Splenic microRNA Expression Profiles and Integration Analyses Involved in Host Responses to Salmonella enteritidis Infection in Chickens
- 1Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, China
- 2Precision Livestock and Nutrition Unit, Gembloux Agro-Bio Tech, University of Liège, Gembloux, Belgium
- 3State Key Laboratory of Animal Nutrition, Beijing, China
- 4College of Animal Science, Jilin University, Changchun, China
To understand the role of miRNAs in regulating genes involved in the host response to Salmonella enteritidis (SE) infection, next generation sequencing was applied to explore the altered splenic expression of microRNAs (miRNAs) and deregulated genes in specific-pathogen-free chickens. Birds were either infected or not (controls, C) and those challenged with SE were evaluated 24 h later and separated into two groups on the basis of the severity of clinical symptoms and blood load of SE: resistant (R, SE challenged-slight clinical symptoms and <105 cfu / 10 μL), and susceptible (S, SE challenged-severe clinical symptoms and >107 cfu/10 μL). Thirty-two differentially expressed (DE) miRNAs were identified in spleen, including 16 miRNAs between S and C, 13 between R and C, and 13 between S and R. Through integration analysis of DE miRNAs and mRNA, a total of 273 miRNA-target genes were identified. Functional annotation analysis showed that Apoptosis and NOD-like receptor signaling pathway and adaptive immune response were significantly enriched (P < 0.05). Interestingly, apoptosis pathway was significantly enriched in S vs. C, while NOD-like receptor pathway was enriched in R vs. C (P < 0.05). Two miRNAs, gga-miR-101-3p and gga-miR-155, in the hub positions of the miRNA-mRNA regulatory network, were identified as candidates potentially associated with SE infection. These 2 miRNAs directly repressed luciferase reporter gene activity via binding to 3′-untranslated regions of immune-related genes IRF4 and LRRC59; over-expressed gga-miR-155 and interference gga-miR-101-3p in chicken HD11 macrophage cells significantly altered expression of their target genes and decreased the production of pro-inflammatory cytokines. These findings facilitate better understanding of the mechanisms of host resistance and susceptibility to SE infection in chickens.
Introduction
Salmonella enteritidis (SE) is a Gram-negative enteric pathogen, infection with which does not cause significant disease or mortality, but birds can carry the bacteria for several weeks without presenting any clinical signs, thereby constituting an insidious risk for public health (Calenge et al., 2010; Barrow et al., 2012; Calenge and Beaumont, 2012). Although, Salmonella contamination can be significantly reduced using control measures in poultry, there was a considerable increase in reported Salmonella cases in the EU (European Food Safety Authority and European Centre for Disease Prevention and Control, 2016) and UK (Inns et al., 2015). SE also tends to be highly resistant to multiple antimicrobials, such as sulfamethoxazole-trimethoprim and nalidixic acid, which has the potential to complicate treatment of animal and human disease (DuPont and Steele, 1987; Goldman, 2004; Kuang et al., 2015). Therefore, to reduce economic losses in poultry production and to protect animal and human health, it is critical to understand the host immune response and mechanisms of resistance against SE infection.
MicroRNAs (miRNAs) have been identified as key regulators of gene expression at the post-transcriptional level. These small RNAs have been demonstrated to have important functions in a variety of biological processes including the cell cycle, differentiation, apoptosis, and pathogenesis (Ambros, 2004; Filipowicz et al., 2008; Krol et al., 2010; Yates et al., 2013). There are increasing evidences that the miRNAs play important roles in regulating the innate immune response induced by bacteria (Eulalio et al., 2012; Staedel and Darfeuille, 2013; Maudet et al., 2014; Das et al., 2016). Previous studies have shown that miRNAs, such as miR-146a, miR-155, and Let-7 and their targets are involved in the regulation of immune response to Salmonella or lipopolysaccharide infection in mice (O'Neill et al., 2011; Schulte et al., 2011; Eulalio et al., 2012) and swine (Bao et al., 2014, 2015; Yao et al., 2016a,b). For instance, few proteins (IRAK1, IRAK2, and TRAF6) within TLR signaling have been confirmed as direct targets of miR-146 (O'Neill et al., 2011); signal molecules MyD88, TAB2, SHIP1, and SOCS1 were targets of miR-155 (Eulalio et al., 2012); and cytokines IL-6 and IL-10 are targeted by Let-7 (Staedel and Darfeuille, 2013).
The role of miRNA in response to bacterial infection has also been investigated in chickens. Several miRNAs (gga-miR-125b-5p, gga-miR-34a-5p, gga-miR-1416-5p, and gga-miR-166) associated with SE infection were identified recently in laying chicken cecum by next generation sequencing (Wu et al., 2017). A novel splenic miRNA, gga-miR-429, involved in the host response to Avian pathogenic Escherichia coli (APEC) was also detected by deep sequencing (Jia et al., 2017). Despite these studies, there is still limited information about the function of miRNAs in the host response and resistance to Salmonella infection in chickens.
The spleen, as the body's major blood filter, plays a major role in detecting cell damage during Salmonella infection and in the pathogenic mechanisms of Salmonella. Further, increasing evidence suggests that the spleen plays a greater role in immune function in avian than in mammalian species, and is responsible for an immediate innate reaction after recognizing pathogens by filtering antigens from the blood (Smith and Hunt, 2004; Tiron and Vasilescu, 2008). Assessing changes in the expression of miRNAs and their targets in spleen on a genome-wide scale, therefore, could provide more comprehensive insight into the immune response to bacterial infection. The objectives of the present study were to identify the miRNAs and miRNA-regulated genes responsible for host resistance and susceptibility to SE infection using next generation sequencing on spleens from three groups of chickens: Controls (C, non-challenged, no detected SE in blood at 24 h, Resistant (R, SE-challenged, slight clinical symptoms, <105 cfu/10 μL SE in blood), and Susceptible (S, SE-challenged, severe clinical symptoms, >107 cfu/10 μL SE in blood) chickens. Subsequently, based on combined analysis of expression profiles of miRNA and potential target mRNA, the functional analysis and candidate miRNAs involved in the host response to SE infection were further characterized with the goal of better understanding the mechanisms of resistance and susceptibility to Salmonella.
Materials and Methods
Ethics Statement
All of the animal experiments were conducted in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China). Animal experiments were approved by the Animal Management Committee (in charge of animal welfare issue) of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (IAS-CAAS, Beijing, China). Ethical approval on animal survival was given by the animal ethics committee of IAS-CAAS (approval number: IASCAAS-AE20140615).
Animals and Sample Collection
Specific-pathogen-free White Leghorn chickens were supplied by the Beijing Laboratory Animal Research Center (BLARC, Beijing, China) and were treated as described in previous studies (Li et al., 2010; Gou et al., 2012). In brief, the SPF chickens were raised in climate-controlled, fully enclosed isolation facilities at the experimental center of China Agriculture University (Beijing, China) under identical management conditions. At 3 d of age, a total of 150 SPF chickens were orally challenged with 1 ml PBS containing 108 cfu of S. enteritidis (50041) and another 75 birds received 1 ml PBS as controls. Blood samples from each of 30 challenged and 15 control chickens were taken at 24 h post infection and birds were killed and the spleens were dissected, snap frozen and held at −80°C. Bacterial burden (expressed as cfu/10 μL blood) was determined indirectly by serovar-specific quantitative real-time PCR (qPCR), and along with clinical severity, was used to evaluate the resistance/susceptibility to SE challenge, as described in previous studies (Deng et al., 2008; Gou et al., 2012). 10 μL EDTA-anticoagulated blood was used for DNA extraction using MiniBEST Whole Blood Genomic DNA Extraction Kit (Takara, Code No. 9781) according to the manufacturer's instructions. Amplification was carried out in a total 25 μL reaction mixture, containing 0.6 μL of each primer (10 μM), 0.75 μL of dNTPs (10 mM), 1.25 U of ExTaq DNA Polymerase (Takara), 5.5 μL of 5 × PCR buffer (Mg2+), 0.8 μL of TaqMan probe (5 μM), and 2 μL of template, with deionized water to 25 μL. Each PCR consisted of a 5 min hot start at 95°C followed by 40 cycles of 30 s at 94°C, 30 s at 55°C, and a fluorescence read step. The probe (5′-FAM-TGCAGCGAGCATGTTCTGGAAAGC-TAMRA-3′) and primers set (forward primer, 5′-TCCCTGAATCTGAGAAAGAAAAACTC-3′; reverse primer, 5′-TTGATGTGGTTGGTTCGTCACT-3′) were designed from the SdfI gene (Gen-Bank Accession No. AF370707.1), as described in Gou et al. (2012). The qPCR assay was calibrated by relating threshold cycle (Ct) values to cfu, as determined by enumeration after plating serial dilutions of S. enteritidis and standard culture.
