistent with higher therapeutic failure rates in humans observed with this antibiotic. Because antibiotic treatment is often administrated several days after symptom onset in tularaemia patients, ciprofloxacin may be a better choice than doxycycline. Moreover, treatment duration with ciprofloxacin of at least 14 days should be recommended, while 3 weeks would be a minimum for doxycycline (Rotem et al., 2012). A longer duration of antibiotic treatment should probably be considered in case treatment is delayed longer than 2 weeks after symptom onset, especially if lymph node suppuration or other local or systemic complications have occurred. The aminoglycosides (especially gentamicin) also have low MICs. They are rapidly bactericidal in acellular media, but their intracellular bactericidal activity only takes place after 72 h of cell exposure owing to their slow penetration through the eukaryotic cell membrane. In the 1994 review by Enderlin et al., streptomycin was considered the most reliable treatment for human tularaemia, with almost no relapse after treatment removal (Enderlin et al., 1994). The aminoglycosides are still considered the most reliable treatment of severe tularaemia cases (e.g., the pneumonic and typhoidal forms of tularaemia, and all other systemic infections). Experimental data suggest that the combination of an aminoglycoside with a fluoroquinolone may currently be the most effective alternative in patients with severe tularaemia, especially to obtain a rapid bactericidal activity against extracellular and intracellular F. tularensis. However, one has always to remember that antimicrobial potency deduced from MIC values, MBC values, in vitro and in vivo bactericidal activity in animal models is only one of many factors (including pharmacokinetic and pharmacodynamic parameters, tolerability, plasma protein binding, tissue penetration, bactericidal activity, and contraindication) that may influence the decision of what drug to use in a clinical setting.
The macrolides (especially azithromycin) could be an alternative in patients infected with erythromycin-susceptible strains of F. tularensis (type A and type B biovar I) and in those for whom the above first-line treatments are contraindicated, including young children and pregnant women. However, the macrolide-resistant type B biovar II strains are currently found in Eastern Europe and in Asia, and co-exist with biovar I strains in Germany, Switzerland and Scandinavia. The empirical use of a macrolide in tularaemia patients cannot be considered reliable in these areas. The use of a macrolide in patients with mild to moderate severity tularaemia is safer in Western Europe, where only type B biovar I strains cause human infections. In this context, quick discrimination between type B biovar I and II strains either by determination of erythromycin susceptibility (using MIC strips or sequencing of rrl gene) or by detection of particular genetic markers (detection of RD23 deletion only found in West European strains) would be of particular interest (Dempsey et al., 2007; Pilo et al., 2009; Karlsson et al., 2016). Since these antibiotics mainly display a bacteriostatic activity against F. tularensis, they cannot be considered reliable to treat severe forms of tularaemia. Azithromycin combined with lymph node resection proved to be effective to cure a pregnant women suffering from oropharyngeal tularaemia with no complications for the fetus (Dentan et al., 2013). Further in vivo data in animal models and humans are needed before azithromycin can be recommended as a reliable alternative for treatment of tularaemia.
Finally, the causes of the high relapse rates observed in tularaemia patients after administration of a fluoroquinolone or a tetracycline remain undetermined. Several hypotheses can be proposed, including low penetration of antibiotics in tissues and eukaryotic cells in vivo; low susceptibility of bacteria in vivo especially because of low replication and persistence; inactivation of antibiotic activity in vivo owing to local conditions (e.g., acidic pH, enzymatic inactivation, etc.) and acquired resistance to antibiotics in vivo in patients under fluoroquinolone or tetracycline treatment. Further evaluation of such hypotheses in in vitro and in vivo experimental models is warranted.
Author Contributions
YC has collected data on antibiotic susceptibility testing (AST) of Francisella tularensis, both in vitro and in animal models, from the English and French literature in the PubMed database, since the introduction of fluoroquinolones in the treatment of tularaemia in 1989 until December 2016. He has written the article. MM has participated to data analysis and corrected the text of the article.
