- 1Department of Endocrinology and Metabolism, The First Hospital of China Medical University, Shenyang, China
- 2Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center, Boston, MA, United States
Objectives: To examine the prevalence of genetic alterations of thyroid-stimulating hormone receptor (TSHR) gene and sodium-iodine symporter (NIS) in a series of thyroid fine needle biopsy (FNB) specimens with indeterminate cytology, and to assess the correlation of the type of genetic changes with clinical features and follow-up results in the target thyroid nodule.
Methods: Between February 2015 and September 2017, 388 consecutive FNBs with indeterminate cytology were evaluated for TSHR mutations and NIS gene overexpression using ThyroSeqV.2 next-generation sequencing (NGS) panel. Medical records were reviewed for target nodules.
Results: Among 388 indeterminate FNBs, TSHR mutations and/or NIS overexpression were detected in 25 (6.4%) nodules. Ten nodules (2.6%) harbored TSHR mutations only, 7 nodules (1.8%) over-expressed NIS gene only, and 8 nodules (2.1%) had both alterations. The TSHR mutations were located between codons 281 and 640, with codon 453 being the most frequently affected. The allelic frequency of the mutated TSHR ranged from 6 to 36%. One nodule with NIS overexpression was simultaneously detected EIF1AX mutation and GNAS mutation. Nodules with TSHR mutations and/or NIS overexpression presented hyperfunctioning (n = 4), hypofunctioning (n = 5), and isofunctioning (n = 3) on the available thyroid scintigraphies. Eight cases accompanied with hyperthyroidism in which only 1 was caused by the target nodule. Evidence of co-existing autoimmune thyroid disease (AITD) and multinodular goiter were found in 52% and 52% of cases, respectively. Seven nodules underwent surgeries and all were benign on final pathology. None of 9 nodules with follow-up by ultrasound (3~33 mon, median 12 mon) showed grow in size.
Conclusions: TSHR mutations and/or NIS overexpression can be detected in pre-operative FNB specimens using the NGS approach. These genetic alterations occurred in 6.4% thyroid nodules in this consecutive series with indeterminate cytology. They present not only in hyperfunctioning nodules but also in hypo- or iso-functional nodules, indicating their prevalence may be higher than previously expected. Co-existing AITD was common in cases with these molecular alterations. None of our patients with TSHR mutations and/or NIS overexpression manifested malignant outcomes. How to use these two molecular markers in thyroid FNBs to guide our clinical practice warrants further investigation.
Introduction
In the human thyroid, thyroid-stimulating hormone (TSH) activates both the cyclic adenosine monophosphate (cAMP) and the phospholipase C-diacylglycerol regulatory cascades. The former cascade positively controls the hormone-producing function and growth of thyrocytes, while the latter cascade contributes to the generation of the intracellular signals myoinositol-1.4.5-triphosphate (1,4,5-PIP3) and diacylglycerol, both of which act on thyrocytes to increase protein iodination and thyroid hormone synthesis (1–6). These fundamental roles of TSH in regulating thyroid growth and function are attained by binding to its receptor, TSH receptor (TSHR), and can be mimicked by activating mutations of the TSHR. Sodium-iodine symporter (NIS) is another known key protein of normal thyroid cell physiology. It is responsible for the active transport of iodide across the basolateral membrane of thyroid cell. NIS function results in a 20- to 40-fold elevation of iodide concentration with respect to the iodide level in circulating blood (7–9).
Given the critical roles of TSHR and NIS in maintaining normal thyroid cell function and proliferation, it is expected that alterations of genes coding these two proteins (TSHR and NIS genes) will be implicated, at least in part, in a variety of thyroid disorders including thyroid nodules In fact, since the 1990s, germline and somatic activating mutations of TSHR have been reported in familial nonautoimmune hyperthyroidism (FNAH), sporadic congenital nonautoimmune hyperthyroidism (SCNAH), and autonomously functioning thyroid nodules (AFTNs) (10); loss-of-function mutations of TSHR have been found to cause hypothyroidism and euthyroidism with elevated serum TSH (so-called “compensated hypothyroidism”) (11, 12). TSHR mutations have also been found occasionally in functional and rarely in non-functional malignant thyroid nodules (1, 13–21). It was suggested that a TSHR mutation concurrent with other thyroid cancer-related genetic alterations such as BRAF, GNAS, RAS, TP53, TRK, PAX8/PPARr, and RET/PTC, or TSHR mutations occurring with very high allelic frequency (allelic frequency >30%) may have a high probability of thyroid cancer (22–25). Similarly, markedly increased levels of NIS expression have been exhibited in active Graves' disease (GD) and AFTN (26). In contrast, defects of NIS expression exist in several cases of congenital hypothyroidism (27–29), and generally low levels of NIS expression is found in non-toxic multinodular goiter (NMNG), diffuse iodine deficiency goiter (IDG) (26), and solitary malignant thyroid nodules (30–32).
In view of these findings, it should be noted that TSHR mutations and NIS overexpression are closely associated with thyroid nodules, in particular with AFTNs. To our knowledge, so far studies of this kind are entirely performed using surgically resected tissues of thyroid nodules. In these studies, functional status and clinicopathological features of the nodule were already confirmed before performing a genetic examination. The major purpose of detecting alterations of TSHR and NIS in confirmed cases was to look for genetic etiologies of AFTNs. Despite available results, the clinical value of a TSHR mutation and/or NIS gene overexpression testing in all pre-operative indeterminate FNB specimens has not been studied.
For those cytologically indeterminate FNB specimens obtained from thyroid nodules, molecular testing has been recommended to assist the identification of thyroid cancer. Starting from February of 2015, we routinely use a large commercial multi-gene next generation sequencing (NGS) gene array to test all indeterminate FNB specimens. This array is called the ThyroSeqV.2 next-generation sequencing panel. With a high capability of sensitive detection, ThyroSeqV.2 can provide abundant information on a number of genes associated with thyroid tumor including TSHR and NIS in a given sample (33, 34). Utilization of ThyroSeqV.2 in all indeterminate cytology gives us an opportunity to find thyroid nodules harboring TSHR mutations and/or NIS overexpression. It allows us to investigate the clinical significance and diagnostic utility of TSHR mutations and/or NIS overexpression detected in pre-operative thyroid nodules with an indeterminate cytology.
Thus, aims of the present clinical utility study included examining the prevalence of genetic alterations of TSHR and NIS in a series of thyroid FNB specimens with indeterminate cytology, as well as identifying various forms of TSHR and NIS alterations. In addition, we intended to assess the correlation of the type of genetic changes with demographic features, sonographic patterns, functioning status, surgical pathology, and follow-up results in the target thyroid nodule.
