- 1Department of Internal Medicine, Section of Gerontology and Geriatrics, Leiden University Medical Center, Leiden, Netherlands
- 2Leyden Academy on Vitality and Ageing, Leiden, Netherlands
- 3Department of Medical Statistics and Bioinformatics, Section Molecular Epidemiology, Leiden University Medical Center, Leiden, Netherlands
- 4Institute for Evidence-Based Medicine in Old Age, IEMO, Leiden, Netherlands
Background: Elevated concentrations of liver enzymes have been associated with an increased risk of developing type 2 diabetes mellitus. However, it remains unclear to which specific aspects of diurnal glucose metabolism these associate most. We aimed to investigate the associations between liver enzyme concentrations and 24 h-glucose trajectories in individuals without diabetes mellitus from three independent cohorts.
Methods: This cross-sectional study included 436 participants without diabetes mellitus from the Active and Healthy Aging Study, the Switchbox Study, and the Growing Old Together Study. Fasting blood samples were drawn to measure gamma-glutamyltransferase (GGT), alanine transaminase, and aspartate transaminase. Measures of glycemia (e.g., nocturnal and diurnal mean glucose levels) and glycemic variability (e.g., mean amplitude of glucose excursions) were derived from continuous glucose monitoring. Analyses were performed separately for the three cohorts; derived estimates were additionally meta-analyzed.
Results: After meta-analyses of the three cohorts, elevated liver enzyme concentrations, and specifically elevated GGT concentrations, were associated with higher glycemia. More specific, participants in the highest GGT tertile (GGT ≥37.9 U/L) had a 0.39 mmol/L (95% confidence interval: 0.23, 0.56) higher mean nocturnal glucose (3:00 to 6:00 a.m.) and a 0.23 mmol/L (0.10, 0.36) higher diurnal glucose (6:00 to 0:00 a.m.) than participants in the lowest GGT tertile (GGT <21.23 U/L). However, elevated liver enzyme concentrations were not associated with a higher glycemic variability.
Conclusion: Though elevated liver enzyme concentrations did not associate with higher glycemic variability in participants without diabetes mellitus, specifically, elevated GGT concentrations associated with higher glycemia.
Introduction
It has been well recognized that the prevalence of type 2 diabetes mellitus (T2DM) is increasing worldwide (1, 2). In literature, studies have focused on the identification of risk factors associated with a higher risk of developing T2DM, in order to identify potential targets for (therapeutic) interventions and to understand the different pathophysiological mechanisms. Several risk factors have been identified for T2DM, which include both inherited factors (e.g., genetic factors) (3) and modifiable risk factors such as adiposity and high caloric intake (4). In addition, disease conditions as non-alcoholic fatty liver disease (NAFLD) or a preclinical higher degree of liver adiposity have also been described to increase the risk of T2DM (5–7). Both NAFLD and increased liver adiposity are reflected by elevated blood concentrations of liver enzymes [notably alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyltransferase (GGT)]. Elevations of blood concentrations of liver enzymes have been repeatedly associated with an increased risk of developing T2DM in multiple settings (8–11). Nevertheless, there are still limited data available on the association between elevated liver enzyme concentrations and the dynamic aspects of glucose metabolism over 24 h, like glycemia and glycemic variability. Such insights will provide additional information about the pathophysiological mechanisms in which elevated liver enzyme concentrations are involved.
Measures of glycemia and glycemic variability, as derived from 24 h glucose trajectories, can be obtained with continuous glucose monitoring (CGM), which uses a minimally invasive device to measures glucose concentrations for a period up to 7 days, while the participants pursue their regular daily life activities (12–14). Using this device, high concentrations of serum ALT have previously been associated with higher glycemia (specifically glycemia during the nocturnal period of the 24 h period) in 322 Chinese individuals without diabetes mellitus (13). However, this study did not investigate the other liver enzymes AST and GGT, nor did it study glycemic variability, and generalization to Western populations has yet to be determined.
To provide further insights in the association between liver enzyme concentrations and 24 h glucose trajectories, we examined the associations between liver enzyme (ALT, AST, and GGT) and CGM-derived measures of glycemia and glycemic variability over 3 days in three independent populations of middle-aged individuals without diabetes mellitus.
Materials and Methods
Study Settings
The present study was conducted using data of the Active and Healthy Aging (AGO) Study, the Switchbox Study, and the Growing Old Together Study (GOTO). The designs and recruitment strategies of the three studies have been described in more detail elsewhere (15–18). The AGO, Switchbox, and GOTO studies have been approved by the medical ethics committee of the Leiden University Medical Center, Leiden, the Netherlands. Written informed consent was obtained from all study participants.