Funding
The French National Reference Center for Tularemia is funded by Santé Publique France, the French Agency for Public Health.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fcimb.2017.00122/full#supplementary-material
References
Abd, H., Johansson, T., Golovliov, I., Sandström, G., and Forsman, M. (2003). Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl. Environ. Microbiol. 69, 600–606. doi: 10.1128/AEM.69.1.600-606.2003
Ahmad, S., Hunter, L., Qin, A., Mann, B. J., and van Hoek, M. L. (2010). Azithromycin effectiveness against intracellular infections of Francisella. BMC Microbiol. 10, 123. doi: 10.1186/1471-2180-10-123
Akalın, H., Helvacı, S., and Gedikoğlu, S. (2009). Re-emergence of tularemia in Turkey. Int. J. Infect. Dis. 13, 547–551. doi: 10.1016/j.ijid.2008.09.020
Aloni-Grinstein, R., Shifman, O., Lazar, S., Steinberger-Levy, I., Maoz, S., and Ber, R. (2015). A rapid real-time quantitative PCR assay to determine the minimal inhibitory extracellular concentration of antibiotics against an intracellular Francisella tularensis Live Vaccine Strain. Front. Microbiol. 6:1213. doi: 10.3389/fmicb.2015.01213
Antunes, N. T., Frase, H., Toth, M., and Vakulenko, S. B. (2012). The class A β-Lactamase FTU-1 is native to Francisella tularensis. Antimicrob. Agents Chemother. 56, 666–671. doi: 10.1128/AAC.05305-11
Baker, C. N., Hollis, D. G., and Thornsberry, C. (1985). Antimicrobial susceptibility testing of Francisella tularensis with a modified Mueller-Hinton broth. J. Clin. Microbiol. 22, 212–215.
Barut, S., and Cetin, I. (2009). A tularemia outbreak in an extended family in Tokat Province, Turkey: observing the attack rate of tularemia. Int. J. Infect. Dis. 13, 745–748. doi: 10.1016/j.ijid.2008.12.002
Bicakci, Z., and Parlak, M. (2008). A neglected cause of cervical lymphadenitis. Oropharyngeal tularemia. Saudi Med. J. 29, 1059–1061.
Bina, X. R., Lavine, C. L., Miller, M. A., and Bina, J. E. (2008). The AcrAB RND efflux system from the live vaccine strain of Francisella tularensis is a multiple drug efflux system that is required for virulence in mice. FEMS Microbiol. Lett. 279, 226–233. doi: 10.1111/j.1574-6968.2007.01033.x
Bina, X. R., Wang, C., Miller, M. A., and Bina, J. E. (2006). The Bla2 β-lactamase from the live-vaccine strain of Francisella tularensis encodes a functional protein that is only active against penicillin-class β-lactam antibiotics. Arch. Microbiol. 186, 219–228. doi: 10.1007/s00203-006-0140-6
Bradford, P. A., Petersen, P. J., Young, M., Jones, C. H., Tischler, M., and O'Connell, J. (2005). Tigecycline MIC testing by broth dilution requires use of fresh medium or addition of the biocatalytic oxygen-reducing reagent oxyrase to standardize the test method. Antimicrob. Agents Chemother. 49, 3903–3909. doi: 10.1128/AAC.49.9.3903-3909.2005
Broman, T., Thelaus, J., Andersson, A.-C., Backman, S., Wikstrom, P., Larsson, E., et al. (2011). Molecular detection of persistent Francisella tularensis Subspecies holarctica in natural waters. Int. J. Microbiol. 2011:851946. doi: 10.1155/2011/851946
Busse, H.-J., Huber, B., Anda, P., Escudero, R., Scholz, H. C., Seibold, E., et al. (2010). Objections to the transfer of Francisella novicida to the subspecies rank of Francisella tularensis-response to Johansson et al. Int. J. Syst. Evol. Microbiol. 60, 1718–1720. doi: 10.1099/00207713-60-8-1718
Caspar, Y., Sutera, V., Boisset, S., Denis, J.-N., and Maurin, M. (2014). Bis-indolic compounds as potential new therapeutic alternatives for tularaemia. Front. Cell. Infect. Microbiol. 4:24. doi: 10.3389/fcimb.2014.00024
Celebi, G., Baruönü, F., Ayoğlu, F., Cinar, F., Karadenizli, A., Uğur, M. B., et al. (2006). Tularemia, a reemerging disease in northwest Turkey: epidemiological investigation and evaluation of treatment responses. Jpn. J. Infect. Dis. 59, 229–234.
