Multiple Ornaments—Multiple Signaling Functions? The Importance of Song and UV Plumage Coloration in Female Superb Fairy-wrens (Malurus cyaneus)
- 1Department of Integrative Biology and Evolution, Konrad Lorenz Institute of Ethology, University of Veterinary Medicine, Vienna, Vienna, Austria
- 2School of Biological Sciences, Flinders University, Adelaide, SA, Australia
- 3Department of Biology, University of Padova, Padova, Italy
Showy ornaments are considered as outcomes of sexual selection processes. They provide a “badge of status” to impress conspecific rivals or potential mating partners. Single ornaments may signal attractiveness or individual quality, yet many species display multiple ornaments. There are several hypotheses that explain the existence of multiple ornaments, suggesting that different ornaments serve as different information sources. They may provide either additive or redundant information on the same quality traits, or are simply evolutionary leftovers with no further relevant information. Although, females of many species display elaborated traits, most studies regarding multiple ornaments focus on males. However, given that in many species females also display multiple ornaments, the question about their functional significance arises. To understand the existence of female multiple ornaments we investigated ornamental features of female Superb Fairy-wrens (Malurus cyaneus), focusing on song and variation in plumage characteristics. Female Superb Fairy-wrens produce complex solo songs, for territorial defense, and have bright blue tail feathers. We examined the relationships between song and plumage coloration characteristics in relation to female quality parameters to investigate whether, and to what extent existing hypotheses on multiple ornaments in males may also apply to females. Based on song recordings and spectrometric measurements of UV-coloration of tail feathers, we derived a series of different song and plumage parameters. Our results indicate interrelationships between the song length (total number of elements in female song) and female body size, but not UV-coloration. Interestingly, song complexity (number of different elements in female song) did not correlate with morphological parameters, UV-Chroma and song length, respectively. This suggests that (i) song and plumage characteristics evolved independently and (ii) even within one trait, namely song, multiple signaling should be considered. To our knowledge, this is the first study investigating multiple traits in female songbirds, raising the idea that multiple signaling of sexually selected traits is not restricted to males only.
Introduction
Showy plumage characteristics and elaborated song in passerines are known to be typical male traits, shaped by sexual selection (Andersson, 1994). There is strong evidence that both traits signal individual quality and are therefore involved in mate choice, as well as competitive interactions (Burley, 1986; Searcy and Andersson, 1986; Andersson, 1994; Nowicki and Searcy, 2004; Hoi and Griggio, 2008). However, the expression of colorful plumage and elaborated song is not restricted to males; females can also display these traits (Webb et al., 2016). As previously stated by Langmore (1998) and Amundsen (2000) this phenomenon has largely been ignored until recently and these traits were either regarded as being the consequence of genetic correlation with male ornamentation, functionless, or a result of physiological abnormalities (Lande, 1980; Amundsen, 2000). However, a growing number of studies focused on the evolution of female ornamentation, including plumage and song characteristics (Amundsen et al., 1997; Langmore, 1997, 1998; Amundsen, 2000; Garamszegi et al., 2007; Doutrelant et al., 2008; Mahr et al., 2012; Tobias et al., 2012; Webb et al., 2016).
Several studies have revealed that female choice can be based on several different traits that signal male quality, such as morphological and behavioral traits (Burley, 1981; Johnstone, 1996; Lozano, 2009; Dolnik and Hoi, 2010; Hoi and Griggio, 2012). Multiple traits may (i) act as amplifiers by offering the same information, (ii) have an additive effect whereby the information of several traits complement each other or, (iii) provide different information e.g., about different qualities of the bearer (Burley, 1981; Grafen, 1990; Zuk et al., 1990, 1992; Johnstone, 1995, 1996; Marchetti, 1998; Rivera-Gutierrez et al., 2010). For example bird song and plumage are traits that can signal the same or different information and both traits are driven by sexual selection in males and females (Lande, 1980; Andersson, 1994; Amundsen et al., 1997; Langmore, 1998; Amundsen, 2000; Ball and MacDougall-Shackleton, 2001; Garamszegi et al., 2007; Hegyi et al., 2007, 2008; Cardoso and Hu, 2011). However, the interaction between both traits has hardly been investigated in females (Garamszegi et al., 2007; Webb et al., 2016).