In this study, the clinical symptoms (diarrhea, drooping wings, and dying) and bacterial load of SE at 24 h after challenge were used together to discriminate susceptible (S, SE-challenged, slight clinical symptoms and >107 cfu/10 μL blood) from resistant (R, SE-challenged, severe clinical symptoms and <105 cfu/10 μL blood) birds. No SE was detected in the Controls (C). Total splenic RNA was extracted from three birds in each of the three groups, S, R, and C, using miRNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. RNA was quantified using the NanoDrop ND-2000 spectrophotometer (NanoDrop Products, Wilmington, DE) and purity was assessed by Bioanalyzer 2100 and RNA 6000 Nano LabChip Kit (Agilent, Santa Clara, CA) with RNA Integrity Number (RIN) number >7.0. Total RNA was stored at −80°C until used.
Small RNA Sequencing and Screening of the Differentially Expressed miRNAs
Total RNA of each sample (~1 μg) was used to prepare the miRNA sequencing library, which included the following steps: (1) 3′-adapter ligation with T4 RNA ligase 2 (truncated); (2) 5′-adapter ligation with T4 RNA ligase; (3) cDNA synthesis with an RT primer; (4) PCR amplification; and (5) extraction and purification of 120–140 bp PCR amplified fragments (corresponding to ~15–25 nt small RNAs) from polyacrylamide gels. An Agilent 2100 Bioanalyzer quantified the libraries, after which the samples were diluted to a final concentration of 8 pM and cluster generation was performed on the Illumina using TruSeq Small RNA Sample Prep Kits (Illumina, San Diego, CA), following the manufacturer's instructions. The 9 miRNA libraries were constructed and single-end sequenced (36 bp) on an Illumina Hiseq 2500 at the LC-BIO (Hangzhou, China) following the vendor's recommended protocol. The raw data of each sample was not <10 M reads. The raw reads were subjected to the Illumina Pipeline filter (Solexa v0.3), and then the dataset was further processed with ACGT101-miRv4.2 (LC Sciences, Houston, TX) to remove adapter dimers, junk, low complexity, common RNA families (rRNA, tRNA, snRNA, snoRNA) and repeats. Subsequently, the 18–25 nt length unique sequences were BLASTed to chicken precursors in miRBase 20.0 (Kozomara and Griffiths-Jones, 2014) (http://www.mirbase.org/) to detect known miRNAs and novel 3p- and 5p- derived miRNAs. One mismatch inside the sequence and length variation at both 3′ and 5′ ends were allowed in the alignments. The unique sequences were mapped to chicken mature miRNAs in hairpin arms recognized as known miRNAs, and mapped to the other arm of known chicken precursor hairpins opposite the annotated mature miRNA-containing arm considered to be novel 5p- or 3p-derived miRNAs. The remaining sequences were mapped to other selected species in miRBase 20.0 by BLAST search, and the mapped pre-miRNAs were further BLASTed against the chicken genomes to identify their genomic positions. The aforementioned miRNAs were considered to be known miRNAs. To identify the novel predicted miRNAs, the unmapped sequences were BLASTed against the chicken genome database, and the hairpin RNA structures comprising sequences were identified using RNAfold software (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi). Modified reads per million (RPM) reads was used to quantify the normalized reads, the formula was: Normalized Expression (NE) = Actual miRNA count/Total count of clean reads. MicroRNAs were regarded as being differentially expressed (DE) based on normalized deep-sequencing levels (with the exclusion of 3 RPM) in S, R and C groups, respectively. The DE miRNAs based on normalized counts were analyzed using Student t-tests (Huang et al., 2015; Li et al., 2016) according to the experimental design and the significance threshold was set as P < 0.05. The normalized read counts of some miRNAs were set to be 0.01 for further calculation if they had no reads in the library.
Differentially Expressed Analysis of mRNA
Nine cDNA libraries were also constructed from splenic RNA (1 μg) of these same birds and sequenced by LC-BIO (Hangzhou, China) on an Illumina HiSeq 2500 platform and 125 bp paired end reads were generated. The raw reads were first processed through FastQC to obtain the clean data, by removing the reads that contain sequencing adapter contaminations or poly-N and the low quality reads, Q-values for which were <20. Some potential residual ribosome RNA data were also removed from the remaining data by alignment. Clean reads were then mapped to the Gallus gallus database using TopHat (Trapnell et al., 2009), and the mapped reads were assembled de novo using Cufflinks (Trapnell et al., 2010). Expression levels of mRNAs were quantified as fragments per kilobase of exon per million mapped reads (FPKM) using the Cufflinks package (Trapnell et al., 2010). Analysis of DE genes between the three groups of chickens was performed using the Cuffdiff with a P < 0.05 and |log2fold change| > 0.58.
Prediction of DE miRNA Targets, Gene Ontology (GO), and KEGG Pathway Analysis
Only target DE genes that were predicted by both TargetScan 6.2 and miRanda 3.3 for all of the DE miRNAs were considered further. Gene Ontology (GO) and KEGG pathway enrichment of target DE genes were analyzed by DAVID 6.8 (http://david.abcc.ncifcrf.gov/), which is based upon a Fisher Exact statistic methodology similar to that previously described (Huang et al., 2009). GO and KEGG results were filtered using P < 0.05.
Correlation Analysis of miRNA and mRNA
In order to build the miRNA-mRNA interaction network, the following method was used, as described in previous studies (Ye et al., 2016): A target gene was identified by the direction of change in a pairwise comparison, for example S to C, being the reverse of changes in the miRNAs. The miRNA-mRNAs interaction network was constructed using Cytoscape v2.8.3 software (http://www.cytoscape.org/).
Mirna Target Validation
The pmiR-RB-Report™ (RiboBio, Guangzhou, China) including double luciferase reporter genes was used to test and validate the target sites for gga-miR-155 and gga-miR-101-3p. The 3′ UTR of IRF4 and LRRC59 containing gga-miR-101-3p and gga-miR-155 binding sites were amplified from chicken genomic DNA. The primers for PCR are provided, as follows: IRF4: GGCGGCTCGAGGATCCTCAGAATAAGTGTT (forward) and AATGCGGCCGCGTTAGAAG-TCCCTAGAAAA (reverse); and LRRC59: GGCGGCTCGAGATGCTACAGCAGAACTCGC (forward) and AATGCGGCCGCCAGACAAATTGATGCGAAA (reverse). All PCR products were cloned into the pmiR-Repor Vector using Xhol and NotI restriction enzymes. Luciferase reporter experiments were performed in 293T (human embryonic kidney) cells, obtained from ATCC. Cells were seeded in 96-well plates at a density of 5 × 104 cells/well and cultured under routine conditions with 10% fetal bovine serum. When the cells reached 70 to 80% confluence, pmiR-3′ UTR (100 ng) was co-transfected with 50 nM of a negative control or a gga-miR-101-3p mimic (GenePharma, Shanghai, China) using 0.30 μL of FugeneHD (Promega, Madison, WI) according to the manufacturer's instructions. The relative luciferase activity was measured 48 h after transfection by the Dual-Glo Luciferase Assay System (Promega).
Over-Expressed gga-miR-155 and Interference gga-miR-101-3p in Chicken HD11 Macrophage Cells
To further validate the biological function of gga-miR-155 and gga-miR-101-3p in a chicken macrophage-like line HD11, 100 μM mimic (gga-miR-155), inhibitor (gga-miR-101-3p) and control oligos (gga-miR-NC) were transfected into HD11 cells using 12-well plates and TransIT®-2020 (Mirus Bio, Madison, WI) per the manufacturer's instructions. HD11 cells were grown at 37°C with 5% CO2 in RPMI-1640 medium that contained 10 mM HEPES, 1 mM sodium pyruvate, 1% glutamine, 1% MEM NEAA, 10% fetal bovine serum, and 5% chicken serum (all reagents from Gibco). After 36-h transfection, the cells were harvested using MiniBEST Universal RNA (Takara, Code No. 9767) to extract the total RNA. For the LPS exposure, macrophages were challenged with 1 mg/ml LPS and harvested at different times for RNA extraction. Cells with no stimulation were collected as the control, and each experiment had three biological replicates.