Subjects and Methods
FNB Samples
Between February 2015 and September 2017, 1,293 consecutive FNBs were performed on thyroid nodules (>1 cm) under ultrasound (US) guidance (Toshiba Xario™ 200 with 5–14 MHz 58 mm Linear Array Ultrasound Transducer or 4.2–10.2 MHz micro convex or linear probe; Toshiba America Medical System, Tustin, CA) by endocrinologists at Boston Medical Center and interpreted by experienced cytopathologists. Cytology diagnosis was determined according to The Bethesda System for Reporting Thyroid Cytopathology (35). Among them, 388 FNB samples with indeterminate were sent consecutively to the CBL Path (Rye Brook, NY) for molecular analysis.
Analysis of TSHR Mutation and NIS Overexpression
At the time of FNB aspiration or capillary technique, the content of the first FNB was preserved for molecular analysis, according to the manufacturer's instructions. TSHR mutations and NIS gene overexpression were detected using the ThyroSeqV.2 next-generation sequencing panel. This panel is applied to test for key thyroid cancer-related genes for base substitutions and small insertion/deletions in targeted regions of 14 genes (AKT1, BRAF, CTNNB, EIF1AX, GNAS, NRAS, HRAS, KRAS, PIK3CA, PTEN, RET, TERT, TP53, TSHR), for more than 42 types of gene fusions (involving ALK, BRAF, IGF2BP3, MET, NTRK1, NTRK3, PPARG, RET, and THADA), and for gene expression of PGK, KRT7, TTF1, TG, CALCA, NIS (SLC5A5), PTH, KRT20 control genes. The analytical sensitivity for detection of all variant types including base substitution and insertion/deletions at >3–5% mutant allele frequency and gene fusions at >1% of tumor nuclei is >99.9% (95% CI: 98–100%). Gene expression profile is reported as negative (normal expression profile) or positive (abnormal expression of one or more genes) with detailed interpretation (33, 34).
Medical Review and Follow-Up of Clinical Characteristics of the Biopsied Nodules
A thorough review of the medical record was performed for each patient with a TSHR mutation and/or NIS overexpression. Patient demographic data, medical history, medical therapy, history of head and neck radiation, thyroid function tests (TFTs), thyroid autoantibody tests, thyroid ultrasound images, and 123I radioactive iodine thyroid scintigraphy scans (NM thyroid uptake and standard three-view planar scan) were collected and recorded. Cytology reports were reviewed to search for details in addition to Bethesda categories.
The biopsied nodules were clinically monitored by periodic bedside thyroid ultrasound performed by an endocrinologist to determine their clinical outcomes. For cases that underwent surgery after FNBs, histologic slides were reviewed by experienced pathologists. The surgical pathology of the thyroid nodule was used as the gold standard for indeterminate Bethesda cytology (Bethesda III, IV, and V) categories. For cases that underwent observation, periodical thyroid ultrasonography, TFTs, and radiological examinations were recommended. The associated reports were collected.
The target nodule was identified by correlating the site and size according to the associated reports with the pre-biopsied US images saved on the radiology picture archiving and communication system (PACS), to ensure that cytology, molecular analysis, radiology, and pathology reports belonged to the same specific nodule.
Statistical Analysis
Data were analyzed using SPSS software (SPSS 20.0, IBM, USA). The parametric variables were compared using the unpaired t-test between two groups and Kruskal–Wallis one-way ANOVA test among three groups. Difference between frequencies was compared using the Yates 2 × 2 Chi square test (when there were <10 numbers in data) or Fisher's test (when there were <4 numbers in data). P level of 0.05 or less was considered significant.
Ethics
The protocol of this study was approved by the Boston University Institutional Review Board. Informed consent was obtained from all patients before performing the procedure.
Results
Genetic Alterations of TSHR and NIS in Indeterminate FNBs
Among 388 indeterminate FNBs, 136 (35.1%) were found at least one genetic alterations by the ThyroSeqV.2 next-generation sequencing panel, including gene mutations involving BRAF, H/N/K-RAS, EIF1AX, PTEN, TERT, TP53, and TSHR, gene fusions involving IGF2BP3, NTRK3, PPARG, and THADA, as well as gene overexpression of MET and NIS. TSHR mutations and/or NIS overexpression were detected in 25 (6.4%) nodules from 24 patients. Ten nodules (2.6%) harbored TSHR mutations only, 7 nodules (1.8%) over-expressed NIS gene only, and 8 nodules (2.1%) had both molecular alterations. The TSHR mutations were located between codons 281–640. Specifically, S281N (n = 2), M453T (n = 7), I468M (n = 1), S505N (n = 1), L512R (n = 1), I568F (n = 1), I568T (n = 2), F631L (n = 2), and T632A (n = 1) were identified, with codon 453 being the most frequently affected (Figure 1 and Table 1). All the mutations are known mutations that were previously reported. They have been documented to be gain of function mutations with increased cAMP production (Figure 1). The allelic frequency of the mutated TSHR ranged from 6 to 36% (Table 1). One nodule (#1) with NIS overexpression had simultaneous EIF1AX and GNAS mutations. Two nodules (#12 and #15) carrying different TSHR mutations (I486M and M453T, respectively) were from the same patient.
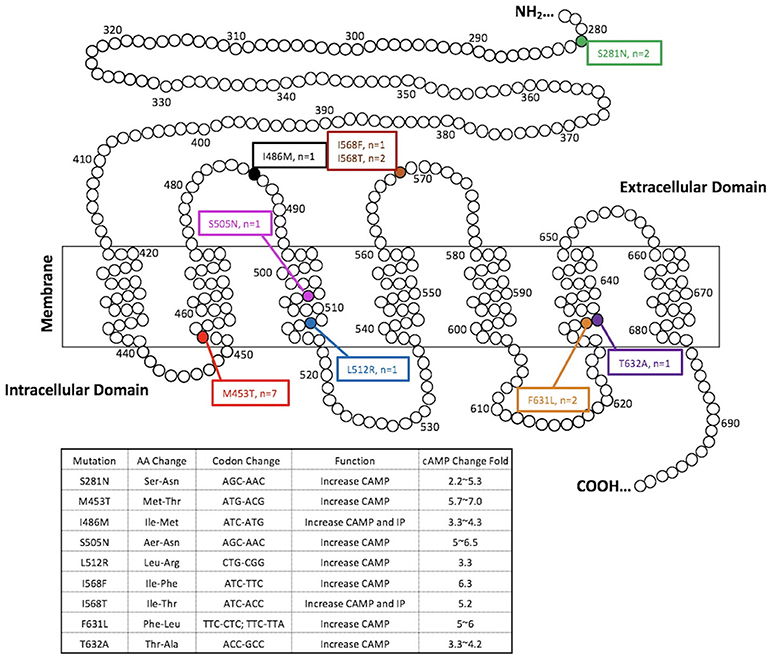
Figure 1. TSHR mutations detected in the present study. Functional characteristics of mutations are available at http://endokrinologie.uniklinikum-leipzig.de/tsh/frame.html.