The Active and Healthy Aging (“Actief en Gezond Oud”; AGO)
The AGO study aimed to investigate the effect of a web-based lifestyle intervention program, with the intention to increase physical activity, on metabolic health. For this study, individuals aged 60–70 years living in the city of Leiden, the Netherlands, were recruited. Individuals with a history of diabetes mellitus, an active lifestyle (more than 3 h of physical exercise or cycling per week), or a contraindication to increase physical activity were not included. In total, 243 individuals were enrolled and were randomized for either the intervention program or the control arm of the AGO study. The AGO study was registered at the Dutch Trial Register (http://www.trialregister.nl) as NTR3045.
The Switchbox Study
The Switchbox Study aimed to investigate the biological mechanisms underlying familial longevity. Individuals were enrolled from the ongoing, and larger, Leiden Longevity Study (19). Participants were eligible when their age was between 55 and 77 years and they had a stable body mass index (BMI) between 19 and 33 kg/m2. Participants were not eligible for participation in the Switchbox study if they had a fasting glucose above 7 mmol/L, if they had significant chronic, renal, hepatic, or endocrine disease, or if they used any medication known to influence lipolysis, thyroid function, glucose metabolism, GH/IGF-1 secretion, or any other hormonal axis. Moreover, participants were excluded if they had a recent trans meridian flight, smoking addition, use of more than 20 U of alcohol, and extreme diet therapies. In total, 135 individuals were enrolled in the Switchbox study.
The Growing Old Together (GOTO) Study
The GOTO study aimed to investigate the effect of a combined physical activity and diet intervention on metabolic and metabolomic phenotypes. Similar to the Switchbox Study, participants were enrolled from the Leiden Longevity Study (19). Individuals of ages between 46 and 75 years and with a BMI between 23 and 35 kg/m2 were eligible to participate. Exclusion criteria were: treated for diabetes mellitus, a fasting glucose level above 7 mmol/L, a weight change of more than 3 kg during the last 6 months, engagement in heavy/intensive physical activity (top sport of physically heavy work), any disease or condition that seriously affects body weight (e.g., cancer, heart failure, COPD), recent immobilization for >1 week, psychiatric or behavioral problems, use of thyroid mediation or immunosuppressive drugs, concurrent participation in any other intervention study or weight management program, or not having a general practitioner. In total, 163 individuals were enrolled in the GOTO study. The GOTO study was registered at the Dutch Trial Register (http://www.trialregister.nl) as NTR3499.
Study Design and Population
The present study was conducted in a cross-sectional setting in participants without T2DM. As AGO and GOTO were both intervention studies, we used only the baseline data (prior to the intervention) for the present study. In AGO, we identified two participants with a potential newly diagnosed T2DM and were, therefore, excluded from the present study. After exclusion of participants with missing data on either CGM or liver enzyme concentrations (N = 15 in AGO; N = 19 in Switchbox), and excluding participants in GOTO who already participated in the Switchbox study, our total study population contained 436 participants with complete data (N = 226 from AGO, N = 116 from Switchbox, N = 94 from GOTO).
Biochemical Analyses
After an overnight fast, blood samples were drawn from all study participants. All measurements were performed with fully automated equipment from Roche Diagnostics (Almere, the Netherlands). ALT, AST, and GGT levels were determined using the Abbott ci8200. ALT and AST were measured using the NADH (with P-5′-P) methodology and GGT was measured using the substrate l-Gamma-Glutamyl-3-carboxy-4-nitroanilide methodology. All measurements were performed at the Department of Clinical Chemistry and Laboratory Medicine, Leiden University Medical Center, Leiden, the Netherlands.
Anthropometrics
Weight and height were measured at the study center by research nurses. BMI was calculated by dividing the weight (in kilograms) by height (in meters) squared. Waist-to-hip ratio was calculated by dividing waist circumference by hip circumference, which were measured at the study center. Percentage of body fat was determined according to a mobile Bioelectrical Impedance Analysis system (Bodystat® 1500 Ltd., Isle of Man, British Isle).
Glucose Measurements
In the three studies, CGM was performed with the Mini-Med® CGM system (Medtronic Minimed Inc., Northridge, CA, USA). For five consecutive days, interstitial glucose levels were monitored with a glucose sensor (Sof-Sensor®, Medtronic, Minimed Inc., Northridge, CA, USA) inserted into the subcutaneous abdominal fat tissue. For calibration of the sensor, participants measured their capillary blood glucose four times a day by means of a finger prick. The participants were supported to continue their normal daily activities. Moreover, they were asked to register their food intake, medication, and physical exercise during the study. In line with the guidelines from the manufacturer, the first and fifth day of the measurement were excluded, as these were considered least accurate, leaving 3 days (covering 72 h) of data for the present study.