Quantitative Real-Time PCR Analysis
To validate and characterize the DE miRNA and DE transcripts identified via high-throughput sequencing, qPCR analyses were performed in an ABI 7500 Detection System (Applied Biosystems, Foster, CA). The miScript SYBR Green PCR kit (Qiagen, Valencia, CA) and PCR Master Mix (SYBR Green) Kit (Toyobo, Osaka, Japan) were used in qPCR to determine the abundance of mRNAs and miRNAs, using β-actin and U6 genes as reference genes, respectively. The relative mRNA and miRNA expression level was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001). All primers are described in Supplementary Tables S8, S9. Three independent replications were used for each assay and data are presented as means ± SD.
Results
miRNA Profiles in the Spleen of Chickens
An average of 6,348,747 high quality clean reads per miRNA sample, which represented 359,232 unique reads in the range of 18–26 nt in the nine libraries were obtained from splenic samples via next generation sequencing (Supplementary Table S1). These high-quality reads were mapped to chicken precursors in miRBase to identify known and novel miRNAs for further analysis. Low levels of large fragments, such as mRNA and rRNA, were also found, which indicated the high-quality and minimal degradation of RNA samples in the present study. For all nine samples, the distribution of the small RNA sequence length was mainly concentrated at 22 nt, followed by 23 and 21 nt (Figure 1A), which is consistent with the typical size range for Dicer-derived products and in agreement with most of the previous reports from other animal species.
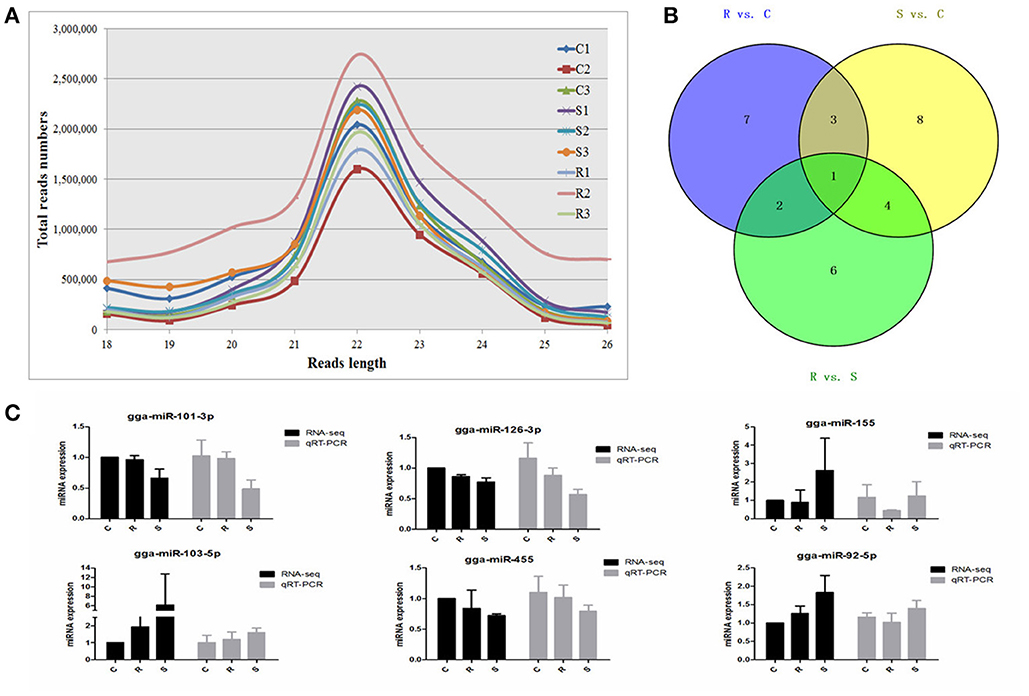
Figure 1. Different expression profiles of miRNAs among C, R, and S chickens. (A) Size distribution of sequenced small RNA reads. (B) Venn diagram demonstrates the overlap of differentially expressed (DE) miRNAs among the three groups; numbers are the DE miRNAs in each comparison. (C) Correspondence of miRNAs obtained by high-throughput sequencing and qPCR. C, Controls; R, Resistant; S, Susceptible.
A total of 2238 miRNAs, classified into five categories (Supplementary Table S2), were detected via BLAST in miRBase. After removing the less expressed miRNAs, i.e., the expression levels were <3 after the normalization of dataset (in at most 3 samples), 744 miRNAs were identified including 439 known chicken miRNAs, and 62 potentially novel miRNAs (defined as PC-3p or PC-5p) in chicken spleen after oral challenge with SE (Supplementary Table S2).
Differential Expression of miRNAs in Response to Salmonella Infection
A total of 32 miRNAs exhibited significantly different expression (DE) among the C, R and S groups. The results showed that, for S vs. C 16 DE miRNAs (7 up- and 9 down-regulated); for R vs. C 13 DE miRNAs were found (4 up- and 9 down-regulated) and 13 were found in the R vs. S comparison (10 up- and 3 down-regulated; Figure 1B, Table 1 and Supplementary Table S3). To validate the expression profiles from sequencing, 6 miRNAs were also examined by q-PCR (Figure 1C). Except for gga-miR-92-5p with a slight difference in the R group, the expression patterns of gga-miR-101-3p, gga-miR-126-3p, gga-miR-155, gga-miR-103-5p, and gga-miR-455 were comparable by both methods. The expression profiles from the deep sequencing were therefore considered as being reliable and appropriate for further analysis.
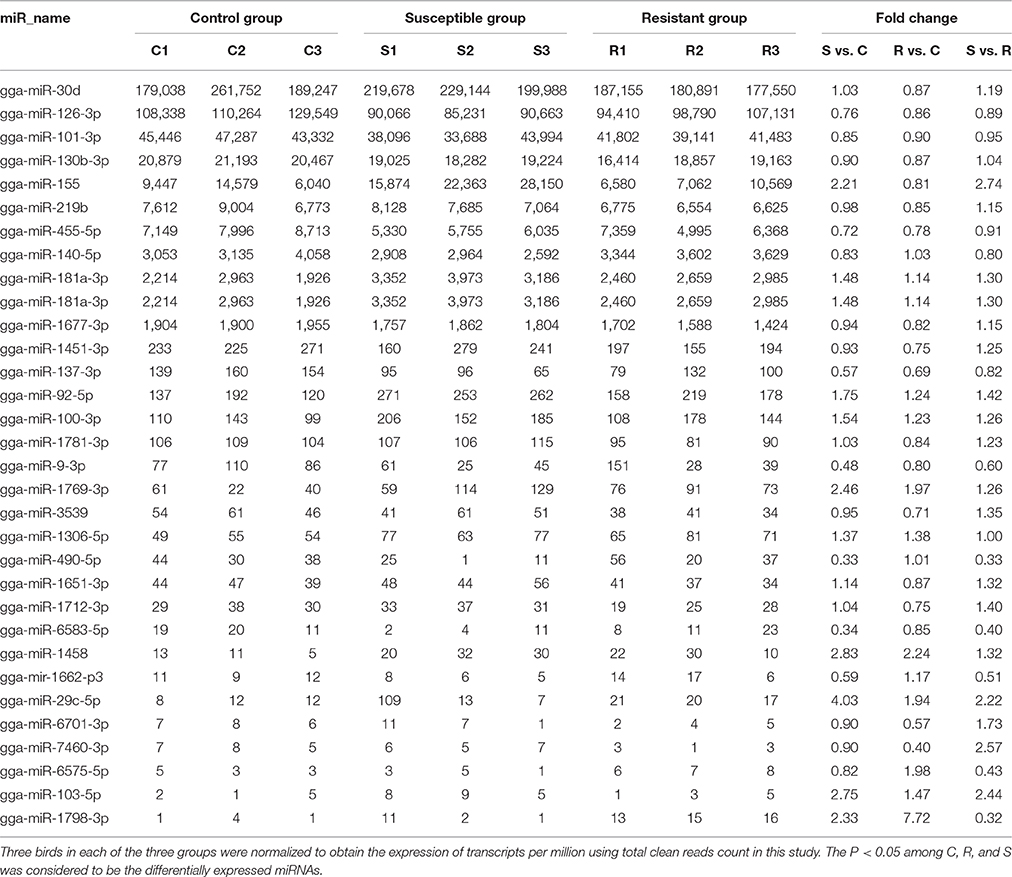
Table 1. Differential expression profile of splenic miRNAs among birds responding differently to SE infection.