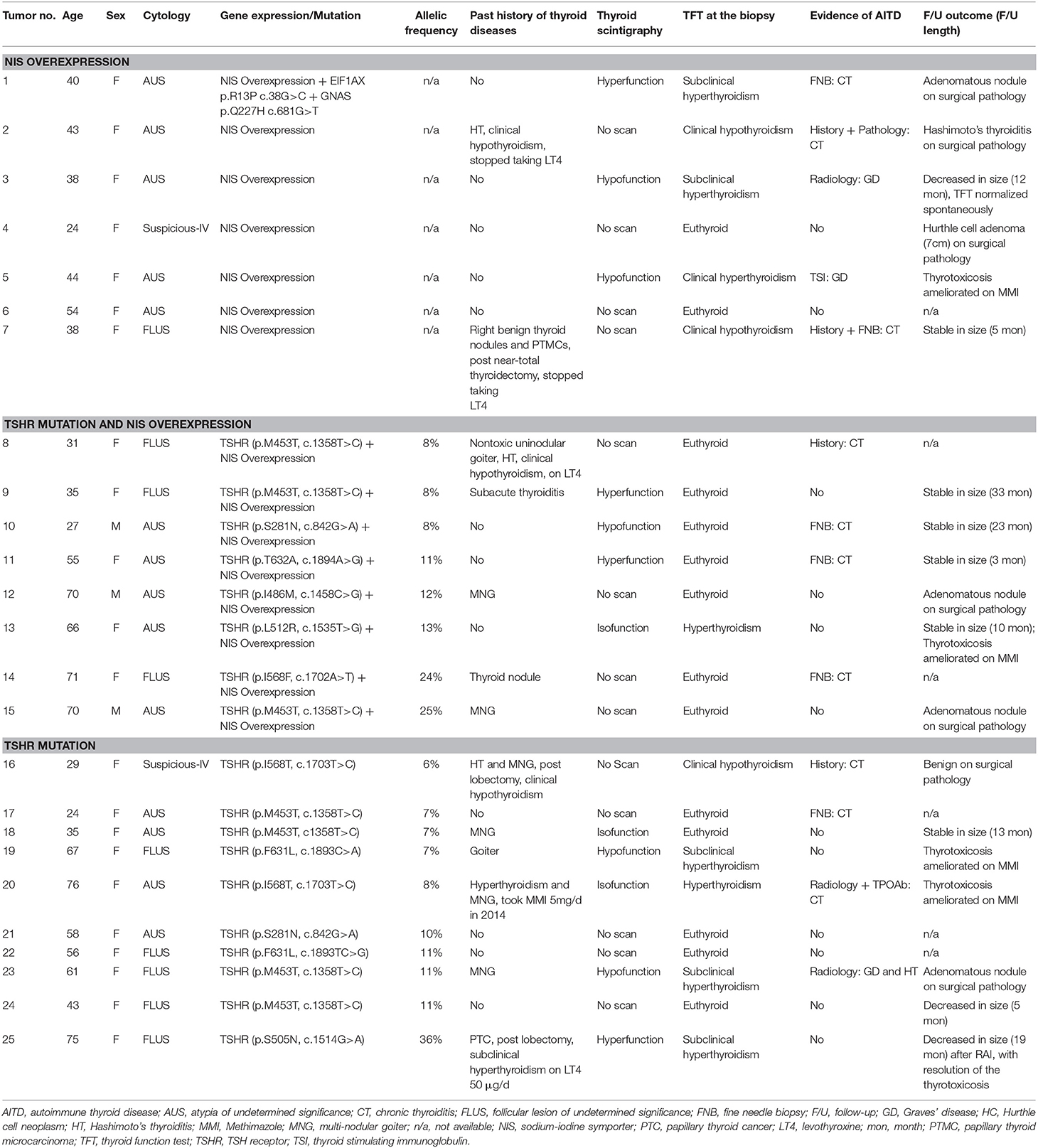
Table 1. Clinical characteristics of the biopsied nodules harboring NIS overexpression and/or TSHR mutations.
Sex and Age of Patients With Nodules Harboring TSHR Mutations and/or NIS Overexpression
Overall, 21 of 24 (87.5%) patients who had indeterminate nodules with TSHR mutations and/or NIS overexpression were female. All the 3 male patients in this case series presented both a TSHR mutation and NIS overexpression. Mean age of the 24 patients was (48.3 ± 16.9) years. Although difference did not show statistical significance, patients who only overexpressed NIS were ~10 years younger than those harboring TSHR mutations with or without NIS overexpression (mean age: 40.1 years vs. 51.7 years, P = 0.13).
Sonographic Patterns of Nodules Harboring TSHR Mutations and/or NIS Overexpression
Sonographic patterns of the 25 nodules with TSHR mutations and/or NIS overexpression are shown in Table 2. Of these indeterminate nodules, 13 (52%) grew in a multinodular goiter (MNG) background. Nodules harboring TSHR mutations with/without NIS overexpression had underlying MNG more commonly than nodules expressing NIS overexpression only (12/18 vs. 1/7, P = 0.03). The greatest dimension of the nodule on ultrasound varied between 1.0 and 5.8 cm. The mean size of nodules harboring TSHR mutations with/without NIS overexpression were smaller than nodules expressing NIS overexpression only (2.27 ± 1.08 vs. 3.31 ± 1.34 cm). The difference was very close to statistical significance (P = 0.055). Thirteen nodules demonstrated hypoechoic echotexture on ultrasound; 7 nodules were isoechoic, and the remaining 3 nodules with associated records available were of heterogeneous echogenicity. Around two thirds (17/24) of nodules were solitary thyroid nodules, whereas the remaining 8 displayed complex composition (n = 6) or spongiform (n = 2) compositions. Worrisome high-risk ultrasound features including microcalcificaion, microlobulated margin, tall shape, and suspicious neck nodes were uncommon in these nodules, shown in 3, 1, 1, and 0 nodules, respectively. Most nodules (19/23) with TSHR mutations and/or NIS overexpression had increased intranodular vascularity. Echogenicity, composition, high risk features and vascularity of the nodule did not correlate with the type of genetic changes the nodule was harboring.