Cerný, Z. (2001). Changes of the epidemiology and the clinical picture of tularemia in Southern Moravia (the Czech Republic) during the period 1936-1999. Eur. J. Epidemiol. 17, 637–642. doi: 10.1023/A:1015551213151
Chanturia, G., Birdsell, D. N., Kekelidze, M., Zhgenti, E., Babuadze, G., Tsertsvadze, N., et al. (2011). Phylogeography of Francisella tularensis subspecies holarctica from the country of Georgia. BMC Microbiol. 11:139. doi: 10.1186/1471-2180-11-139
Christova, I., Velinov, T., Kantardjiev, T., and Galev, A. (2004). Tularaemia outbreak in Bulgaria. Scand. J. Infect. Dis. 36, 785–789. doi: 10.1080/00365540410021199
Clemens, D. L., and Horwitz, M. A. (2007). Uptake and intracellular fate of Francisella tularensis in human macrophages. Ann. N. Y. Acad. Sci. 1105, 160–186. doi: 10.1196/annals.1409.001
CLSI. M45-A2 (2010). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guideline-Second Edition. M45A2. Wayne.
Cooper, C. L., Caeseele, P. V., Canvin, J., and Nicolle, L. E. (1999). Chronic prosthetic device infection with Francisella tularensis. Clin. Infect. Dis. 29, 1589–1591. doi: 10.1086/313550
Cross, J. T., and Jacobs, R. F. (1993). Tularemia: treatment failures with outpatient use of ceftriaxone. Clin. Infect. Dis. 17, 976–980. doi: 10.1093/clinids/17.6.976
Cross, J. T., Schutze, G. E., and Jacobs, R. F. (1995). Treatment of tularemia with gentamicin in pediatric patients. Pediatr. Infect. Dis. J. 14, 151–152. doi: 10.1097/00006454-199502000-00014
Dempsey, M. P., Dobson, M., Zhang, C., Zhang, M., Lion, C., Gutiérrez-Martín, C. B., et al. (2007). Genomic deletion marking an emerging subclone of Francisella tularensis subsp. holarctica in France and the Iberian Peninsula. Appl. Environ. Microbiol. 73, 7465–7470. doi: 10.1128/AEM.00646-07
Dennis, D. T., Inglesby, T. V., Henderson, D. A., Bartlett, J. G., Ascher, M. S., Eitzen, E., et al. (2001). Tularemia as a biological weapon: medical and public health management. JAMA 285, 2763–2773. doi: 10.1001/jama.285.21.2763
Dentan, C., Pavese, P., Pelloux, I., Boisset, S., Brion, J.-P., Stahl, J.-P., et al. (2013). Treatment of tularemia in pregnant woman, France. Emerging Infect. Dis. 19, 996–998. doi: 10.3201/eid1906.130138
El-Etr, S. H., Margolis, J. J., Monack, D., Robison, R. A., Cohen, M., Moore, E., et al. (2009). Francisella tularensis type A strains cause the rapid encystment of Acanthamoeba castellanii and survive in amoebal cysts for three weeks postinfection. Appl. Environ. Microbiol. 75, 7488–7500. doi: 10.1128/AEM.01829-09
Enderlin, G., Morales, L., Jacobs, R. F., and Cross, J. T. (1994). Streptomycin and alternative agents for the treatment of tularemia: review of the literature. Clin. Infect. Dis. 19, 42–47. doi: 10.1093/clinids/19.1.42
Erdem, H., Ozturk-Engin, D., Yesilyurt, M., Karabay, O., Elaldi, N., Celebi, G., et al. (2014). Evaluation of tularaemia courses: a multicentre study from Turkey. Clin. Microbiol. Infect. 20, O1042–O1051. doi: 10.1111/1469-0691.12741
Feldman, K. A., Enscore, R. E., Lathrop, S. L., Matyas, B. T., McGuill, M., Schriefer, M. E., et al. (2001). An outbreak of primary pneumonic tularemia on Martha's Vineyard. N. Engl. J. Med. 345, 1601–1606. doi: 10.1056/NEJMoa011374
Francis, E., Mayne, B., and Lake, G. C. (1922). Tularæmia Francis 1921 a New Disease of Man. Washington, DC: Government Printing office.