The differences in splenic expression between the controls, resistant and susceptible birds were examined. Four miRNAs were significantly differently expressed in both S vs. C and R vs. C, and 5 in both S vs. C and S vs. R, as well as 3 in both R vs. C and R vs. S (Figure 1B, Table 1). Only 1 miRNA (gga-mir-1677) was significantly differently expressed in all three groups of birds (Figure 1B). Several miRNAs previously reported to be involved in immune responses such as miR-155, miR-9, miR-30, miR-126, and miR-29 families were identified. Also identified here were several new candidate miRNAs associated with SE infection, such as gga-miR-29c-5p (up-regulated, P = 0.01) and gga-miR-137-3p (down-regulated, P = 0.009).
Differential Expression of miRNA-Targeted Genes in Response to SE Infection
In order to validate the roles of DE miRNAs in affecting expression of their target genes, mRNA in the same samples was also profiled by sequencing. Based on both TargetScan and miRanda systems, a total of 273 DE genes can be targeted by the 32 DE miRNAs in the three groups (Supplementary Table S4). As shown in Figure 2A, 148 (S vs. C), 40 (R vs. C), and 85 (R vs. S) DE miRNA-targeted genes were identified with fold change (FC) > 1.50 or < 0.67 and P < 0.05 (Supplementary Table S5). The heat map and hierarchical clustering demonstrated distinct profiles of the unique miRNA-targeted genes in the three comparisons (Figures 2B–D). Several immune-related genes were found to be significantly DE in spleen after challenge with SE. For example, the expression of IL8, CXCR4, and IRF4 were significantly up-regulated following SE challenge (FC 5.21, 3.69, and 2.02, respectively). To validate the expression profiles from sequencing, transcript abundances of eight genes were measured by qPCR (Figure 2E); overall, there was good concordance between the two methods.
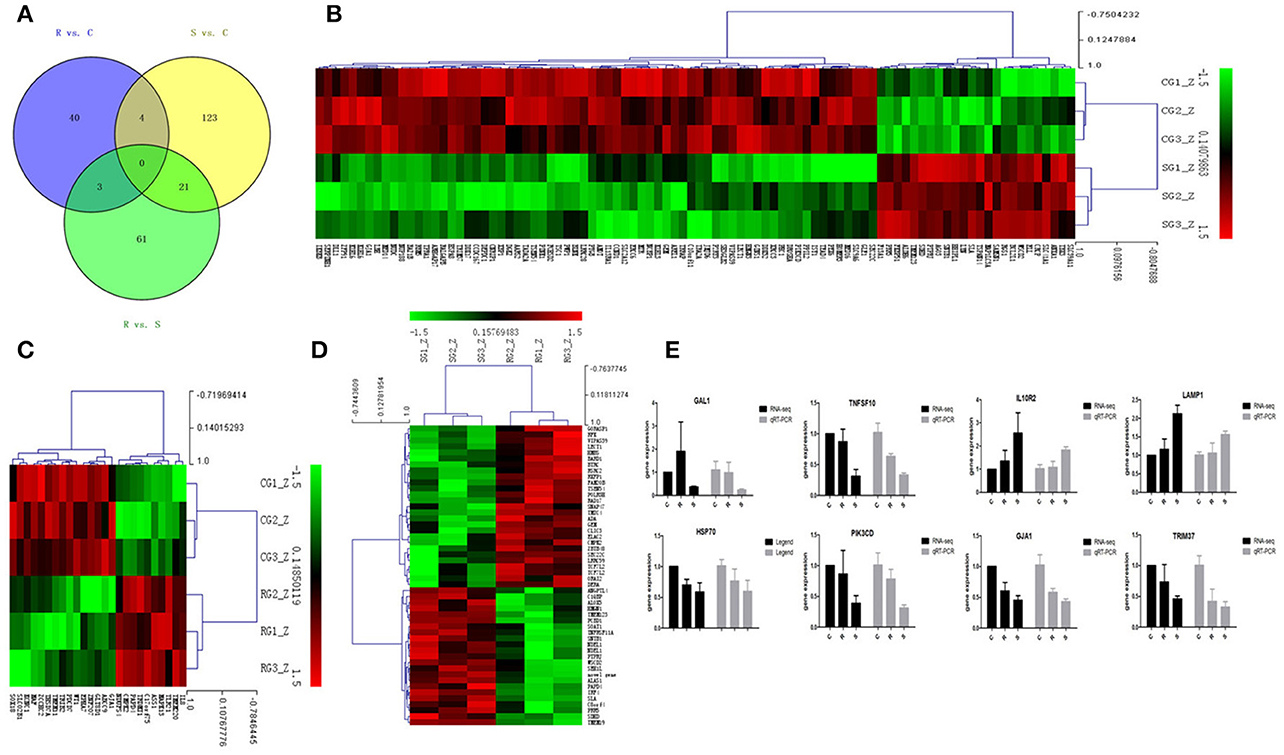
Figure 2. Differential expression of miRNAtargeted genes in response to SE infection. (A) Venn diagram demonstrates the overlap of targeted genes for the DE miRNAs among the three groups of chickens. Numbers in each section indicate the numbers of differently expressed miRNAs in the comparison. (B–D) The heat map of unique targets of DE miRNAs in S vs. C, R vs. C, and R vs. S, respectively. (E) Correspondence of the targeted genes for the DE miRNAs by high-throughput sequencing and qPCR. Data for each method were from the same samples of splenic tissues (C, R, and S chickens); TargetScan 6.2 and miRanda 3.3 were used to predict the miRNA targets and only targets predicted by both methods were used for further analysis. The heat map and clustering was constructed by Multi Experiment Viewer v4.8 using Row Z-Score (Murie et al., 2014) [(ΔΔCt–means)/SD] (Supplementary Table S7). In the figures, red represents up-regulation, green shows down-regulation, and black is no change.
Potential Function Analysis of DE miRNA Targets
The ultimate function of miRNAs is at the level of the activity of target genes. In this study, functional annotation and pathway enrichment analysis of 273 target DE genes were performed using Gene Ontology (GO) and KEGG. Potential function analysis of these genes showed that 2 immune-related KEGG pathways and 1 biological process were significantly enriched (P < 0.05), including Apoptosis, NOD-like receptor signaling pathway, and adaptive immune response (GO:0002250) (Table 2). The present results suggest that the changed miRNAs may regulate these immune-related targets in chicken spleen during SE infection.
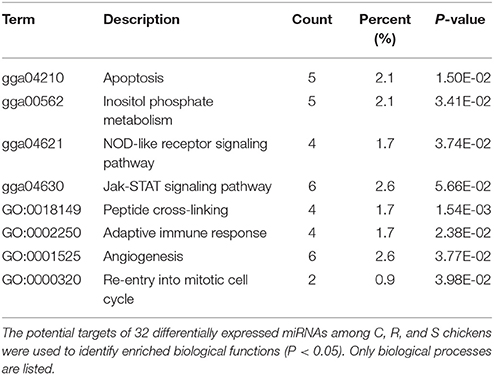
Table 2. Functional annotation and pathway enrichment analysis of all target genes were performed using GO and KEGG.
Potential functional analyses for host immune responses to SE infection between R and S chickens were further characterized, based on the target genes of significant DE miRNAs between these two groups and the controls. For S vs. C, 4 pathways were enriched (P < 0.05), viz. Apoptosis, Spliceosome, mTOR signaling pathway, Insulin signaling and Jak-STAT signaling pathway; 2 biological processes were significantly enriched (P < 0.05); regulation of inflammatory response and heart looping. In the R vs. C comparisons, NOD-like receptor signaling pathway was significantly enriched (P < 0.05); defense response to bacterium (GO:0042742), immune-related biological processes, was enriched but not significantly (P = 0.06). Except for regulation of proteasomal protein catabolic process (GO:0061136) being enriched (P < 0.05), no pathways were found when R was compared with S (Table 3). These results are consistent with the susceptible birds being more likely to exhibit apoptosis due to an inflammatory response, while the resistant birds showed more of an innate immune response to SE infection.