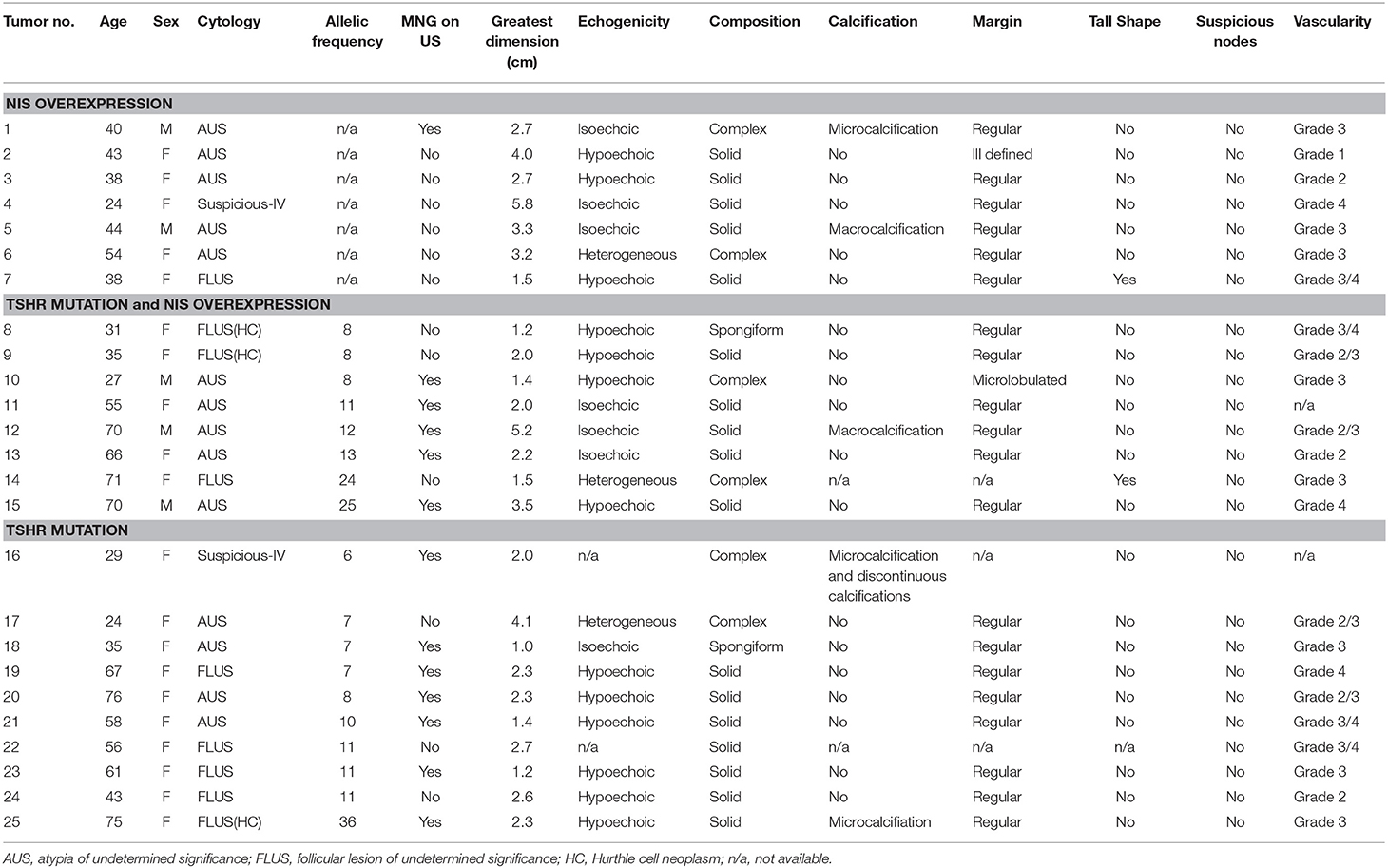
Table 2. Sonographic patterns of the biopsied nodules harboring NIS overexpression and/or TSHR mutations.
Functioning Status of Nodules Harboring TSHR Mutations and/or NIS Overexpression
Among 25 nodules with TSHR mutations and/or NIS overexpression, 12 (48.0%) had NM thyroid update and scan checked, 5 of which were done between 21 days and 3 years before the FNB and otherwise after the FNB. Four of them exhibited hyperfunctioning nodules on thyroid scintigraphy, while 5 were hypofunctioning, and 3 were isofunctioning nodules compared to surrounding normal thyroid parenchyma. Accumulation of radioiodine by the nodule did not correlate with the type of genetic changes in the target nodule.
Biochemical Thyroid Function and Co-existing Autoimmune Thyroid Disease (AITD) in Patients With Nodules Harboring TSHR Mutations and/or NIS Overexpression
Biochemical thyroid function tests were available for review in 23 (95.8%) patients. None of the patients were taking thyroid medication including levothyroxine, thyroid extract, antithyroid drugs, or iodine contained preparations at the time of evaluation, except for 1 case of Graves' disease (GD) treated with Methimazole (MMI) indicated in Figure 2. Of the 24 indeterminate nodules from 23 patients, more than half (n = 14, 56%) existed with normal biochemical TFTs, 8 had concurrent hyperthyroidism in which only 2 were caused by the autonomous function of the biopsied nodules, and 3 had concurrent hypothyroidism as the consequence of previous surgeries or chronic thyroiditis (CT). Evidence of co-existing AITD in the target nodule and/or its surrounding thyroid parenchyma were indicated, by medical history, cytology, thyroid autoantibody measurements, ultrasound in 52% (n = 13) of nodules (Table 1 and Figure 2). Patients with and without AITD did not show significant difference in age and sex distribution (44.4 ± 16.7 vs. 53.0 ± 16.6, P = 0.11; 12:1 vs. 10:1, P = 0.26). Biochemical thyroid function and co-existing AITD did not correlate with the type of genetic changes in the target nodule.
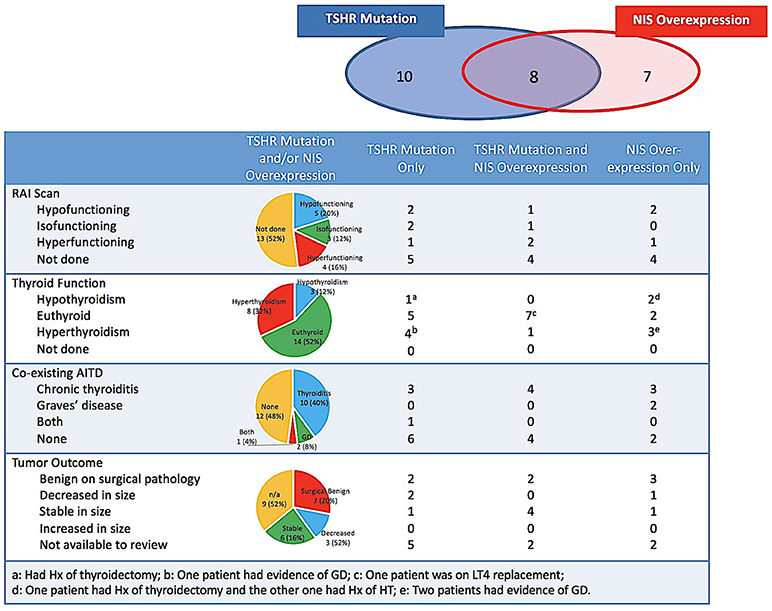
Figure 2. Accumulation of radioiodine, biochemical thyroid function, co-existing AITD and tumor outcome of the biopsied nodules harboring TSHR mutations and/or NIS overexpression. AITD, autoimmune thyroid disease; GD, Graves' disease; HT, Hashimoto's thyroiditis; Hx, history; NIS, sodium-iodine symporter; LT4, levothyroxine; RAI, radioiodine; TSHR, TSH receptor.