On the basis of the retrieved glucose trajectories, we calculated multiple measures of glycemia and glycemic variability for each participant separately. As measures of glycemia, we calculated the 72-h mean glucose concentration, the mean diurnal glucose concentration (6:00 to 0:00 a.m.), and the mean nocturnal glucose level (3:00 to 6:00 a.m.; a period when all participants were considered to be sleeping). As measures of glycemic variability, we calculated the 72-h SD mean amplitude of glucose excursion (MAGE), and the mean of daily difference (MODD). The MAGE determines intraday glycemic variability (20), whereas the MODD determines interday variability (21). These calculations for glycemia and glycemic variability have been validated in non-diabetic individuals before (14), and have already been used in previous studies (12, 22).
Statistical Analyses
Characteristics of the study populations were presented separately for the AGO, Switchbox, and GOTO study populations as mean (SD), number of cases (percentage), or median (interquartile range; non-normally distributed data only).
We divided the three study populations in three groups based on their blood liver enzyme concentration, because it has previously been shown that the association between elevated GGT and T2DM was not linear (10). Mean liver enzyme concentrations as well as the variation in liver enzyme concentrations between the three cohorts were observed to be considerably different. To obtain tertiles that were comparable between the cohorts in terms of mean liver enzyme concentrations, we divided the participants from AGO (as being the largest cohort contributing to the present study) in tertiles based on their blood liver enzyme concentrations and applied the boundaries of these strata to the other two study populations. By using the same boundaries for all groups, it was possible to compare and to subsequently meta-analyze the results of the three study populations.
All statistical analyses assessing the associations between the blood liver enzyme concentrations and CGM-derived measures of glycemia and glycemic variability were conducted using multivariable linear regression analyses with the strata of the liver enzyme concentrations as independent variables and the measures of glycemia and glycemic variability as dependent variables. Analyses were done separately for the AGO, Switchbox, and GOTO study populations. For the analyses, we considered participants in the group with the lowest blood liver enzyme concentration as the reference group. Analyses were adjusted for age, sex, and BMI using STATA v12.0 (StataCorp LP, College Station, TX, USA). In sensitivity analyses, we adjusted the analyses on the measures of glycemic variability for the 72-h mean glucose concentration. Results of the study populations were combined using a fixed effect inverse-variance weighted meta-analysis as implemented in the rmeta package for the R statistical environment (23). All results were presented as mean difference with respect to the reference group and with a 95% confidence interval (CI). A two-sided p-value < 0.05 was considered statistically significant.
Results
Characteristics of the Three Study Populations
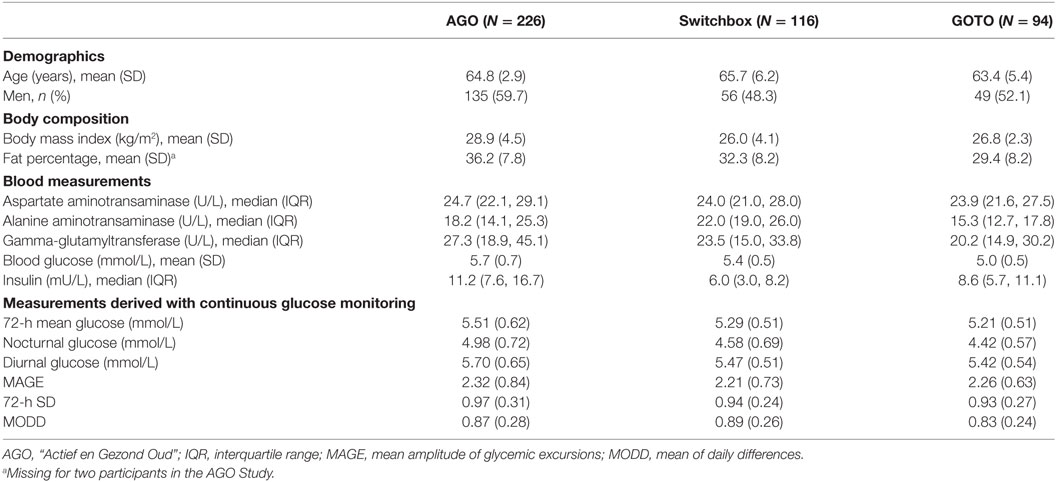
For the present study, we included 226 participants from the AGO study, 116 participants from the Switchbox study, and 94 participants from the GOTO study (Table 1). The three independent cohorts had a similar mean age (±65 years). Specifically participants from the AGO study had a higher percentage of men (59.7 versus 48.3 and 52.1%), had a higher mean BMI (28.9 versus 26.0 and 26.8 kg/m2), and had a higher percentage of body fat (36.2 versus 32.3 and 29.4%) compared with the Switchbox and GOTO studies. In addition, participants from AGO had a higher mean GGT (27.3 versus 23.5 and 20.2 U/L), a higher mean fasting glucose (5.7 versus 5.4 and 5.0 mmol/L), and a higher median fasting insulin (11.2 versus 6.0 and 8.6 mU/L) compared with the Switchbox and GOTO studies.