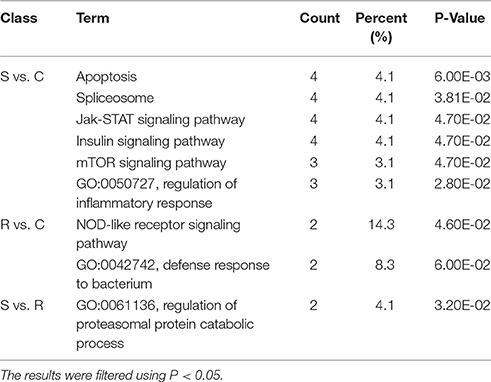
Table 3. GO and KEGG enrichment of unique miRNA target genes were analyzed between S vs. C, R vs. C, and S vs. R.
miRNA-mRNA Regulatory Relationships in Spleen after SE Infection
Most descriptions of miRNA function have focused on their roles as post-transcriptional regulators of target mRNAs. Based on the putative miRNA-mRNA regulatory pairs, it was found that 91 SE-related genes can be targeted by 29 of the 32 DE miRNAs (Supplementary Table S6). The potentially important interaction networks for immune-related miRNA-mRNA pairs are shown in Figure 3. The relative expression of innate/inflammatory marker genes such as PIK3CD was significantly up-regulated following SE infection. Some mRNAs are highly connected and regulated by multiple miRNAs. For example, CXCR4 is involved in cytokine-cytokine receptor interaction and was identified as a potential target of gga-miR-155 and gga-miR-9-3p. IFR4 was predicted to be regulated by gga-miR-30d and gga-miR-101-3p. LRRC59 was predicted as a potential target of gga-miR-103-5p and gga-miR-155. One Salmonella-regulated miRNA of particular interest identified through the present study is gga-miR-101-3p. Although, the expression levels of miR-101-3p were relatively moderate, it is highly connected (>8 SE-related target genes) within the miRNA-mRNA network. These have not been previously reported to be associated with Salmonella infection, and are predicted here to regulate several immune-related genes.
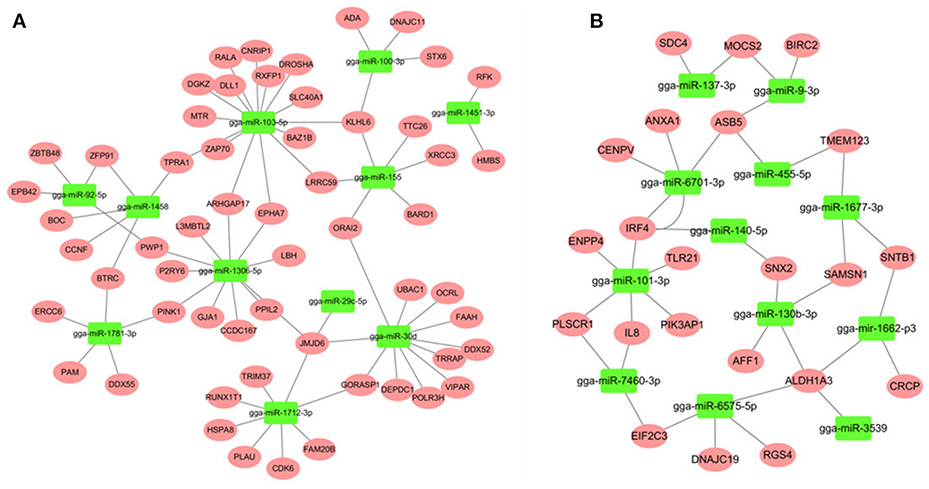
Figure 3. miRNA-mRNA interactions in spleen associated with SE infection. (A) miRNA-mRNA network among up-regulated miRNAs and down-regulated mRNAs (B) miRNA-mRNA network among down-regulated miRNAs and up-regulated mRNAs.
Validations of miRNA-mRNA Interactions Using gga-miR-101-3p-IRF4 and gga-miR-155-LRRC59 mimics
The luciferase reporter gene system was used to validate the above-stated predicted interactions. The 3′ UTRs of IRF4 and LRRC59 were cloned into luciferase reporter plasmids to test gga-miR-101-3p and gga-miR-155 functions in vitro. Transfection with a gga-miR-101-3p mimic resulted in significant (P < 0.01) reduction in relative luciferase activity for IRF4 plasmids (Figure 4), compared with negative control miRNA (random miRNA sequence) and a no-insert control. Similarly, transfections with mimics resulted in significant (P < 0.05) reduction in relative luciferase activity for LRRC59 (Figure 5) compared with the negative miRNA and no-insert controls. These results indicate that similar responses are likely to be happening in the host during SE infection, that is, the down-regulation of gga-miR-101-3p may result in increased expression of IRF4 during Salmonella infection, and up-regulation of gga-miR-155 may inhibit expression of LRRC59.
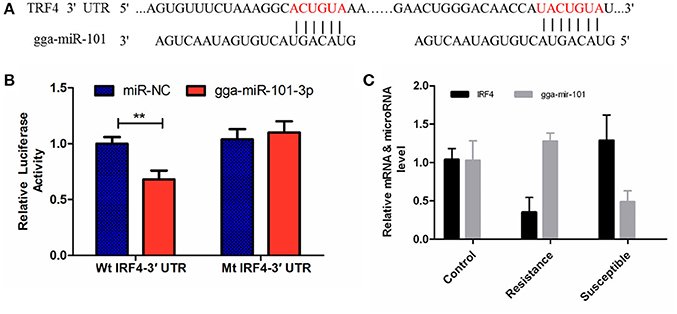
Figure 4. Regulation of IRF4 by gga-miR-101-3p. (A) Predicted gga-miR-101-3p binding sites at distinct positions in IRF4; nucleotides of the gga-miR-101-3p seed region are in red. (B) Luciferase activity in 293T cells transfected with miRNA mimics and plasmids carrying the 3′UTR of IRF4. NC miRNA = negative control miRNA. (C) Expression change of IRF4 and gga-miR-101-3p after infection. **represents P-value < 0.01.
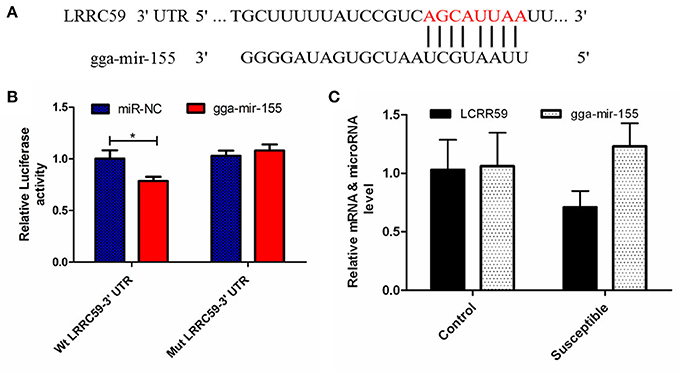
Figure 5. Regulation of LRRC59 by gga-miR-155. (A) Predicted gga-miR-155 binding sites at distinct positions in LRRC59; nucleotides of the gga-miR-155 seed region are in red. (B) Luciferase activity in 293T cells transfected with miRNA mimics and plasmids carrying the 3′UTR of LRRC59. NC miRNA = negative control miRNA. (C) Expression change of LRRC59 and gga-miR-155 after infection. *represents P-value < 0.05.
Validations of Biological Function of gga-miR-155 and gga-miR-101-3p in Chicken HD11 Macrophage Cells
After 36-h treatment with mimic, elevating gga-miR-155 significantly repressed the mRNA expression levels of LRRC59 compared to the miR-NC and negative controls (P < 0.05); In contrast, after 36-h treatment with gga-miR-101-3p inhibitor, the mRNA expression levels of IRF4 were significantly increased (P < 0.05) compared to the controls (Figure 6). In order to address the effect of miR-155 and miR-101 on the induction of pro-inflammatory cytokines in response to LPS, the expression levels of IL-6 and TNF-α were measured in a macrophage inflammatory response model. The results showed that miR-155 overexpression markedly decreased the expression of IL-6 and TNF-α compared with control miRNA or miR-155 inhibitor (Figure 7A; P < 0.01), while miR-101 knockdown significantly decreased the expression of IL-6 and TNF-α compared with control miRNA inhibitor (Figure 7B; P < 0.05). These data demonstrate that gga-miR-155 and gga-miR-101 could regulate the production of pro-inflammatory cytokines, IL-6 and TNF-a, which may play a negative role in response to LPS stimulation in chickens.