Fujita, O., Uda, A., Hotta, A., Okutani, A., Inoue, S., Tanabayashi, K., et al. (2008). Genetic diversity of Francisella tularensis subspecies holarctica strains isolated in Japan. Microbiol. Immunol. 52, 270–276. doi: 10.1111/j.1348-0421.2008.00036.x
García del Blanco, N., Gutiérrez Martín, C. B., de la Puente Redondo, V. A., and Rodríguez Ferri, E. F. (2004). In vitro susceptibility of field isolates of Francisella tularensis subsp. holarctica recovered in Spain to several antimicrobial agents. Res. Vet. Sci. 76, 195–198. doi: 10.1016/j.rvsc.2003.12.002
Georgi, E., Schacht, E., Scholz, H. C., and Splettstoesser, W. D. (2012). Standardized broth microdilution antimicrobial susceptibility testing of Francisella tularensis subsp. holarctica strains from Europe and rare Francisella species J. Antimicrob. Chemother. 67, 2429–2433. doi: 10.1093/jac/dks238
Golovliov, I., Baranov, V., Krocova, Z., Kovarova, H., and Sjöstedt, A. (2003). An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71, 5940–5950. doi: 10.1128/IAI.71.10.5940-5950.2003
Gyuranecz, M., Birdsell, D. N., Splettstoesser, W., Seibold, E., Beckstrom-Sternberg, S. M., Makrai, L., et al. (2012). Phylogeography of Francisella tularensis subsp. holarctica, Europe. Emerg. Infect. Dis. 18, 290–293. doi: 10.3201/eid1802.111305
Gyuranecz, M., Rigó, K., Dán, A., Földvári, G., Makrai, L., Dénes, B., et al. (2011). Investigation of the ecology of Francisella tularensis during an inter-epizootic period. Vector Borne Zoonotic. Dis. 11, 1031–1035. doi: 10.1089/vbz.2010.0091
Hall, I. H., Schwab, U. E., Stacy Ward, E., Butts, J. D., Wolford, E. T., and Ives, T. J. (2002). Disposition and intracellular activity of azithromycin in human THP-1 acute monocytes. Int. J. Antimicrob. Agents 20, 348–360. doi: 10.1016/S0924-8579(02)00187-5
Hand, W. L., and Hand, D. L. (2001). Characteristics and mechanisms of azithromycin accumulation and efflux in human polymorphonuclear leukocytes. Int. J. Antimicrob. Agents 18, 419–425. doi: 10.1016/S0924-8579(01)00430-7
Hauri, A. M., Hofstetter, I., Seibold, E., Kaysser, P., Eckert, J., Neubauer, H., et al. (2010). Investigating an airborne tularemia outbreak, Germany. Emerging Infect. Dis. 16, 238–243. doi: 10.3201/eid1602.081727
Helvaci, S., Gedikoğlu, S., Akalin, H., and Oral, H. B. (2000). Tularemia in Bursa, Turkey: 205 cases in ten years. Eur. J. Epidemiol. 16, 271–276. doi: 10.1023/A:1007610724801
Hofinger, D. M., Cardona, L., Mertz, G. J., and Davis, L. E. (2009). TUlaremic meningitis in the united states. Arch. Neurol. 66, 523–527. doi: 10.1001/archneurol.2009.14
Hotta, A., Fujita, O., Uda, A., Sharma, N., Tanabayashi, K., Yamamoto, Y., et al. (2013). In vitro antibiotic susceptibility of Francisella tularensis isolates from Japan. Jpn. J. Infect. Dis. 66, 534–536. doi: 10.7883/yoken.66.534
Huber, B., Escudero, R., Busse, H.-J., Seibold, E., Scholz, H. C., Anda, P., et al. (2010). Description of Francisella hispaniensis sp. nov., isolated from human blood, reclassification of Francisella novicida (Larson et al. 1955) Olsufiev et al. 1959 as Francisella tularensis subsp. novicida comb. nov. and emended description of the genus Francisella. Int. J. Syst. Evol. Microbiol. 60, 1887–1896. doi: 10.1099/ijs.0.015941-0
Ikäheimo, I., Syrjälä, H., Karhukorpi, J., Schildt, R., and Koskela, M. (2000). In vitro antibiotic susceptibility of Francisella tularensis isolated from humans and animals. J. Antimicrob. Chemother. 46, 287–290. doi: 10.1093/jac/46.2.287
Jellison, W. L. (1974). Tularemia in North America, 1930-1974. University of Montana, University of Montana Foundation.
Jellison, W. L., and Owen, C. R. (1961). Tularemia and Animal Populations Ecology and Epizootiology. West Salem, Wis.