Associations between Liver Enzyme Concentrations and CGM-Derived Measures of Glycemia
A graphical visualization of the mean glucose trajectories over 72 h of participants in the group of the lowest and highest GGT concentration is presented in Figure 1.
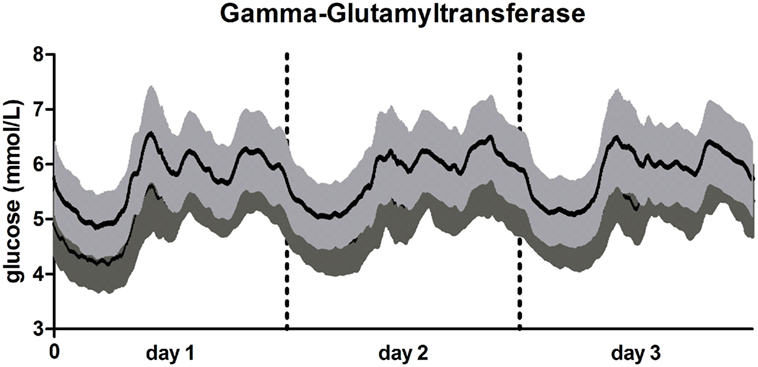
Figure 1. 72-h glucose trajectories in participants with low and high gamma-glutamyltransferase (GGT). Line in light gray: participants with high GGT (≥37.9 U/L; N = 111). Line in dark gray: participants with low high GGT (<21.23 U/L; N = 179). Data presented as mean glucose concentration with SE for every 5 min over a 72 h period.
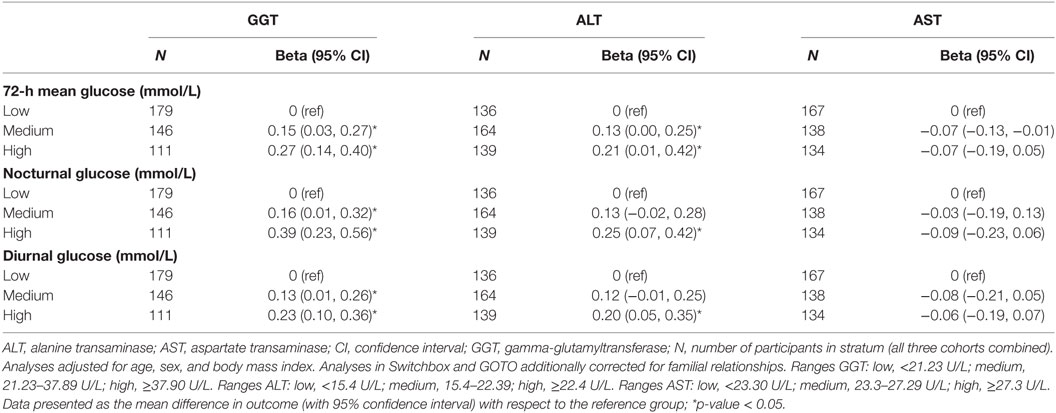
After meta-analyzing the results of the three studies (Table 2), participants allocated to the group with the highest GGT concentration (GGT ≥37.90 U/L) had a 0.27 mmol/L (95% CI: 0.14, 0.40) higher 72-h mean glucose concentration, a 0.39 mmol/L (95% CI: 0.23, 0.56) higher mean nocturnal glucose concentration, and a 0.23 mmol/L (95% CI: 0.10, 0.36) higher diurnal glucose concentration, compared with participants allocated to the group with the lowest GGT blood concentration (GGT < 21.23 U/L). Similar results were observed in the three individual studies (Table S1 in Supplementary Material), although sometimes with large confidence intervals due to the small number of participants in some of the subgroups.
Similar results, although with somewhat smaller effect sizes than with GGT (especially for the association with nocturnal glucose), were observed with ALT (Table 2). For example, participants allocated to the group with the highest ALT blood concentrations (ALT ≥22.40 U/L) had a 0.21 (95% CI: 0.07, 0.20) mmol/L higher 72-h mean glucose concentration than participants in the group with the lowest ALT blood concentrations (ALT <15.40 U/L). Again, these observations were similarly observed in the three study populations separately (Table S2 in Supplementary Material). We found no evidence, also not after meta-analyses of the three study populations, that high AST blood concentrations were associated with any of the investigated CGM-derived measures of glycemia (Table 2) neither in one of the three individual cohorts (Table S3 in Supplementary Material).