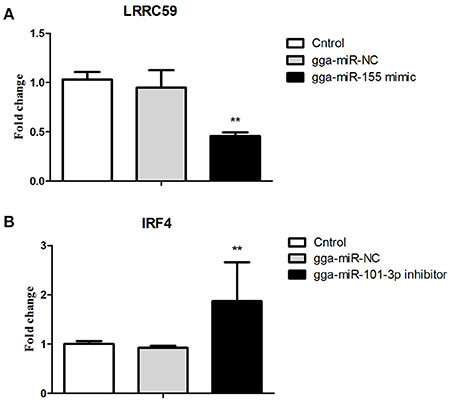
Figure 6. Validations of biological function of gga-miR-155 and gga-miR-101-3p in chicken HD11 macrophages. (A) gga-miR-155 mimic significantly repressed the mRNA expression of LRRC59. (B) gga-miR-101-3p inhibitor significantly promoted mRNA expression of IRF4. The fold-change values were calculated using the comparative 2−ΔΔCT. The P-values are indicated with asterisks when lower than 0.01 (**) when compared to control (non-transfected) and NC (gga-miR-NC).
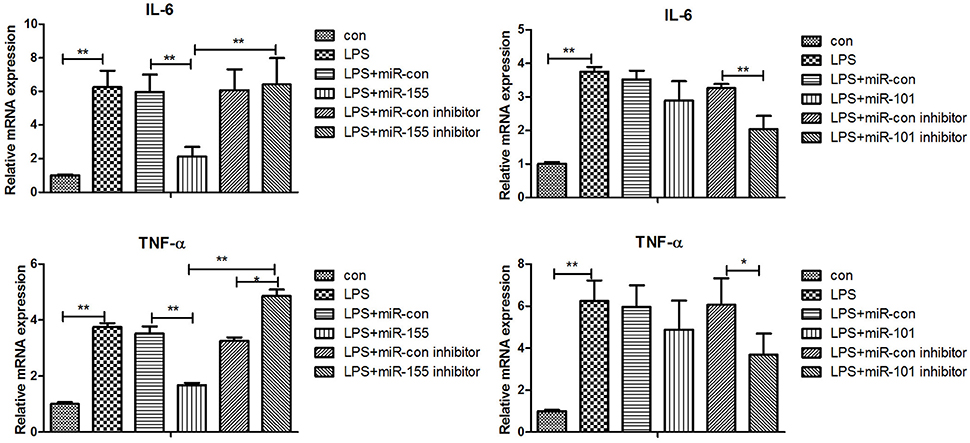
Figure 7. Gga-miR-155 and gga-miR-101-3p regulate expression of pro-inflammatory cytokine genes induced by LPS. mRNA expression of IL-6 and TNF-α in chicken HD11 6 h after LPS treatment, or 24-h post transfection with miRNA control (50 nM), miRNA inhibitor control (100 nM), miRNA-155/101 (50 nM) and miRNA-155/101 inhibitor (100 nM) then the cells were stimulated with LPS (1 μg/mL) for 6 h. Relative transcript abundances of the genes were analyzed by qPCR. Data are presented as the mean ± SE from three independent experiments performed in triplicate. The P-values are indicated with asterisks when lower than 0.05 (*) or 0.01 (**) when compared to control.
Discussion
MiRNAs are important regulators of innate and adaptive immunity (Sonkoly et al., 2008; O'Neill et al., 2011; Olivieri et al., 2013) but their specific roles in regulating the responses to Salmonella infection in chicken are incompletely understood. It is necessary, therefore, to identify and characterize the critical miRNAs in the chicken immune response to Salmonella with the aim of understanding pathogenesis, improving animal welfare, reducing losses in poultry production and in keeping food safe. Here, next generation sequencing was used to detect differences in splenic expression profiles of miRNAs in chickens challenged with SE. A total of 439 known and 62 potentially novel miRNAs were detected, including those expressed at low levels such as gga-miR-7460 (normalized average 7 and 2 reads for C and R, respectively). Through DEG analysis, 32 miRNAs were found to be differentially expressed among C, R and S groups, representing differences between both infected and non-infected animals and heavy and light bacterial burdens resulting from a single-dose infection with SE. For these miRNAs, gga-miR-155 had the most abundant expression and it was significantly up-regulated in susceptible chickens (both S vs. C and S vs. R). Similarly, gga-miR-92-5p was highly up-regulated in resistant birds (R vs. C and R vs. S). Another highly expressed miRNA, gga-miR-1306-5p, was increased in both R and S compared with C, but with no significant difference between R and S. This suggests that these miRNAs in spleen might be involved as components of the immune response to SE. These results of the present study also suggested that deep sequencing technology has utility in the discovery of functional miRNAs, including those expressed at low levels, in the SE pathogenic processes. Also in this study, three groups were defined to increase the power of detecting miRNA DE, according to the severity of clinical symptoms and host carrier-state level (quantified as cfu/ unit volume of blood), allowing comparisons to be made between birds demonstrating resistance or vulnerability to SE, in addition to simply comparing challenged and non-challenged birds. This is clearly a useful approach to identify the candidate genes involved to host resistance to SE. The present study of splenic miRNA and mRNA profiles from chickens after Salmonella challenge has identified differential expression of several miRNAs linked to immune responses, including miR-155, miR-9, miR-30 which have been reported previously and several miRNAs, such as miR-101-3p and miR-130b-3p, which were shown here to be associated with the immune response to infection with SE.
It is useful to predict miRNA function and construct regulation networks by the prediction of their targets and annotation of their biological function. Two immune-related KEGG pathways and one biological process were significantly enriched: Apoptosis, NOD-like receptor signaling pathway, and adaptive immune response. Interestingly, apoptosis pathway and regulation of inflammatory response were mainly enriched in the S vs. C comparison, while NOD-like receptor pathway and defense response to bacterium were enriched in the R vs. C comparison. These results indicated that miRNAs may play different regulatory roles associated with the extent of pathogen load in response to infection with SE, that is, between the susceptible and resistant birds.
Through the integration of miRNA and mRNA expression data and miRNA-RNA target prediction analysis, a number of putative miRNA-mRNA interactions were identified. Since hub nodes have been found to play important roles in many networks (He and Zhang, 2006), the presence of hub miRNAs was sought and, several were identified including gga-miR-155 and gga-miR-101-3p (Figure 3). It has been shown that miR-155 is involved in the TLRs signaling pathway and play important roles in the innate immune response (Quinn and O'Neill, 2011; Elton et al., 2013; Li and Shi, 2013). In contrast, gga-miR-101-3p has not been previously linked to Salmonella infection; the present finding in chicken spleen is novel.
The leucine-rich repeat (LRR) containing protein (LRRC) 59/p34 is a type II transmembrane protein with a short C-terminal domain facing the lumen of the endoplasmic reticulum (ER) and four LRRs and coiled-coil domain facing the cytosol. LRRC59 resides in the ER and nuclear membrane, and is reported to have the function of nuclear import of fibroblast growth factor (Skjerpen et al., 2002) and CIP2A (Pallai et al., 2015) at the nuclear membrane. Although, little is known about the function of LRRC59, it is becoming clear that this family of proteins, could have far-reaching effects on the immune response. A recent study showed that LRRC59 dependent trafficking of nucleic acid-sensing TLRs might be beneficial for augmentation of antimicrobial immune responses from the endoplasmic reticulum via association with Uncoordinated 93 homolog B1 (UNC93B1) (Tatematsu et al., 2015). MiR-155 has been reported to play important roles in both innate and adaptive immunity in mammals. Its expression is up-regulated after activation of the innate response in murine macrophages by lipopolysaccharide, CpG and poly (I:C) and it can down-regulate these signaling pathways by targeting key signaling molecules (Elton et al., 2013; Li and Shi, 2013; Olivieri et al., 2013; Maudet et al., 2014). In the current study, gga-miR-155 was significantly induced by SE infection, which was consistent with the above mammalian studies. Interestingly, the expression of gga-mir-155 was significantly higher in the S chickens compared with R birds. The expression of LRRC59 here was significantly down-regulated (P = 0.02) in S vs. R chickens. The in vitro experiment showed that gga-miR-155 directly repressed the expression of LRRC59; In addition miR-155 overexpression markedly decreased the expression of IL-6 and TNF-α compared with control miRNA or miR-155 inhibitor (P < 0.01). These results indicate that gga-mir-155 could target gene LRRC59 and then suppress the production of pro-inflammatory cytokines in response to LPS challenge.