Follow-Up of Nodules With TSHR Mutations and/or NIS Overexpression
Seven nodules underwent surgeries and all were reported benign on final pathology, including one Hurthle cell adenoma, 4 adenomatous nodules, 1 benign thyroid nodule and 1 fibrous variant Hashimoto's thyroiditis. Nine nodules without surgery were followed up by ultrasound between 3 and 33 months (median 12 months): 6 nodules were stable and 3 had decreased in size, respectively. Ultrasound follow-ups were not available in the additional 9 nodules, either because of short time since biopsy (< 6 months, n = 2) or because of loss to ultrasound follow-up at the Boston Medical Center. Thus, TSHR mutations and NIS overexpression have not shown any solid association with the malignancy in our patient cohort.
Featured Cases
It is worthwhile to highlight three cases. The first case is a 40-year female. Testing of her nodule detected NIS overexpression, as well as simultaneous EIF1AX and GNAS mutation. This is the only case with multiple gene mutations in our cohort. The nodule showed microcalcification and otherwise no suspicious features on ultrasound. It manifested as hyperfunctioning on the NM scan and a low TSH level consistent with subclinical hyperthyroidism. The patient decided to be put on medical therapy since August, 2017. Her TFT returned to normal after taking MMI 5–10 mg daily for 2 months and the nodule did not develop suspicious sonographic features or grew significantly (>20% in 2 dimensions) on periodic ultrasound examination. The second case is a 70-year male originally from Vietnam, a country with mild iodine deficiency (http://www.ign.org/vietnam.htm). In a background of big MNG, 2 nodules were identified with indeterminate FNBs. Both nodules had TSHR mutations, but the codons affected were different (p.I468M and p.M453T, respectively). The patient underwent a total thyroidectomy by a high-volume surgeon and surgical pathology confirmed that both nodules were adenomatous nodules. A papillary microcarcinoma (0.5 cm, conventional subtype, BRAF V600E mutation absent) and a 0.3 cm non-invasive follicular neoplasm with papillary-like nuclear features (NIFTP) loci were incidentally found in the contralateral thyroid lobe according to the post-surgical pathology report. Levothyroxine was given to keep his TSH low-normal level after surgery. Seven months after the total-thyroidectomy, ultrasound monitoring indicated thyroid tissue remnants in both thyroid beds measuring (2.8 × 1.1 × 1.1) cm and (0.7 × 0.5 × 0.8) cm, respectively. The third case is a 75-year female with a hot nodule harboring TSHR p.S505N mutation in high allelic frequency (36%). She presented with subclinical hyperthyroidism due to the hot nodule, without any thyroid active medication. Her past medical history included a lobectomy for MNG and papillary thyroid carcinoma more than 30 years ago. Although complete thyroidectomy was recommended, she refused for the concern about risks of reoperation. She was treated with radioiodine in January, 2017 and 3 months later, her nodule decreased in size with resolution of the thyrotoxicosis.
Discussion
In the present study, we have investigated the clinical significance and diagnostic utility of TSHR mutations and/or NIS overexpression in thyroid FNB specimens with indeterminate cytology. Unlike previous studies which checked somatic genetic alterations of TSHR and NIS using surgically resected thyroid tissue from known autonomous nodules, the distinction of our study was having these molecular alterations detected in pre-operative thyroid nodules with indeterminate biopsy. Accordingly, the purpose of detecting TSHR mutations and/or NIS overexpression in thyroid nodules was different between our study and former researches. Instead of verifying the etiological involvement of TSHR and NIS alterations in autonomously hyperfunctioning nodules, we determined in a clinical utility study the true prevalence of TSHR mutations and NIS overexpression with patients with indeterminate thyroid cytology.
Our study demonstrated that TSHR mutations and/or NIS overexpression occurred in a higher than expected 6.4% of 388 consecutively biopsied thyroid nodules with indeterminate cytology. In regards to the prevalence of TSHR mutations in this nodule series, it was 4.7%. All the TSHR mutations are known mutations that were previously reported in AFTNs, and are somatic gain-of-function mutations (Figure 1) (36–39). In previous studies, somatic mutations of TSHR gene have been reported in toxic adenomas and toxic multinodular goiters, with a frequency ranging from 8 to 82% of cases (40, 41). It is not surprising that the mutation rate described in our study was much lower when compared with existing data, because we were not doing the molecular testing in selected functioning nodular diseases. Interestingly, the most frequently detected TSH mutation in our FNB series was M453T, which is also the most prevalent mutation in tissue samples from known AFTNs (42). It indicated that this mutation would account for a major genetic alteration of TSHR in thyroid nodules and be more common than we used to anticipate. Given the fact that gain-of-function mutations of the TSHR can constitutively stimulate TSH-adenylcyclase-cAMP pathway to increase the uptake of iodine by thyrocytes and the production of thyroid hormones (4), we expected that NIS overexpression would commonly happen along with TSHR mutations. However, more than half (10/18) of TSHR mutations were not accompanied with over-expressed NIS gene. Type and allelic frequency of the TSHR mutation did not influence the occurrence of NIS overexpression. On the other hand, we found 7 nodules having NIS overexpression without any TSHR mutations. A proposed explanation may relate to other known or unknown molecular alterations and alternate cellular pathways besides TSHR that are mediating the increase in NIS expression. The nodule that overexpressed NIS had concurrent EIF1AX and GNAS mutations found in our study was a clue for this hypothesis. Iodine deficiency may be another influence on the occurrence of TSHR mutations and NIS overexpression in our study. Previous studies have suggested that AFTNs and toxic nodules in a MNG in iodine-deficient regions tend to have an elevated frequencies of TSHR mutations (43–45). Iodine status is a major factor that affects the expression of NIS gene. NIS expression in thyrocytes is elevated when iodine is deficient and this in turn retains the ability to accumulate iodine to maintain thyroid hormone production; while iodine excess down-regulates NIS expression (46). However, a study done in Turkey did not find that TSHR mutation was influenced by iodine intake (16), and the mutant TSHR was a common genetic cause of TA in Japanese, despite their high iodine intake (42). The effect of iodine intake on genetic alterations of TSHR and NIS remains unclear and attractive. Unfortunately, in the present study, former and current iodine intakes of each case was not available for further analysis.