Associations between Liver Enzyme Concentrations and CGM-Derived Measures of Glycemic Variability
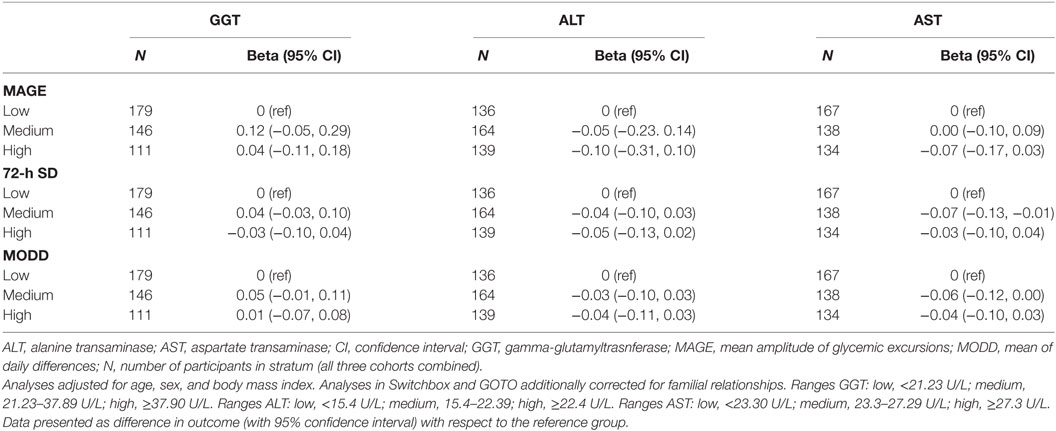
After meta-analyses of the results of the three study populations (Table 3), we found no evidence that participants in the group with the highest GGT blood concentration (GGT ≥ 37.90 U/L) had, compared to participants in the group with the lowest GGT blood concentration (GGT < 21.23 U/L), a higher MAGE (difference: 0.04; 95% CI: −0.11, 0.18), a higher 72-h SD (difference: −0.03; 95% CI: −0.13, 0.02), or a higher MODD (difference: 0.01; 95% CI: −0.07, 0.08). Similar results were obtained for ALT and AST (Table 3), as well as for the three individual studies (Tables S4–S6 in Supplementary Material). The results did not materially change when we additionally adjusted for 72-h mean glucose concentration (results not shown).
Discussion
Within the present study, we aimed to investigate the associations between liver enzyme concentrations and measures of glycemia and glycemic variability in a cross-sectional study comprising middle-aged individuals without T2DM. The results of the present study provided evidence that, using data from three independent studies, elevated liver enzyme concentrations, and specifically elevated GGT concentrations, were associated with higher glycemia during day and night. However, the present study did not provide evidence that elevated liver enzyme concentrations were associated with higher glycemic variability.
The results of the associations between elevated liver enzyme concentrations and increased CGM-derived measures of glycemia are in line with previous studies on the association between elevated liver enzyme concentrations and fasting glucose concentrations (24–27). Our study showed strong associations between elevated GGT concentrations and measures of glycemia. In line, there is strong consensus that elevated blood GGT concentrations are associated with an increased risk to develop T2DM (10). The results of the present study indicate that the previously observed association between GGT and elevated fasting blood glucose concentrations persists over the day, which was reflected in our analyses on the mean nocturnal, as well as on the mean diurnal glucose concentrations. This might indicate that the biological mechanism behind the association between elevated liver enzyme concentrations and measures of glycemia involves a decreased glucose disposal.
The three investigated liver enzyme concentrations have been shown to be well correlated with each other in previous studies (27). However, the present study also found associations between elevated ALT concentrations and measures of glycemia, but no associations were found between elevated AST concentrations and measures of glycemia. Nevertheless, the associations for AST were generally less strong than with GGT although the results with ALT also reached the level of statistical significance. In line, there are a number of publications that showed that elevated ALT concentrations are associated with increased fasting blood glucose concentrations (13, 26–28). For example, in a Chinese-ancestry population, an elevated blood ALT concentration was associated with an increased fasting blood glucose concentration, but this effect was only observed in women (26). The only previous study using CGM-derived measures of glycemia observed associations between ALT and mean nocturnal and diurnal glucose concentrations (13). Nevertheless, a study conducted in participants of Taiwanese ancestry observed an association between ALT and metabolic syndrome, but not with elevated fasting glucose concentrations and T2DM (29). Together with our results, this supports that the investigated liver enzymes reflect different aspects of liver function. This indicates that blood ALT and AST concentrations are less related to glucose metabolism than blood GGT concentrations.