Interferon regulatory factor 4 (IRF4) is a transcription factor of the IRF family that plays pivotal roles in the negative regulation of TLR signaling. Several previous studies have demonstrated that, in macrophages, IRF4 negatively regulates the production of pro-inflammatory cytokines such as IL-6 and TNF-α in response to TLR ligands (Honma et al., 2005; Negishi et al., 2005). IRF4 interacts with MyD88 and acts as a negative regulator of TLR signaling by competing with IRF5 (Negishi et al., 2005). It is well recognized that the innate immune response is critical to controlling the replication of pathogenic microorganisms, especially in young mammals and birds (Kawai and Akira, 2011; Keestra et al., 2013). In this study, the expression of IRF4 was significantly up-regulated in S compared to uninfected C birds (FC = 1.92, P = 0.03) and in S vs. R comparisons (FC = 2.62, P < 0.01). The expression of gga-miR-101-3p was significantly down-regulated in S vs. C (P < 0.01). In addition, gga-miR-101-3p directly inhibited IRF4 expression and miR-101-KO significantly decreased the expression of IL-6 and TNF-α compared with control miRNA inhibitor (P < 0.05).
Based on the foregoing observations and interpretations, it is reasonable to propose that gga-miR-155 and gga-miR-101-3p contribute to SE-induced pathogenesis and regulate the production of pro-inflammatory cytokines through directly down-regulating LRRC59 and up-regulating IRF4 genes, respectively.
In conclusion, this paper presents the first characterization of the splenic miRNA expression profile of the chicken in response to SE infection. A total of 32 DE miRNAs were identified among three phenotypic groups of chickens consisting of non-challenged controls, birds that were resistant to challenge with SE, and those that were susceptible to SE with heavy pathogen loads at 24 h after infection. Through integration analysis of DE miRNAs and DE mRNAs, a total of 273 miRNAs-targeted genes were identified. Immune-related Apoptosis and NOD-like receptor signaling pathway were found to be significantly enriched. Two miRNAs, gga-miR-155 and gga-miR-101-3p, could directly alter the expression of target IRF4 and LRRC59 and regulate the production of pro-inflammatory cytokines, respectively. These investigations indicate that miRNAs in spleen play a major role in the SE infection process. The findings will facilitate understanding resistance and susceptibility to Salmonella infection through miRNA-induced systems, provide guidance on potential vaccine targets, and may assist breeding for genetic resistance to SE in poultry.
Author Contributions
PL and WF performed experiments and data analysis and draft writing; QL and RL revised the manuscript and contributed to experiments and data analysis. JWa contributed to the animal study and data analysis. NE contributed to experimental design and revised the manuscript. JL, YZ, MZ, and HC contributed to animal experiments and data analysis and interpretation. GZ and JWe designed the experiments and supervised and coordinated the study. All authors reviewed the manuscript.
Funding
The research was supported by grants from National Natural Science Foundation of China (No. 31572393) and National Key Technology R&D Program (2015BAD03B03) and China Agricultural Science and Technology Innovation Project (ASTIP-IAS04) and the earmarked fund for modern agro-industry technology research system (CARS-42).
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Wang et al., 2017) in BIG Data Center Members (2017), Beijing Institute of Genomics (BIG), Chinese Academy of Sciences, under accession numbers CRA000315 that are publicly accessible at http://bigd.big.ac.cn/gsa.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank W. Bruce Currie, Emeritus Professor, Cornell University, for his contributions to presentation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fcimb.2017.00377/full#supplementary-material
References
Bao, H., Kommadath, A., Liang, G., Sun, X., Arantes, A. S., Tuggle, C. K., et al. (2015). Genome-wide whole blood microRNAome and transcriptome analyses reveal miRNA-mRNA regulated host response to foodborne pathogen Salmonella infection in swine. Sci. Rep. 5:12620. doi: 10.1038/srep12620
Bao, H., Kommadath, A., Plastow, G. S., Tuggle, C. K., Guan, L. L., and Stothard, P. (2014). MicroRNA Buffering and altered variance of gene expression in response to Salmonella infection. PLoS ONE 9:e94352. doi: 10.1371/journal.pone.0094352
Barrow, P. A., Jones, M. A., Smith, A. L., and Wigley, P. (2012). The long view: Salmonella - the last forty years. Avian Pathol. 41, 413–420. doi: 10.1080/03079457.2012.718071
BIG Data Center Members (2017). The BIG Data Center: from deposition to integration to translation. Nucleic Acids Res. 45, D18–D24. doi: 10.1093/nar/gkw1060
Calenge, F., and Beaumont, C. (2012). Toward integrative genomics study of genetic resistance to Salmonella and Campylobacter intestinal colonization in fowl. Front. Genet. 3:261. doi: 10.3389/fgene.2012.00261
Calenge, F., Kaiser, P., Vignal, A., and Beaumont, C. (2010). Genetic control of resistance to salmonellosis and to Salmonella carrier-state in fowl: a review. Genet. Sel. Evol. 42:11. doi: 10.1186/1297-9686-42-11
Das, K., Garnica, O., and Dhandayuthapani, S. (2016). Modulation of Host miRNAs by Intracellular Bacterial Pathogens. Front. Cell. Infect. Microbiol. 6:79. doi: 10.3389/fcimb.2016.00079
Deng, S. X., Cheng, A. C., Wang, M. S., and Cao, P. (2008). Serovar-specific real-time quantitative detection of Salmonella enteritidis in the gastrointestinal tract of ducks after oral challenge. Avian Dis. 52, 88–93. doi: 10.1637/8102-090107-Reg
DuPont, H. L., and Steele, J. H. (1987). The human health implication of the use of antimicrobial agents in animal feeds. Vet. Q. 9, 309–320. doi: 10.1080/01652176.1987.9694119
EFSA and ECDC. (European Food Safety Authority and European Centre for Disease Prevention and Control) (2016). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 14, 4634–4634. doi: 10.2903/j.efsa.2016.4634
Elton, T. S., Selemon, H., Elton, S. M., and Parinandi, N. L. (2013). Regulation of the MIR155 host gene in physiological and pathological processes. Gene 532, 1–12. doi: 10.1016/j.gene.2012.12.009
Eulalio, A., Schulte, L. N., and Vogel, J. (2012). The mammalian microRNA response to bacterial infections. RNA Biol. 9, 742–750. doi: 10.4161/rna.20018
Filipowicz, W., Bhattacharyya, S. N., and Sonenberg, N. (2008). Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114. doi: 10.1038/nrg2290
Goldman, E. (2004). Antibiotic abuse in animal agriculture: exacerbating drug resistance in human pathogens. Hum. Ecol. Risk Assess. 10, 121–134. doi: 10.1080/10807030490281016
Gou, Z., Liu, R., Zhao, G., Zheng, M., Li, P., Wang, H., et al. (2012). Epigenetic modification of TLRs in Leukocytes is associated with increased susceptibility to Salmonella enteritidis in chickens. PLoS ONE 7:e33627. doi: 10.1371/journal.pone.0033627
He, X., and Zhang, J. (2006). Why do hubs tend to be essential in protein networks? PLoS Genet. 2, 826–834. doi: 10.1371/journal.pgen.0020088
Honma, K., Udono, H., Kohno, T., Yamamoto, K., Ogawa, A., Takemori, T., et al. (2005). Interferon regulatory factor 4 negatively regulates the production of proinflammatory cytokines by macrophages in response to LPS. Proc. Natl. Acad. Sci. U.S.A. 102, 16001–16006. doi: 10.1073/pnas.0504226102
Huang, D. W., Sherman, B. T., and Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. doi: 10.1038/nprot.2008.211