Johansson, A., Berglund, L., Gothefors, L., Sjöstedt, A., and Tärnvik, A. (2000). Ciprofloxacin for treatment of tularemia in children. Pediatr. Infect. Dis. J. 19, 449–453. doi: 10.1097/00006454-200005000-00011
Johansson, A., Celli, J., Conlan, W., Elkins, K. L., Forsman, M., Keim, P. S., et al. (2010). Objections to the transfer of Francisella novicida to the subspecies rank of Francisella tularensis. Int. J. Syst. Evol. Microbiol. 60, 1717–1720. doi: 10.1099/ijs.0.022830-0
Johansson, A., Lärkeryd, A., Widerström, M., Mörtberg, S., Myrtännäs, K., Ohrman, C., et al. (2014). A respiratory tularemia outbreak caused by diverse clones of Francisella tularensis. Clin. Infect. Dis. 59, 1546–1553. doi: 10.1093/cid/ciu621
Johansson, A., Urich, S. K., Chu, M. C., Sjöstedt, A., and Tärnvik, A. (2002). In vitro susceptibility to quinolones of Francisella tularensis subspecies tularensis. Scand. J. Infect. Dis. 34, 327–330. doi: 10.1080/00365540110080773
Kantardjiev, T., Ivanov, I., Velinov, T., Padeshki, P., Popov, B., Nenova, R., et al. (2006). Tularemia outbreak, Bulgaria, 1997-2005. Emerging Infect. Dis. 12, 678–680. doi: 10.3201/eid1204.050709
Karlsson, E., Golovliov, I., Lärkeryd, A., Granberg, M., Larsson, E., Öhrman, C., et al. (2016). Clonality of erythromycin resistance in Francisella tularensis. J. Antimicrob. Chemother. 71, 2815–2823. doi: 10.1093/jac/dkw235
Karlsson, E., Svensson, K., Lindgren, P., Byström, M., Sjödin, A., Forsman, M., et al. (2013). The phylogeographic pattern of Francisella tularensis in Sweden indicates a Scandinavian origin of Eurosiberian tularaemia. Environ. Microbiol. 15, 634–645. doi: 10.1111/1462-2920.12052
Keim, P., Johansson, A., and Wagner, D. M. (2007). Molecular epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. 1105, 30–66. doi: 10.1196/annals.1409.011
Kilic, S., Birdsell, D. N., Karagöz, A., Çelebi, B., Bakkaloglu, Z., Arikan, M., et al. (2015). Water as source of Francisella tularensis infection in humans, Turkey. Emerg. Infect. Dis. 21, 2213–2216. doi: 10.3201/eid2112.150634
Kiliç, S., Çelebi, B., Acar, B., and Ataş, M. (2013). In vitro susceptibility of isolates of Francisella tularensis from Turkey. Scand. J. Infect. Dis. 45, 337–341. doi: 10.3109/00365548.2012.751125
Kingry, L. C., and Petersen, J. M. (2014). Comparative review of Francisella tularensis and Francisella novicida. Front. Cell. Infect. Microbiol. 4:35. doi: 10.3389/fcimb.2014.00035
Klimpel, G. R., Eaves-Pyles, T., Moen, S. T., Taormina, J., Peterson, J. W., Chopra, A. K., et al. (2008). Levofloxacin rescues mice from lethal intra-nasal infections with virulent Francisella tularensis and induces immunity and production of protective antibody. Vaccine 26, 6874–6882. doi: 10.1016/j.vaccine.2008.09.077
Kreizinger, Z., Makrai, L., Helyes, G., Magyar, T., Erdélyi, K., and Gyuranecz, M. (2013). Antimicrobial susceptibility of Francisella tularensis subsp. holarctica strains from hungary, Central Europe. J. Antimicrob. Chemother. 68, 370–373. doi: 10.1093/jac/dks399
Kudelina, R. I., and Olsufiev, N. G. (1980). Sensitivity to macrolide antibiotics and lincomycin in Francisella tularensis holarctica. J. Hyg. Epidemiol. Microbiol. Immunol. 24, 84–91.