The present study is the first to examine the associations between liver enzyme concentrations and measures of glycemic variability. A high glycemic variability is a generally known risk factor for micro- and macrovascular complications in patients diagnosed with T2DM (30). However, we found that elevated liver enzyme concentrations were not associated with a higher glycemic variability. The lack of an association with glycemic variability has previously also been observed for genetic variation in the TCF7L2 gene (31), one of the strongest genetic risk factors for T2DM. This might indicate that glycemia rather than glycemic variability is the most important risk factor for the development of T2DM. Therefore, the importance (if any) of glycemic variability in populations of individuals without diabetes mellitus needs to be further elucidated.
The results of the present study suggest that increased liver enzyme concentrations are associated with decreased glucose disposal. In the literature, there is currently debate on whether the association between GGT and increased glycemia and T2DM are causal (8, 32, 33). The observations done in the present study could reflect a common cause, rather than a causal relation. Several mechanisms have been proposed to underpin the observed associations between GGT and risk of T2DM (34, 35). As a potential mechanism explaining the results of the present study, we hypothesize that liver fat content might be the common cause of elevated blood GGT concentrations and elevated levels of glycemia. Liver fat content was shown to correlate with blood GGT concentrations (36), as well as to associate with several established risk factors for T2DM, including obesity, oxidative stress, and inflammation. However, additional studies are required to further elucidate on this hypothesis.
The present study has a number strengths and limitations. The main strength of the present study is that data on glucose levels were collected every 5 min over a 72-h period during which participants were asked to continue their normal daily life activities. This provided the opportunity for a detailed analysis of nocturnal and diurnal patterns, but also of the variation in measurements. Furthermore, the present study was conducted using a large sample size in which CGM data have been collected. Also, the present study was conducted using data collected from three independent study populations with different inclusion criteria and population characteristics. Notably, the AGO study population was recruited with the intention to improve lifestyle (16), and using different in- and exclusion criteria, participants from Switchbox and GOTO were enrolled from the Leiden Longevity Study, which included participants based on their propensity to become long-lived together with their partners as controls (15). As the results were similar in the three study populations, this emphasizes the robustness of our findings across populations with different characteristics. Nevertheless, because of the observational nature of the study, no causality was ascertained and results might have been harmed by residual confounding and/or reverse causality. However, since observations were similar in three distinct populations with different study characteristics, the effect of residual confounding in the analyses was considered minimal.
In summary, the results of the present study from individuals without diabetes mellitus indicate that elevated liver enzyme concentrations, specifically elevated GGT blood concentrations, are associated with increased glycemia, but not with increased glycemic variability.
Ethics Statement
The three cohorts described in the present study have been approved by the medical ethics committee of the Leiden University Medical Center, Leiden, the Netherlands.
Author Contributions
Study design (RN, DV, HD, PS, SM, DH); acquisition of data (CW, AA, SK, NH, SJ, BS, MB); data analyses (RN, DV, HD); drafted the manuscript (RN, DV, HD, DH); critically commented and final approval of the manuscript (RN, DV, HD, CW, AA, SK, NH, SJ, BS, MB, PS, SM, DH).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, LB, and the handling editor declared their shared affiliation.
Acknowledgments
The AGO Study thanks M. van der Elst, R. du Puy, L. ten Brinke, M. Kersbergen, and M. van Schie-Troost for their valuable contributions. The Switchbox study thanks the secretarial staff (M. van der Star and E. Bemer-Oorschot), the research nurse (R. de Wilde), the research assistant (B. Ladan), database manager (S. Henquet), and laboratory personnel (J. Verhagen, G. van Steen, S. Buitendijk, M. van Schie-Troost) for their valuable contributions. The GOTO study thanks all staff members and Bachelor’s and Master’s students who contributed to the preparation, design, and performance of the GOTO intervention trial and/or assisted on the project. Our greatest gratitude goes to all participants who did their very best to adhere to the intervention guidelines and underwent all measurements.
Funding
The AGO study was financially supported by Philips Consumer Lifestyle, and the Netherlands Genomics Initiative/Netherlands Organization for scientific research (NGI/NWO; 05040202 and 050-060-810). The Switchbox Study was funded by the European Commission project Switchbox (FP7, Health-F2-2010-259772). The GOTO study was financially supported by the Netherlands Consortium for Healthy Ageing (grant 050-060-810), in the framework of the Netherlands Genomics Initiative, Netherlands Organization for Scientific Research (NWO). The present study was funded by the European Commission funded project HUMAN (Health-2013-INNOVATION-1-602757). All the funders had no role in the design and performance of the study, nor in the analyses or interpretation of the data, or in the drafting of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fendo.2017.00236/full#supplementary-material.