Huang, H. Y., Liu, R. R., Zhao, G. P., Li, Q. H., Zheng, M. Q., Zhang, J. J., et al. (2015). Integrated analysis of microRNA and mRNA expression profiles in abdominal adipose tissues in chickens. Sci. Rep. 5, 16132–16132. doi: 10.1038/srep16132
Inns, T., Lane, C., Peters, T., Dallman, T., Chatt, C., McFarland, N., et al. (2015). A multi-country Salmonella enteritidis phage type 14b outbreak associated with eggs from a German producer: ‘near real-time’ application of whole genome sequencing and food chain investigations, United Kingdom, May to September 2014. Eurosurveillance 20, 15–22. doi: 10.2807/1560-7917.ES2015.20.16.21098
Jia, X., Nie, Q., Zhang, X., Nolan, L. K., and Lamont, S. J. (2017). Novel microRNA involved in host response to avian pathogenic Escherichia coli identified by deep sequencing and integration analysis. Infect. Immun. 85:e00688-16. doi: 10.1128/iai.00688-16
Kawai, T., and Akira, S. (2011). Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650. doi: 10.1016/j.immuni.2011.05.006
Keestra, A. M., de Zoete, M. R., Bouwman, L. I., Vaezirad, M. M., and van Putten, J. P. M. (2013). Unique features of chicken Toll-like receptors. Dev. Comp. Immunol. 41, 316–323. doi: 10.1016/j.dci.2013.04.009
Kozomara, A., and Griffiths-Jones, S. (2014). miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42, D68–D73. doi: 10.1093/nar/gkt1181
Krol, J., Loedige, I., and Filipowicz, W. (2010). The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 11, 597–610. doi: 10.1038/nrg2843
Kuang, X., Hao, H., Dai, M., Wang, Y., Ahmad, I., Liu, Z., et al. (2015). Serotypes and antimicrobial susceptibility of Salmonella spp. isolated from farm animals in China. Front. Microbiol. 6:602. doi: 10.3389/fmicb.2015.00602
Li, P., Xia, P., Wen, J., Zheng, M., Chen, J., Zhao, J., et al. (2010). Up-regulation of the MyD88-dependent pathway of TLR signaling in spleen and caecum of young chickens infected with Salmonella serovar Pullorum. Vet. Microbiol. 143, 346–351. doi: 10.1016/j.vetmic.2009.12.008
Li, X., Shahid, M. Q., Wu, J., Wang, L., Liu, X., and Lu, Y. (2016). Comparative small RNA analysis of pollen development in autotetraploid and diploid rice. Int. J. Mol. Sci. 17:499. doi: 10.3390/IJMS17040499
Li, Y., and Shi, X. (2013). MicroRNAs in the regulation of TLR and RIG-I pathways. Cell. Mol. Immunol. 10, 65–71. doi: 10.1038/cmi.2012.55
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Maudet, C., Mano, M., and Eulalio, A. (2014). MicroRNAs in the interaction between host and bacterial pathogens. FEBS Lett. 588, 4140–4147. doi: 10.1016/j.febslet.2014.08.002
Murie, C., Barette, C., Lafanechere, L., and Nadon, R. (2014). Control-Plate Regression (CPR) Normalization for high-throughput screens with many active features. J. Biomol. Screen. 19, 661–671. doi: 10.1177/1087057113516003
Negishi, H., Ohba, Y., Yanai, H., Takaoka, A., Honma, K., Yui, K., et al. (2005). Negative regulation of Toll-like-receptor signaling by IRF-4. Proc. Natl. Acad. Sci. U.S.A. 102, 15989–15994. doi: 10.1073/pnas.0508327102
Olivieri, F., Rippo, M. R., Prattichizzo, F., Babini, L., Graciotti, L., Recchioni, R., et al. (2013). Toll like receptor signaling in “inflammaging”: microRNA as new players. Immun. Ageing 10:11. doi: 10.1186/1742-4933-10-11
O'Neill, L. A., Sheedy, F. J., and McCoy, C. E. (2011). MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat. Rev. Immunol. 11, 163–175. doi: 10.1038/nri2957
Pallai, R., Bhaskar, A., Barnett-Bernodat, N., Gallo-Ebert, C., Pusey, M., Nickels, J. T. Jr., et al. (2015). Leucine-rich repeat-containing protein 59 mediates nuclear import of cancerous inhibitor of PP2A in prostate cancer cells. Tumor Biol. 36, 6383–6390. doi: 10.1007/s13277-015-3326-1
Quinn, S. R., and O'Neill, L. A. (2011). A trio of microRNAs that control Toll-like receptor signalling. Int. Immunol. 23, 421–425. doi: 10.1093/intimm/dxr034
Schulte, L. N., Eulalio, A., Mollenkopf, H.-J., Reinhardt, R., and Vogel, J. (2011). Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. EMBO J. 30, 1977–1989. doi: 10.1038/emboj.2011.94
Skjerpen, C. S., Wesche, J., and Olsnes, S. (2002). Identification of ribosome-binding protein p34 as an intracellular protein that binds acidic fibroblast growth factor. J. Biol. Chem. 277, 23864–23871. doi: 10.1074/jbc.M112193200
Smith, K. G., and Hunt, J. L. (2004). On the use of spleen mass as a measure of avian immune system strength. Oecologia 138, 28–31. doi: 10.1007/s00442-003-1409-y
Sonkoly, E., Stahle, M., and Pivarcsi, A. (2008). MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin. Cancer Biol. 18, 131–140. doi: 10.1016/j.semcancer.2008.01.005
Staedel, C., and Darfeuille, F. (2013). MicroRNAs and bacterial infection. Cell. Microbiol. 15, 1496–1507. doi: 10.1111/cmi.12159
Tatematsu, M., Funami, K., Ishii, N., Seya, T., Obuse, C., and Matsumoto, M. (2015). LRRC59 regulates trafficking of nucleic acid-sensing TLRs from the endoplasmic reticulum via association with UNC93B1. J. Immunol. 195, 4933–4942. doi: 10.4049/jimmunol.1501305
Tiron, A., and Vasilescu, C. (2008). Spleen and immunity. Immune implications of splenectomy. Chirurgia (Bucur) 103, 255–263.
Trapnell, C., Pachter, L., and Salzberg, S. L. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111. doi: 10.1093/bioinformatics/btp120
Trapnell, C., Williams, B. A., Pertea, G., Mortazavi, A., Kwan, G., van Baren, M. J., et al. (2010). Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, U511–U174. doi: 10.1038/nbt.1621
Wang, Y., Song, F., Zhu, J., Zhang, S., Yang, Y., Chen, T., et al. (2017). GSA: GENOME Sequence Archive. Genomics Proteomics Bioinform. 15, 14–18. doi: 10.1016/j.gpb.2017.01.001
Wu, G., Qi, Y., Liu, X., Yang, N., Xu, G., Liu, L., et al. (2017). Cecal microRNAome response to Salmonella enterica serovar enteritidis infection in white Leghorn Layer. BMC Genomics 18:77. doi: 10.1186/s12864-016-3413-8
Yao, M., Gao, W., Tao, H., Yang, J., Liu, G., and Huang, T. (2016a). Regulation signature of miR-143 and miR-26 in porcine Salmonella infection identified by binding site enrichment analysis. Mol. Genet. Genomics 291, 789–799. doi: 10.1007/s00438-015-1146-z
Yao, M., Gao, W., Yang, J., Liang, X., Luo, J., and Huang, T. (2016b). The regulation roles of miR-125b, miR-221 and miR-27b in porcine Salmonella infection signalling pathway. Biosci. Rep. 36:e00375. doi: 10.1042/bsr20160243
Yates, L. A., Norbury, C. J., and Gilbert, R. J. C. (2013). The long and short of MicroRNA. Cell 153, 516–519. doi: 10.1016/j.cell.2013.04.003
Keywords: MicroRNA, Salmonella enteritidis, next generation sequencing, chicken, clinical symptoms, spleen, miRNA-target genes
Citation: Li P, Fan W, Li Q, Wang J, Liu R, Everaert N, Liu J, Zhang Y, Zheng M, Cui H, Zhao G and Wen J (2017) Splenic microRNA Expression Profiles and Integration Analyses Involved in Host Responses to Salmonella enteritidis Infection in Chickens. Front. Cell. Infect. Microbiol. 7:377. doi: 10.3389/fcimb.2017.00377
Received: 16 April 2017; Accepted: 08 August 2017;
Published: 24 August 2017.
Edited by:
Brian Ahmer, The Ohio State University Columbus, United StatesReviewed by:
César López-Camarillo, Universidad Autónoma de la Ciudad de México, MexicoRiti Sharan, Texas A&M, Health Science Center, United States
Copyright © 2017 Li, Fan, Li, Wang, Liu, Everaert, Liu, Zhang, Zheng, Cui, Zhao and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guiping Zhao, zhaoguiping@caas.cn
Jie Wen, wenjie@iascaas.net.cn
 Peng Li
Peng Li Wenlei Fan1
Wenlei Fan1  Nadia Everaert
Nadia Everaert