For many years, AFTNs have been considered a very low risk for malignancy and FNB of such nodules is therefore normally unnecessary. The discovery of TSHR mutations and NIS overexpression (26, 47) in AFTNs not only supports an important molecular mechanism underlying the pathogenesis of non-autoimmune thyroid autonomy, but also encourages the idea that TSHR and NIS alterations can be used as molecular markers to predict AFTNs and subsequently reduce FNBs on them. On the other hand, although genetic analysis of the TSHR gene in thyroid cancers supported the notion that TSHR mutations do not play a role in the pathogenesis of nonfunctioning differentiated thyroid carcinoma (48), as TSHR mediates the proliferation of thyrocytes through the TSH-AC-cAMP pathway, we cannot rule out that its constant activation by a gain-of-function mutation is a logical carcinogenic factor (48, 49). Based on prior reported cases and experimental studies (25, 49), TSHR mutations, especially those occurring with a high allelic frequency (>30%) and/or at specific codons (e.g., between 620 and 631), are associated with the uncommon but relatively increased risk of functioning thyroid carcinomas. Therefore, we should precisely evaluate the predictive value of TSHR mutations for AFTNs and malignancies according to more detailed mutation information, e.g., type, allelic frequency, other co-existing mutation, etc. The NGS approach to TSHR mutations and NIS expression detection in thyroid FNB specimens with indeterminate cytology allows us for the first time to correlate details of these genetic alterations with functional status and outcome of the target thyroid nodules. This gives us a better understanding of the clinical significance of using TSHR mutations and NIS overexpression as molecular makers. In our study, out of 25 nodules harboring mutant TSHR and/or NIS overexpression, radioiodine scintigraphy was performed in 12 and TFT results were available in 24. Surprisingly, these data indicated that accumulation of radioiodine by the nodule and biochemical thyroid function of the resulting phenotype were quite variable, and did not correlate the TSHR mutation and NIS overexpression in the target nodule. Thus, TSHR mutations and NIS overexpression detected in pre-operative FNBs are not good molecular indicators of hyperfunctioning nodules. But we observed that nodules with multiple genetic alterations (NIS overexpression, EIF1AX, and GNAS mutations) and mutant TSHR with a high allelic frequency (36%) were both hyperfunctioning. It likely suggests that co-existence of other mutations and abundant mutant TSHR may be necessary to attain enough activation and induce autonomous function in the target nodule. In regards to the value of TSHR mutations and/or NIS overexpression in distinguishing benign and malignant nodules, based on surgical pathology and ultrasound follow-up, these genetic alterations did not predict any malignant diagnoses. This result is in accordance with our expectation, given that NIS expression represents well-differentiated status of thyrocytes, and only one case of a TSHR mutation with high allelic frequency was detected. We agree with the current opinion that TSHR mutations (except for those found at a high level) and/or NIS overexpression do not raise the probability of thyroid cancer or a pre-cancerous tumor, noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP).
Previous studies showed that activating TSHR mutations only existed in clinical and histological subtypes of autonomous nodules, but had never been found in non-functioning nodules. It has been also reported that cold nodules express very low levels of NIS mRNA (26). However, the results of our study directly contradict the former finding. Nodules harboring mutant TSHR associated with elevated cAMP production and/or NIS overexpression could display hyperfunction, isofunction, or even hypofunction, on thyroid scintigraphy. Taking into account that the allelic frequency of TSHR mutations in the majority of these nodules was relatively low (between 7 and 13%), our finding may suggest that autonomous function requires higher allelic frequency of the mutant TSHR levels; on the other hand, a negative finding in non-functioning nodules from previous studies may be due to less sensitive methods they employed for detecting these genetic alterations compared to the highly sensitive NGS used in this study.
Our study also illustrated for the first time that co-existing AITD was common in patients with a nodule harboring TSHR mutations and/or NIS overexpression. Although more than half of the patients were found with evidence of AITD, i.e., Graves' disease and/or chronic autoimmune thyroiditis, 71% (5/7) with NIS overexpression demonstrated AITD. This figure is much higher than the prevalence of AITD in the general population (50). Mechanisms underlying this phenomenon are not clear. Certainly, high thyroidal radioactive iodine uptake is seen in early Hashimoto's thyroiditis but usually under the condition of an elevated TSH and TSHR activation. Theoretically, modifications in the primary structure of the TSHR might be an autoimmune antigen causing an immunoreaction. However, a couple of somatic TSHR variants that had been proposed as the cause of autoimmunity, including Asp36His and Pro52Thr, were later demonstrated to be polymorphisms that were also frequently present in healthy people (51, 52). It would be interesting to explore the possibility that variant TSHR, when associated with particular HLA haplotype(s) or other genetically linked anomalies in the immune system can grant higher susceptibility to developing AITD. Other than mutant TSHR, co-existing AITD may not result from NIS overexpression, but is more likely one of factors that causes elevated NIS expression. Nonetheless, we should realize that AITD, when concurrent with genetic alterations, is a confounding factor on the phenotype of biochemical thyroid function and ultrasound sonography. For example, Graves disease plus a functioning nodule with a TSHR mutation may cause more apparent hyperthyroidism, while Hashimoto thyroiditis may mask the hyperthyroidism caused by the nodule harboring a gain-of-function TSHR mutation. This can be one explanation to why some autonomous functioning nodules present in patients with normal thyroid function.
Though our study provided a novel perspective to understand the clinical significance of TSHR mutations and/or NIS overexpression in thyroid nodules, due to some limitations, results of this study need to be applied with caution. First, as we mentioned above, iodine intake and iodine nutrition status of these cases were not available. Thus, the influence of iodine on these genetic alterations could not be analyzed but NHANES studies have seen a stable median iodine intake based on urinary iodine has been adequate in the United States for >30 years (NHAS references). Secondly, only some patients performed thyroid scintigraphy and most patients did not undergo surgery, so the predictive value of these genetic alterations may be over- or under-evaluated. Thirdly, radioiodine scintigraphy was performed in planar technique. This may not be sensitive enough to define thyroid autonomy of nodules without TSH suppression, leading to potential misjudgment on function of target nodule. Finally, length of follow-up was not long enough, hence clinical development of the target nodule has not been fully exposed.
In conclusion, TSHR mutations and/or NIS overexpression can be detected in pre-operative FNB specimens using the NGS approach. These genetic alterations occurred in 6.4% thyroid nodules in this consecutive series with indeterminate cytology. One third of these nodules had both a TSHR mutation and NIS overexpression. Co-existing AITD was common in cases with these molecular alterations. Thyroid function and accumulation of radioiodine varied and did not correlate with these genetic changes in indeterminate nodules. None of our patients with TSHR mutations and/or NIS overexpression manifested malignant outcomes. How to use these two molecular markers in thyroid FNBs to guide our clinical practice warrants further investigation.
Author Contributions
SL designed the study. HG, DM, and GT reviewed clinical records, collected data, and did data input. HG prepared the manuscript, tables and figures. SL revised the manuscript. All authors reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Rainjade Chung for her linguistic assistance during the preparation of this manuscript. HG received a scholarship from the China Scholarship Council (201708210108). A conference abstract based on preliminary data of the present work was published at the ENDO 2018, which was held by the Endocrine Society at Chicago, IL between March 17 and March 20, 2018.