Although, song and plumage traits may carry the same information, these traits can act on different time and spatial scales (Taff et al., 2012). Song usually acts as a long distance signal whereas plumage ornaments act as a short distance signal. When both signals carry the same information, one would predict a positive relationship in the expression of the traits. Alternatively, the expression of both ornamental features might underlie different mechanisms and require different preconditions to maintain an honest signaling function. Furthermore, different production costs may arise, which may consequently represent different qualities. In this case, one would predict that trade-offs between both traits could result in either negative correlations between traits or independent development of different traits, like song and plumage characteristics. However, to our knowledge this trade-off has only been investigated on a phylogenetic scale (Badyaev et al., 2002; Mason et al., 2014; Soma and Garamszegi, 2015).
Whether this also applies to females has, to our knowledge, only been investigated in two comparative studies, focusing on song and plumage development in passerine species. Garamszegi et al. (2007) suggested that singing behavior often occurs in the presence of carotenoid based ornamentation, which is supported by very recent findings from Webb et al. (2016). This positive association might indicate that both traits are generally used in a similar or the same functional context and hence carry the same information content. Nevertheless, only a few case studies examined the interaction between both traits within breeding populations in male songbirds (Møller et al., 1998; Chiver et al., 2008; Taff et al., 2012), and to our knowledge, no study on female birds exists so far. In this study we used the female Superb Fairy-wren (Malurus cyaneus) to examine (i) the signaling function of song characteristics and plumage coloration and (ii) the interaction between these two female ornamental features. To determine whether these ornaments reflect female quality we used female body size and body condition as covariates.
The Superb Fairy-wren is an ideal model species to answer our questions because both males and females sing solo chatter songs year-round for territorial defense (Cooney and Cockburn, 1995; Cain and Langmore, 2015), and the structure and complexity of female chatter song is similar to male chatter song (Kleindorfer et al., 2013). Mate attraction may be a secondary function of male chatter song (Dalziell and Cockburn, 2008), but to our knowledge there is no study investigating whether this function applies to female song. In contrast to song, Superb Fairy-wrens have a strong sexual plumage dichromatism. Whereas males have bright blue plumage, females are more cryptic, displaying only an orange lore and a blue tail that reflects in the UV range (own data represented in the Supplementary Material). Maluridae are sensitive to UV and females frequently wave their tail during foraging and social interactions (own unpublished data). This raises the question whether the UV reflecting tail of females is a sexually selected trait (Ödeen et al., 2012).
Previous studies demonstrated a decrease of UV reflectance in worn feathers and from dust accumulation (Örnborg et al., 2002; Zampiga et al., 2004; Griggio et al., 2010, 2011). There is a trade-off between the removal of ectoparasites and dirt from feathers, preening and activities like foraging or increased vigilance against predators (Redpath, 1988; Cucco and Malacarne, 1997; Shawkey et al., 2003; Kapun et al., 2011; Moreno-Rueda and Hoi, 2012). Interestingly there is evidence that similar mechanisms also apply to song features, in particular song rate, which is regarded as a highly variable trait depending on current physiological condition and time of the reproductive cycle (Gil and Gahr, 2002). Therefore, both plumage maintenance and singing behavior force individuals into a trade-off that individuals in poorer condition cannot afford, being forced to invest either more in one or equally, but less in both traits (Andersson et al., 2002).
In many songbird species, song complexity is regarded as stable over the year. The ability to produce complex songs can be an honest signal of quality, because during the development of the neural song system, the expression of neuronal structures and development of the syringeal muscles can be affected by early developmental stress such as under-nourishment (Nowicki et al., 2000; Spencer et al., 2003; Buchanan et al., 2004; Nowicki and Searcy, 2004). Hence, in contrast to plumage characteristics, song complexity can be regarded as less sensitive toward the change of individual condition after the crystallization and determination of singing behavior (Gil and Gahr, 2002).