References
1. International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation (2015).
2. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care (2004) 27(5):1047–53. doi:10.2337/diacare.27.10.2569-a
3. Mahajan A, Go MJ, Zhang W, Below JE, Gaulton KJ, Ferreira T, et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet (2014) 46(3):234–44. doi:10.1038/ng.2897
4. Bi Y, Wang T, Xu M, Xu Y, Li M, Lu J, et al. Advanced research on risk factors of type 2 diabetes. Diabetes Metab Res Rev (2012) 28(Suppl 2):32–9. doi:10.1002/dmrr.2352
5. Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab (2003) 285(4):E906–16. doi:10.1152/ajpendo.00117.2003
6. Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia (2008) 51(11):1947–53. doi:10.1007/s00125-008-1135-4
7. Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med (2004) 164(19):2169–75. doi:10.1001/archinte.164.19.2169
8. Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care (2009) 32(4):741–50. doi:10.2337/dc08-1870
9. Sala M, Kroft LJ, Roell B, van der Grond J, Slagboom PE, Mooijaart SP, et al. Association of liver enzymes and computed tomography markers of liver steatosis with familial longevity. PLoS One (2014) 9(3):e91085. doi:10.1371/journal.pone.0091085
10. Kunutsor SK, Abbasi A, Adler AI. Gamma-glutamyl transferase and risk of type II diabetes: an updated systematic review and dose-response meta-analysis. Ann Epidemiol (2014) 24(11):809–16. doi:10.1016/j.annepidem.2014.09.001
11. Andre P, Balkau B, Born C, Royer B, Wilpart E, Charles MA, et al. Hepatic markers and development of type 2 diabetes in middle aged men and women: a three-year follow-up study. The D.E.S.I.R. Study (Data from an Epidemiological Study on the Insulin Resistance syndrome). Diabetes Metab (2005) 31(6):542–50. doi:10.1016/S1262-3636(07)70229-X
12. Wijsman CA, van Heemst D, Hoogeveen ES, Slagboom PE, Maier AB, de Craen AJ, et al. Ambulant 24-h glucose rhythms mark calendar and biological age in apparently healthy individuals. Aging Cell (2013) 12(2):207–13. doi:10.1111/acel.12042
13. Zhou J, Mo Y, Li H, Ran X, Yang W, Li Q, et al. Alanine aminotransferase is associated with an adverse nocturnal blood glucose profile in individuals with normal glucose regulation. PLoS One (2013) 8(2):e56072. doi:10.1371/journal.pone.0056072
14. Akintola AA, Noordam R, Jansen SW, de Craen AJ, Ballieux BE, Cobbaert CM, et al. Accuracy of continuous glucose monitoring measurements in normo-glycemic individuals. PLoS One (2015) 10(10):e0139973. doi:10.1371/journal.pone.0139973
15. Schoenmaker M, de Craen AJ, de Meijer PH, Beekman M, Blauw GJ, Slagboom PE, et al. Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. Eur J Hum Genet (2006) 14(1):79–84. doi:10.1038/sj.ejhg.5201508
16. Wijsman CA, Westendorp RG, Verhagen EA, Catt M, Slagboom PE, de Craen AJ, et al. Effects of a web-based intervention on physical activity and metabolism in older adults: randomized controlled trial. J Med Internet Res (2013) 15(11):e233. doi:10.2196/jmir.2843
17. van de Rest O, Schutte BA, Deelen J, Stassen SA, van den Akker EB, van Heemst D, et al. Metabolic effects of a 13-weeks lifestyle intervention in older adults: the Growing Old Together Study. Aging (2016) 8(1):111–26. doi:10.18632/aging.100877
18. Jansen SW, Akintola AA, Roelfsema F, van der Spoel E, Cobbaert CM, Ballieux BE, et al. Human longevity is characterised by high thyroid stimulating hormone secretion without altered energy metabolism. Sci Rep (2015) 5:11525. doi:10.1038/srep11525
19. Westendorp RG, van Heemst D, Rozing MP, Frolich M, Mooijaart SP, Blauw GJ, et al. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study. J Am Geriatr Soc (2009) 57(9):1634–7. doi:10.1111/j.1532-5415.2009.02381.x
20. Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes (1970) 19(9):644–55. doi:10.2337/diab.19.9.644
21. Molnar GD, Taylor WF, Ho MM. Day-to-day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia (1972) 8(5):342–8. doi:10.1007/BF01218495
22. Mastrototaro JJ. The MiniMed continuous glucose monitoring system. Diabetes Technol Ther (2000) 2(Suppl 1):S13–8. doi:10.1089/15209150050214078
23. Sonksen PH, Judd SL, Lowy C. Home monitoring of blood-glucose. Method for improving diabetic control. Lancet (1978) 1(8067):729–32.