References
1. Camacho P, Gordon D, Chiefari E, Yong S, DeJong S, Pitale S, et al. A Phe 486 thyrotropin receptor mutation in an autonomously functioning follicular carcinoma that was causing hyperthyroidism. Thyroid (2000) 10:1009–12. doi: 10.1089/thy.2000.10.1009
2. Dumont JE, Lamy F, Roger P, Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev. (1992) 72:667–97. doi: 10.1152/physrev.1992.72.3.667
3. Nagayama Y, Nagataki S. [The TSH receptor gene and the pathogenesis of Graves' disease]. Nihon Naibunpi Gakkai Zasshi (1992) 68:584–91. doi: 10.1507/endocrine1927.68.6_584
4. Paschke R, Ludgate M. The thyrotropin receptor in thyroid diseases. N Engl J Med. (1997) 337:1675–81. doi: 10.1056/NEJM199712043372307
5. De Groot LJ, Kopp P. Thyrotoxicosis of other etiologies. In: Thyroid Disease Manager (2010). Available online at: www.thyroidmanager.org
6. Kopp P. The TSH receptor and its role in thyroid disease. Cell Mol Life Sci. (2001) 58:1301–22. doi: 10.1007/PL00000941
7. Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature (1996) 379:458–60. doi: 10.1038/379458a0
8. Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, Jhiang SM. Cloning of the human sodium lodide symporter. Biochem Biophys Res Commun. (1996) 226:339–45.
9. Levy O, De la Vieja, A, Carrasco N. The Na+/I- symporter (NIS): recent advances. J Bioenerg Biomembr. (1998) 30:195–206.
10. Hebrant A, van Staveren WC, Maenhaut C, Dumont JE, Leclere J. Genetic hyperthyroidism: hyperthyroidism due to activating TSHR mutations. Eur J Endocrinol. (2011) 164:1–9. doi: 10.1530/EJE-10-0775
11. Stein SA, Oates EL, Hall CR, Grumbles RM, Fernandez LM, Taylor NA, et al. Identification of a point mutation in the thyrotropin receptor of the hyt/hyt hypothyroid mouse. Mol Endocrinol. (1994) 8:129–38.
12. Sunthornthepvarakul T, Hayashi Y, Refetoff S. Polymorphism of a variant human thyrotropin receptor (hTSHR) gene. Thyroid (1994) 4:147–9. doi: 10.1089/thy.1994.4.147
13. Niepomniszcze H, Suarez H, Pitoia F, Pignatta A, Danilowicz K, Manavela M, et al. Follicular carcinoma presenting as autonomous functioning thyroid nodule and containing an activating mutation of the TSH receptor (T620I) and a mutation of the Ki-RAS (G12C) genes. Thyroid (2006) 16:497–503. doi: 10.1089/thy.2006.16.497
14. Fuhrer D, Tannapfel A, Sabri O, Lamesch P, Paschke R. Two somatic TSH receptor mutations in a patient with toxic metastasising follicular thyroid carcinoma and non-functional lung metastases. Endocr Relat Cancer (2003) 10:591–600. doi: 10.1677/erc.0.0100591
15. Spambalg D, Sharifi N, Elisei R, Gross JL, Medeiros-Neto G, Fagin JA. Structural studies of the thyrotropin receptor and Gs alpha in human thyroid cancers: low prevalence of mutations predicts infrequent involvement in malignant transformation. J Clin Endocrinol Metab. (1996) 81:3898–901.
16. Gozu H, Avsar M, Bircan R, Sahin S, Ahiskanali R, Gulluoglu B, et al. Does a Leu 512 Arg thyrotropin receptor mutation cause an autonomously functioning papillary carcinoma? Thyroid (2004) 14:975–80. doi: 10.1089/thy.2004.14.975
17. Mircescu H, Parma J, Huot C, Deal C, Oligny LL, Vassart G, et al. Hyperfunctioning malignant thyroid nodule in an 11-year-old girl: pathologic and molecular studies. J Pediatr. (2000) 137:585–7. doi: 10.1067/mpd.2000.108437
18. Russo D, Arturi F, Schlumberger M, Caillou B, Monier R, Filetti S, et al. Activating mutations of the TSH receptor in differentiated thyroid carcinomas. Oncogene (1995) 11:1907–11.
19. Russo D, Tumino S, Arturi F, Vigneri P, Grasso G, Pontecorvi A, et al. Detection of an activating mutation of the thyrotropin receptor in a case of an autonomously hyperfunctioning thyroid insular carcinoma. J Clin Endocrinol Metab. (1997) 82:735–8.
20. Russo D, Wong MG, Costante G, Chiefari E, Treseler PA, Arturi F, et al. A Val 677 activating mutation of the thyrotropin receptor in a Hurthle cell thyroid carcinoma associated with thyrotoxicosis. Thyroid (1999) 9:13–7.
21. Lado-Abeal J, Celestino R, Bravo SB, Garcia-Rendueles ME, de la Calzada J, Castro I, et al. Identification of a paired box gene 8-peroxisome proliferator-activated receptor gamma (PAX8-PPARgamma) rearrangement mosaicism in a patient with an autonomous functioning follicular thyroid carcinoma bearing an activating mutation in the TSH receptor. Endocr Relat Cancer (2010) 17:599–610. doi: 10.1677/ERC-09-0069
22. Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. (2009) 94:2092–8. doi: 10.1210/jc.2009-0247
23. Nikiforov YE, Yip L, Nikiforova MN. New strategies in diagnosing cancer in thyroid nodules: impact of molecular markers. Clin Cancer Res. (2013) 19:2283–8. doi: 10.1158/1078-0432.CCR-12-1253
24. Nikiforova MN, Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid (2009) 19:1351–61. doi: 10.1089/thy.2009.0240
25. Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. (2013) 98:E1852–60. doi: 10.1210/jc.2013-2292
26. Joba W, Spitzweg C, Schriever K, Heufelder AE. Analysis of human sodium/iodide symporter, thyroid transcription factor-1, and paired-box-protein-8 gene expression in benign thyroid diseases. Thyroid (1999) 9:455–66.