Our study focuses on the relationship between song complexity and the number of elements females are using (rather than song rate) and UV-reflectance in the blue tail-feathers of female Superb Fairy-wrens. Given both traits may provide different information the question arises whether females use multiple traits to signal quality and condition to male and female conspecifics. Studies investigating relationships between multiple traits within populations are rare and to our knowledge this is the first study focusing on the relationship between features of song, plumage coloration, and morphological traits in a female passerine.
Methods
Study Sites
The study was carried out during the breeding season between September and November 2012 and 2013 at three study sites on Kangaroo Island: Flinders Chase National Park (35°54′S, 136°47′E), Vivonne Bay Conservation Park (36°00′S, 137°09′E), and Kelly Hill Conservation Park (35°97′S, 136°90′E) and at two study sites on the mainland in South Australia (SA): Cleland Wildlife Conservation Park (35°05′S, 138°41′E) and Newlandhead Conservation Park (35°37′S, 138°29′E). All study sites and territories were chosen on the basis of long term monitoring of Superb Fairy-wren populations, conducted by the BirdLab at Flinders University (Colombelli-Négrel et al., 2010; Kleindorfer et al., 2013).
General Methods
All birds were caught with mist-nets using conspecific playback stimuli and banded with numbered aluminum rings provided by Australian Bird and Bat Banding Scheme (ABBBS) and a unique combination of darvic color rings. Standard measurements of the flattened wing chord length and tail length (to the nearest 0.5 mm) were taken with a ruler, whereas bill length was measured with a caliper (peak to skull, to the nearest 0.1 mm). Body mass was recorded to the nearest 0.1 g.
The research was approved by the Animal Welfare Committee of Flinders University (permit numbers E312 and E386). Permit to undertake scientific research in SA was granted by SA Department of Environment, Water and Natural resources (permit number Z24699-9). All birds were banded under permit number 2601 from the Australian Bird and Bat Banding Scheme.
To assess whether song complexity or plumage coloration is related to female quality, we used female size and body-condition as a determinant of female intrinsic quality and conducted a principal component analyses on these traits (detailed descriptions are attached in the Supplementary Material). Body condition was determined by using residuals of body mass not explained by size (tarsus length; detailed descriptions are attached in the Supplementary Material).
Only fertile females were included in the analyses to control for variation in singing behavior due to reproductive state. Fertility status was verified according to the following three parameters: (i) development of the brood patch (not fully developed), (ii) nest building status (females were considered fertile until the first egg was laid), and (iii) sexual behavior patterns (copulatory behavior, male display, female solicitation behavior; Mulder, 1992 in Cooney and Cockburn, 1995).
Song Recordings and Analyses
Solo songs of color-banded birds occur naturally between 08:00 and 12:00 h (after the dawn chorus) and were recorded from a distance between 5 and 15 m using a parabolic microphone (Telinga Microphones, Sweden) connected to a portable Sound Devices 722 digital audio recorder (Sound Devices LCC, U.S.A). All sound files were recorded as broadcast wave files (24 bit 48 kHz).
Recordings were transcribed to an Apple MacPro (Apple Corporation, U.S.A) and edited with Amadeus Pro 2.1.2 (Hairersoft Inc, Switzerland). Spectrograms were created using Raven 1.5 on the Hann algorithm display type (filter bandwidth 270 Hz, size 256 samples, time grid overlap 50%, grid resolution 2.67 ms, 188 Hz, DFT 256 samples). Only songs that could be confidently assigned to observed color-banded females were analyzed. In total, 82 songs from 28 females were analyzed. For each song, we measured the total number of elements per song (“song length”), and the number of different elements per song (“song complexity”). We define a song as a complex vocalization composed of several different element types (as described by Langmore and Mulder, 1992), and defined an element in the song as a single, continuous trace on a spectrogram. We categorized the different element types according to previously classified element types (A, F, O, P, Q, R, T, U, V, W) developed by Langmore and Mulder (1992), Colombelli-Négrel et al. (2010), Kleindorfer et al. (2013), and Evans and Kleindorfer (2016), and newly identified element types (FL, H, K, L, Z, ZN). For the analysis we determined the element frequency per song as (i) the total number of elements per song (we refer to this variable as “song length”), and (ii) the number of different element types per song (we refer to this variable as “song complexity”).