Kugeler, K. J., Mead, P. S., Janusz, A. M., Staples, J. E., Kubota, K. A., Chalcraft, L. G., et al. (2009). Molecular epidemiology of Francisella tularensis in the United States. Clin. Infect. Dis. 48, 863–870. doi: 10.1086/597261
Larssen, K. W., Afset, J. E., Heier, B. T., Krogh, T., Handeland, K., Vikøren, T., et al. (2011). Outbreak of tularaemia in central Norway, January to March 2011. Euro Surveill. 16:19828. Available online at: www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19828
Larssen, K. W., Bergh, K., Heier, B. T., Vold, L., and Afset, J. E. (2014). All-time high tularaemia incidence in Norway in 2011: report from the national surveillance. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1919–1926. doi: 10.1007/s10096-014-2163-2
Livermore, D. M. (2003). Linezolid in vitro: mechanism and antibacterial spectrum. J. Antimicrob. Chemother 51, ii9–ii16. doi: 10.1093/jac/dkg249
Madrid, P. B., Chopra, S., Manger, I. D., Gilfillan, L., Keepers, T. R., Shurtleff, A. C., et al. (2013). A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS ONE 8:e60579. doi: 10.1371/journal.pone.0060579
Mailles, A., Madani, N., Maurin, M., Garin-Bastuji, B., and Vaillant, V. (2010). Unexpected increase of human and animal tularemia cases during winter 2007/2008 in France: emergence or short-lasting episode?. Med. Mal. Infect. 40, 279–284. doi: 10.1016/j.medmal.2009.11.006
Matzneller, P., Krasniqi, S., Kinzig, M., Sörgel, F., Hüttner, S., Lackner, E., et al. (2013). Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrob. Agents Chemother. 57, 1736–1742. doi: 10.1128/AAC.02011-12
Maurin, M., and Gyuranecz, M. (2016). Tularaemia: clinical aspects in Europe. Lancet Infect. Dis. 16, 113–124. doi: 10.1016/S1473-3099(15)00355-2
Maurin, M., Mersali, N. F., and Raoult, D. (2000). Bactericidal activities of antibiotics against intracellular Francisella tularensis. Antimicrob. Agents Chemother. 44, 3428–3431. doi: 10.1128/AAC.44.12.3428-3431.2000
Maurin, M., Pelloux, I., Brion, J. P., Banõ, J.-N. D., and Picard, A. (2011). Human tularemia in France, 2006–2010. Clin. Infect. Dis. 53, e133–e141. doi: 10.1093/cid/cir612
Maurin, M., and Raoult, D. (2001). Use of aminoglycosides in treatment of infections due to intracellular bacteria. Antimicrob. Agents Chemother. 45, 2977–2986. doi: 10.1128/AAC.45.11.2977-2986.2001
Mccoy, G. W., and Chapin, C. W. (1912). Further observations on a plague-like disease of rodents with a preliminary note on the causative agent, bacterium tularense. J. Infect. Dis. 10, 61–72. doi: 10.1093/infdis/10.1.61
McDonald, P. J., and Pruul, H. (1991). Phagocyte uptake and transport of azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 10, 828–833. doi: 10.1007/BF01975835
Mengeloglu, Z., Duran, A., Hakyemez, I. N., Ocak, T., Kücükbayrak, A., Karadag, M., et al. (2014). Evaluation of patients with Tularemia in bolu province in northwestern Anatolia, Turkey. J. Infect. Dev. Ctries. 8, 315–319. doi: 10.3855/jidc.3952
Meric, M., Willke, A., Finke, E.-J., Grunow, R., Sayan, M., Erdogan, S., et al. (2008). Evaluation of clinical, laboratory, and therapeutic features of 145 tularemia cases: the role of quinolones in oropharyngeal tularemia. APMIS 116, 66–73. doi: 10.1111/j.1600-0463.2008.00901.x
Muller, W., Hotzel, H., Otto, P., Karger, A., Bettin, B., Bocklisch, H., et al. (2013). German Francisella tularensis isolates from European brown hares (Lepus europaeus) reveal genetic and phenotypic diversity. BMC Microbiol. 13:61. doi: 10.1186/1471-2180-13-61
Nano, F. E., and Schmerk, C. (2007). The francisella pathogenicity Island. Ann. N. Y. Acad. Sci. 1105, 122–137. doi: 10.1196/annals.1409.000
Olsufiev, N. G., Emelyanova, O. S., and Dunayeva, T. N. (1959). Comparative study of strains of B. tularense in the old and new world and their taxonomy. J. Hyg. Epidemiol. Microbiol. Immunol. 3, 138–149.
Olsufjev, N. G. (1970). Taxonomy and characteristic of the genus Francisella Dorofeev, 1947. J. Hyg. Epidemiol. Microbiol. Immunol. 14, 67–74.
Olsufjev, N. G., and Meshcheryakova, I. S. (1982). Infraspecific taxonomy of tularemia agent Francisella tularensis McCoy et Chapin. J. Hyg. Epidemiol. Microbiol. Immunol. 26, 291–299.