27. Fujiwara H, Tatsumi K, Miki K, Harada T, Miyai K, Takai S, et al. Congenital hypothyroidism caused by a mutation in the Na+/I- symporter. Nat Genet. (1997) 16:124–5. doi: 10.1038/ng0697-124
28. Matsuda A, Kosugi S. A homozygous missense mutation of the sodium/iodide symporter gene causing iodide transport defect. J Clin Endocrinol Metab. (1997) 82:3966–71. doi: 10.1210/jc.82.12.3966
29. Pohlenz J, Medeiros-Neto G, Gross JL, Silveiro SP, Knobel M, Refetoff S. Hypothyroidism in a Brazilian kindred due to iodide trapping defect caused by a homozygous mutation in the sodium/iodide symporter gene. Biochem Biophys Res Commun. (1997) 240:488–91. doi: 10.1006/bbrc.1997.7594
30. Ward LS, Santarosa PL, Granja F, da Assumpção LVM, Savoldi M, Goldman GH. Low expression of sodium iodide symporter identifies aggressive thyroid tumors. Cancer Lett. (2003) 200:85–91. doi: 10.1016/s0304-3835(03)00392-6
31. Trouttet-Masson S, Selmi-Ruby S, Bernier-Valentin F, Porra V, Berger-Dutrieux N, Decaussin M, et al. Evidence for transcriptional and posttranscriptional alterations of the sodium/iodide symporter expression in hypofunctioning benign and malignant thyroid tumors. Am J Pathol. (2004) 165:25–34. doi: 10.1016/S0002-9440(10)63272-5
32. Sodre AK, Rubio IG, Galrao AL, Knobel M, Tomimori EK, Alves VA, et al. Association of low sodium-iodide symporter messenger ribonucleic acid expression in malignant thyroid nodules with increased intracellular protein staining. J Clin Endocrinol Metab. (2008) 93:4141–5. doi: 10.1210/jc.2007-0353
33. Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer (2014) 120:3627–34. doi: 10.1002/cncr.29038
34. Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, et al. Impact of the multi-gene thyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid (2015) 25:1217–23. doi: 10.1089/thy.2015.0305
35. Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid (2009) 19:1159–65. doi: 10.1089/thy.2009.0274
36. Lueblinghoff J, Eszlinger M, Jaeschke H, Mueller S, Bircan R, Gozu H, et al. Shared sporadic and somatic thyrotropin receptor mutations display more active in vitro activities than familial thyrotropin receptor mutations. Thyroid (2011) 21:221–9. doi: 10.1089/thy.2010.0312
37. Lueblinghoff J, Mueller S, Sontheimer J, Paschke R. Lack of consistent association of thyrotropin receptor mutations in vitro activity with the clinical course of patients with sporadic non-autoimmune hyperthyroidism. J Endocrinol Invest. (2010) 33:228–33. doi: 10.1007/BF03345784
38. Kosugi S, Hai N, Okamoto H, Sugawa H, Mori T. A novel activating mutation in the thyrotropin receptor gene in an autonomously functioning thyroid nodule developed by a Japanese patient. Eur J Endocrinol. (2000) 143:471–7. doi: 10.1530/eje.0.1430471
39. Nanba K, Usui T, Minamiguchi S, Mori Y, Watanabe Y, Honda K, et al. Two rare TSH receptor amino acid substitutions in toxic thyroid adenomas. Endocr J. (2012) 59:13–9. doi: 10.1507/endocrj.EJ11-0202
40. Vicchio TM, Giovinazzo S, Certo R, Cucinotta M, Micali C, Baldari S, et al. Lack of association between autonomously functioning thyroid nodules and germline polymorphisms of the thyrotropin receptor and Galphas genes in a mild to moderate iodine-deficient Caucasian population. J Endocrinol Invest. (2014) 37:625–30. doi: 10.1007/s40618-014-0081-x
41. Krohn K, Fuhrer D, Bayer Y, Eszlinger M, Brauer V, Neumann S, et al. Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev. (2005) 26:504–24. doi: 10.1210/er.2004-0005
42. Nishihara E, Amino N, Maekawa K, Yoshida H, Ito M, Kubota S, et al. Prevalence of TSH receptor and Gsalpha mutations in 45 autonomously functioning thyroid nodules in Japan. Endocr J. (2009) 56:791–8. doi: 10.1507/endocrj.K09E-073
43. Georgopoulos NA, Sykiotis GP, Sgourou A, Papachatzopoulou A, Markou KB, Kyriazopoulou V, et al. Autonomously functioning thyroid nodules in a former iodine-deficient area commonly harbor gain-of-function mutations in the thyrotropin signaling pathway. Eur J Endocrinol. (2003) 149:287–92. doi: 10.1530/eje.0.1490287
44. Palos-Paz F, Perez-Guerra O, Cameselle-Teijeiro J, Rueda-Chimeno C, Barreiro-Morandeira F, Lado-Abeal JG, et al. Prevalence of mutations in TSHR, GNAS, PRKAR1A and RAS genes in a large series of toxic thyroid adenomas from Galicia, an iodine-deficient area in NW Spain. Eur J Endocrinol. (2008) 159:623–31. doi: 10.1530/EJE-08-0313
45. Corvilain B, Van Sande J, Dumont JE, Bourdoux P, Ermans AM. Autonomy in endemic goiter. Thyroid (1998) 8:107–13.
46. Lee SY, Rhee CM, Leung AM, Braverman LE, Brent GA, Pearce EN. A review: radiographic iodinated contrast media-induced thyroid dysfunction. J Clin Endocrinol Metab. (2015) 100:376–83. doi: 10.1210/jc.2014-3292
47. Celano M, Sponziello M, Tallini G, Maggisano V, Bruno R, Dima M, et al. Increased expression of pro-angiogenic factors and vascularization in thyroid hyperfunctioning adenomas with and without TSH receptor activating mutations. Endocrine (2013) 43:147–53. doi: 10.1007/s12020-012-9747-3
48. Cetani F, Tonacchera M, Pinchera A, Barsacchi R, Basolo F, Miccoli P, et al. Genetic analysis of the TSH receptor gene in differentiated human thyroid carcinomas. J Endocrinol Invest. (1999) 22:273–8. doi: 10.1007/BF03343556
49. Ludgate M, Gire V, Crisp M, Ajjan R, Weetman A, Ivan M, et al. Contrasting effects of activating mutations of GalphaS and the thyrotropin receptor on proliferation and differentiation of thyroid follicular cells. Oncogene (1999) 18:4798–807. doi: 10.1038/sj.onc.1202864
50. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. (2006) 354:2783–93. doi: 10.1056/NEJMoa054022
51. Gustavsson B, Eklof C, Westermark K, Westermark B, Heldin NE. Functional analysis of a variant of the thyrotropin receptor gene in a family with Graves' disease. Mol Cell Endocrinol. (1995) 111:167–73.
Keywords: thyroid nodule, genetic diagnosis, fine needle biopsy, TSHR, NIS, genetic alterations
Citation: Guan H, Matonis D, Toraldo G and Lee SL (2018) Clinical Significance of Thyroid-Stimulating Hormone Receptor Gene Mutations and/or Sodium-Iodine Symporter Gene Overexpression in Indeterminate Thyroid Fine Needle Biopsies. Front. Endocrinol. 9:566. doi: 10.3389/fendo.2018.00566
Received: 15 June 2018; Accepted: 05 September 2018;
Published: 25 September 2018.
Edited by:
Susanne Neumann, National Institutes of Health (NIH), United StatesReviewed by:
Eijun Nishihara, Kuma Hospital, JapanRocco Bruno, Independent Researcher, Matera, Italy
Copyright © 2018 Guan, Matonis, Toraldo and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haixia Guan, hxguan@vip.126.com
Stephanie L. Lee, stlee@bmc.org
 Haixia Guan
Haixia Guan Danielle Matonis2
Danielle Matonis2 Stephanie L. Lee
Stephanie L. Lee