24. Akehi Y, Tsutsumi Y, Tatsumoto A, Yoshida R, Ohkubo K, Takenoshita H, et al. Serum gamma-glutamyltransferase, triglyceride and total cholesterol are possible prediabetic risk markers in young Japanese men. Endocr J (2010) 57(11):981–9. doi:10.1507/endocrj.K10E-174
25. Liang J, Gong Y, Wang Y, Qiu Q, Zou C, Dou L, et al. Serum gamma-glutamyltransferase is associated with impaired fasting glucose in Chinese adults: the Cardiometabolic Risk in Chinese (CRC) study. Cell Biochem Biophys (2014) 70(3):1823–8. doi:10.1007/s12013-014-0136-9
26. Xie JH, Liu Q, Yang Y, Liu ZL, Hu SH, Zhou XR, et al. Correlation of liver enzymes with diabetes and pre-diabetes in middle-aged rural population in China. J Huazhong Univ Sci Technolog Med Sci (2016) 36(1):53–8. doi:10.1007/s11596-016-1541-7
27. Succurro E, Arturi F, Grembiale A, Iorio F, Fiorentino TV, Andreozzi F, et al. One-hour post-load plasma glucose levels are associated with elevated liver enzymes. Nutr Metab Cardiovasc Dis (2011) 21(9):713–8. doi:10.1016/j.numecd.2011.02.002
28. Fraser A, Ebrahim S, Smith GD, Lawlor DA. A comparison of associations of alanine aminotransferase and gamma-glutamyltransferase with fasting glucose, fasting insulin, and glycated hemoglobin in women with and without diabetes. Hepatology (2007) 46(1):158–65. doi:10.1002/hep.21667
29. Yueh CY, Chen JH, Lee LW, Lu CW, Parekh B, Chi CC. Elevated alanine aminotransferase is associated with metabolic syndrome but not consistently associated with impaired fasting glucose or type 2 diabetes mellitus. Diabetes Res Clin Pract (2011) 94(1):64–70. doi:10.1016/j.diabres.2011.05.038
30. Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab (2010) 12(4):288–98. doi:10.1111/j.1463-1326.2009.01160.x
31. van der Kroef S, Noordam R, Deelen J, Akintola AA, Jansen SW, Postmus I, et al. Association between the rs7903146 polymorphism in the TCF7L2 gene and parameters derived with continuous glucose monitoring in individuals without diabetes. PLoS One (2016) 11(2):e0149992. doi:10.1371/journal.pone.0149992
32. Lee YS, Cho Y, Burgess S, Davey Smith G, Relton CL, Shin SY, et al. Serum gamma-glutamyl transferase and risk of type 2 diabetes in the general Korean population: a Mendelian randomization study. Hum Mol Genet (2016) 25(17):3877–86. doi:10.1093/hmg/ddw226
33. Noordam R, Smit RA, Postmus I, Trompet S, van Heemst D. Assessment of causality between serum gamma-glutamyltransferase and type 2 diabetes mellitus using publicly available data: a Mendelian randomization study. Int J Epidemiol (2016) 45(6):1953–60. doi:10.1093/ije/dyw306
34. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol (2004) 24(5):816–23. doi:10.1161/01.ATV.0000122852.22604.78
35. Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord (2003) 27(Suppl 3):S53–5. doi:10.1038/sj.ijo.0802502
Keywords: continuous glucose monitoring, liver enzymes, glycemia, glycemic variability, cross-sectional cohort study
Citation: Noordam R, Vermond D, Drenth H, Wijman CA, Akintola AA, van der Kroef S, Jansen SWM, Huurman NC, Schutte BAM, Beekman M, Slagboom PE, Mooijaart SP and van Heemst D (2017) High Liver Enzyme Concentrations are Associated with Higher Glycemia, but not with Glycemic Variability, in Individuals without Diabetes Mellitus. Front. Endocrinol. 8:236. doi: 10.3389/fendo.2017.00236
Received: 20 June 2017; Accepted: 28 August 2017;
Published: 13 September 2017
Edited by:
Jan Polák, Charles University, CzechiaReviewed by:
Ludmila Brunerova, Charles University, CzechiaDubravka Jurišić Eržen, Clinical Hospital Centre Rijeka, Croatia
Copyright: © 2017 Noordam, Vermond, Drenth, Wijman, Akintola, van der Kroef, Jansen, Huurman, Schutte, Beekman, Slagboom, Mooijaart and van Heemst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raymond Noordam, r.noordam@lumc.nl
†These authors have contributed equally to this work.
 Raymond Noordam
Raymond Noordam Debbie Vermond
Debbie Vermond Hermijntje Drenth1,2†
Hermijntje Drenth1,2† Abimbola A. Akintola
Abimbola A. Akintola Marian Beekman
Marian Beekman Diana van Heemst
Diana van Heemst