Olsufjev, N. G., and Meshcheryakova, I. S. (1983). Subspecific Taxonomy of Francisella tularensis McCoy and Chapin 1912. Int. J. Syst. Bacteriol. 33, 872–874. doi: 10.1099/00207713-33-4-872
Origgi, F. C., Frey, J., and Pilo, P. (2014). Characterisation of a new group of Francisella tularensis subsp. holarctica in Switzerland with altered antimicrobial susceptibilities, 1996 to 2013. Euro Surveill. 19:20858. doi: 10.2807/1560-7917.ES2014.19.29.20858
Payne, L., Arneborn, M., Tegnell, A., and Giesecke, J. (2005). Endemic tularemia, Sweden, 2003. Emerging Infect. Dis. 11, 1440–1442. doi: 10.3201/eid1109.041189
Pérez-Castrillón, J. L., Bachiller-Luque, P., Martín-Luquero, M., Mena-Martín, F. J., and Herreros, V. (2001). Tularemia epidemic in Northwestern Spain: clinical description and therapeutic response. Clin. Infect. Dis. 33, 573–576. doi: 10.1086/322601
Petersen, J. M., Carlson, J. K., Dietrich, G., Eisen, R. J., Coombs, J., Janusz, A. M., et al. (2008). Multiple Francisella tularensis subspecies and clades, tularemia outbreak, utah. Emerg. Infect. Dis. 14, 1928–1930. doi: 10.3201/eid1412.080482
Piercy, T., Steward, J., Lever, M. S., and Brooks, T. J. G. (2005). In vivo efficacy of fluoroquinolones against systemic tularaemia infection in mice. J. Antimicrob. Chemother. 56, 1069–1073. doi: 10.1093/jac/dki359
Pilo, P., Johansson, A., and Frey, J. (2009). Identification of Francisella tularensis cluster in central and Western Europe. Emerg. Infect. Dis. 15, 2049–2051. doi: 10.3201/eid1512.080805
Raymond, C. R., and Conlan, J. W. (2009). Differential susceptibility of Sprague-Dawley and Fischer 344 rats to infection by Francisella tularensis. Microb. Pathog. 46, 231–234. doi: 10.1016/j.micpath.2009.01.002
Reintjes, R., Dedushaj, I., Gjini, A., Jorgensen, T. R., Cotter, B., Lieftucht, A., et al. (2002). Tularemia outbreak investigation in Kosovo: case control and environmental studies. Emerg. Infect. Dis. 8, 69–73. doi: 10.3201/eid0801.010131
Rick Lyons, C., and Wu, T. H. (2007). Animal models of Francisella tularensis infection. Ann. N. Y. Acad. Sci. 1105, 238–265. doi: 10.1196/annals.1409.003
Rotem, S., Bar-Haim, E., Cohen, H., Elia, U., Ber, R., Shafferman, A., et al. (2012). Consequences of delayed ciprofloxacin and doxycycline treatment regimens against Francisella tularensis airway infection. Antimicrob. Agents Chemother. 56, 5406–5408. doi: 10.1128/AAC.01104-12
Russell, P., Eley, S. M., Fulop, M. J., Bell, D. L., and Titball, R. W. (1998). The efficacy of ciprofloxacin and doxycycline against experimental tularaemia. J. Antimicrob. Chemother. 41, 461–465. doi: 10.1093/jac/41.4.461
Scheel, O., Hoel, T., Sandvik, T., and Berdal, B. P. (1993). Susceptibility pattern of scandinavian Francisella tularensis isolates with regard to oral and parenteral antimicrobial agents. APMIS 101, 33–36. doi: 10.1111/j.1699-0463.1993.tb00077.x
Schmitt, D. M., O'Dee, D. M., Cowan, B. N., Birch, J. W.-M., Mazzella, L. K., Nau, G. J., et al. (2013). The use of resazurin as a novel antimicrobial agent against Francisella tularensis. Front. Cell. Infect. Microbiol. 3:93. doi: 10.3389/fcimb.2013.00093
Siret, V., Barataud, D., Prat, M., Vaillant, V., Ansart, S., Le Coustumier, A., et al. (2006). An outbreak of airborne tularaemia in France, August 2004. Euro Surveill. 11, 58–60.
Sjostedt, A. (2007). Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105, 1–29. doi: 10.1196/annals.1409.009
Stundick, M. V., Albrecht, M. T., Houchens, C. R., Smith, A. P., Dreier, T. M., and Larsen, J. C. (2013). Animal models for Francisella tularensis and Burkholderia Species scientific and regulatory gaps toward approval of antibiotics under the FDA animal rule. Vet. Pathol. 50, 877–892. doi: 10.1177/0300985813486812
Sutera, V., Caspar, Y., Boisset, S., and Maurin, M. (2014). A new dye uptake assay to test the activity of antibiotics against intracellular Francisella tularensis. Front. Cell. Infect. Microbiol. 4:36. doi: 10.3389/fcimb.2014.00036
Svensson, K., Back, E., Eliasson, H., Berglund, L., Granberg, M., Karlsson, L., et al. (2009a). Landscape epidemiology of tularemia outbreaks in Sweden. Emerg. Infect. Dis. 15, 1937–1947. doi: 10.3201/eid1512.090487
Svensson, K., Granberg, M., Karlsson, L., Neubauerova, V., Forsman, M., and Johansson, A. (2009b). A real-time PCR array for hierarchical identification of francisella isolates. PLoS ONE 4:e8360. doi: 10.1371/journal.pone.0008360
Tärnvik, A., and Berglund, L. (2003). Tularaemia. Eur. Respir. J. 21, 361–373. doi: 10.1183/09031936.03.00088903
Tärnvik, A., and Chu, M. C. (2007). New approaches to diagnosis and therapy of tularemia. Ann. N. Y. Acad. Sci. 1105, 378–404. doi: 10.1196/annals.1409.017
Tomaso, H., Al Dahouk, S., Hofer, E., Splettstoesser, W. D., Treu, T. M., Dierich, M. P., et al. (2005). Antimicrobial susceptibilities of Austrian Francisella tularensis holarctica biovar II strains. Int. J. Antimicrob. Agents 26, 279–284. doi: 10.1016/j.ijantimicag.2005.07.003
Urich, S. K., and Petersen, J. M. (2008). In vitro susceptibility of isolates of Francisella tularensis types A and B from North America. Antimicrob. Agents Chemother. 52, 2276–2278. doi: 10.1128/AAC.01584-07
Valade, E., Vaissaire, J., Mérens, A., Hernandez, E., Gros, C., Doujet, C. L., et al. (2008). Susceptibility of 71 French isolates of Francisella tularensis subsp. holarctica to eight antibiotics and accuracy of the Etest® method. J. Antimicrob. Chemother. 62, 208–210. doi: 10.1093/jac/dkn146
Velinov, K., Kantardjiev, T., Nicolova, M., and Kuzmanov, A. (2011). In vitro antimicrobial susceptibility of Francisella tularensis isolated in Bulgaria. Probl. Infect. Parasit. Dis. 39, 7–9.
Vogler, A. J., Birdsell, D., Price, L. B., Bowers, J. R., Beckstrom-Sternberg, S. M., Auerbach, R. K., et al. (2009). Phylogeography of Francisella tularensis: global expansion of a highly fit clone. J. Bacteriol. 191, 2474–2484. doi: 10.1128/JB.01786-08
Wang, Y., Hai, R., Zhang, Z., Xia, L., Cai, H., Liang, Y., et al. (2011). Genetic relationship between Francisella tularensis strains from China and from other countries. Biomed. Environ. Sci. 24, 310–314. doi: 10.3967/0895-3988.2011.03.015
Wang, Y., Peng, Y., Hai, R., Xia, L., Li, H., Zhang, Z., et al. (2014). Diversity of Francisella tularensis Subsp. holarctica Lineages, China. Emerg. Infect. Dis. 20, 1191–1194. doi: 10.3201/eid2007.130931
Wherry, W. B., and Lamb, B. H. (1914). Infection of man with bacterium tularense. J. Infect. Dis. 15, 331–340. doi: 10.1093/infdis/15.2.331
Keywords: Francisella tularensis, antimicrobial susceptibility, MIC, MBC, intracellular, animal model, tularaemia, antibiotic therapy
Citation: Caspar Y and Maurin M (2017) Francisella tularensis Susceptibility to Antibiotics: A Comprehensive Review of the Data Obtained In vitro and in Animal Models. Front. Cell. Infect. Microbiol. 7:122. doi: 10.3389/fcimb.2017.00122
Received: 25 January 2017; Accepted: 27 March 2017;
Published: 11 April 2017.
Edited by:
Jing-Ren Zhang, Tsinghua University, ChinaReviewed by:
Roger Derek Pechous, University of Arkansas for Medical Sciences, USAGregory T. Robertson, Colorado State University, USA
Copyright © 2017 Caspar and Maurin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yvan Caspar, ycaspar@chu-grenoble.fr
 Yvan Caspar
Yvan Caspar Max Maurin
Max Maurin