Spectrometry
We measured the tail coloration of females (N = 41), using a JAZ-2000 spectrophotometer and a Xenon-pulsed light source, connected through a bifurcated fiber-optic probe (Ocean Optics, Eerbek, Netherlands). To exclude disturbance by outer light sources and to ensure a standardized distance and angle (90°), a black rubber cylinder was fitted to the top of the probe. Before each measurement the spectrophotometer was recalibrated using a standard white (Avantes, Eerbek, Netherlands); for calibration of black the probe was removed from the light source and the cap of the plug closed (Mahr et al., 2012). Standard descriptors of reflectance spectra were used for quantification of colors. Measurements were taken from five areas on the tail feathers. Calculations were carried out for reflectance in the 320–700 nm range, which is regarded as visual spectrum of most passerine species (Hill and McGraw, 2006). To quantify the UV-reflectance of the blue tail we chose a commonly used variable, namely UV-Chroma (Johnsen et al., 2005; Griggio et al., 2010; Mahr et al., 2012), which is defined as proportion of UV-reflectance on total reflectance (UV:R320–R415/R320–R700; Hill and McGraw, 2006).
Statistical Analyses
To test for the relationships between song length and morphological parameters and plumage characteristics we applied a General Linear Mixed Effects Model (GLMM). Song complexity was analyzed using a Generalized Linear Mixed Effects Model (GZLMM) with a Poisson-distribution as model residuals did not achieve normal distribution even after transformation. Both initial models included UV-Chroma, size, and condition as covariates. As Kangaroo Island and mainland populations are considered to represent different subspecies, we included study site (“location”) in all the initial models as a fixed factor to assess local variation in morphology and ornament expression (Dudaniec et al., 2011; Kleindorfer et al., 2013). Also, all the initial models included the interaction between UV-Chroma and location as well as body-size and location because we aimed to test for differences between the populations in regard to UV-Chroma and body-size. Female ID was included as random factor to control for non-independence of multiple measurements from the same female. We had to exclude six females from the analyses since there was not sufficient data available.
The relation between morphological parameters and plumage characteristics was tested separately, due to a difference in the sample size. Analyses were carried out using a General Linear Model (GLM). This GLM included the factor location and the covariates condition, size and the interaction of condition and size. UV-Chroma and condition can show variation during the breeding season, therefore we also included capture month into the GLM. Since the analyses revealed no significant effects of capture date on UV-Chroma and condition, this factor was not included in the GLMM and GZLMM.
We tested for a correlation between song length and complexity using a Spearman's rank correlation test. Song length and complexity were not correlated (Spearman's rank correlation: ρ = 0.14, S = 0.45, p = 0.24), thus we treated these variables independently.
All statistical analyses were performed using “R” (version 2.14.1; R Development Core Team, 2011). We implemented linear mixed effects models using the “lme” function of the “nlme” package. All models were conducted using stepwise forward and backward introduction of terms. Beginning with the interactions, non-significant terms were step by step eliminated from the model. Each eliminated term was re-entered in the final model to obtain statistics (Grafen and Hails, 2002; Engqvist, 2005). In addition to model selection based on p-values we performed model averaging using AIC to assess comparability and reliability of both methods. AIC model averaging was implemented using the “model.avg” function of the “MuMIn” package. No differences in the significant results became apparent, and details on the results from model averaging based on AIC-values can be found in the Supplemental Material.
Results
The GLM revealed no significant differences in UV coloration between mainland and island populations [mainland: N = 18, island: N = 23; F(1, 40) = 2.29, p = 0.13], furthermore no significant effect of month of capture was found on plumage color [F(1, 37) = 0.87, p = 0.46]. Female size was not related to plumage [F(1, 39) = 0.72, p = 0.40], and no relation between UV coloration and female condition was detectable [F(1, 39) = 0.18, p = 0.67]. Also, there were no significant interactions between location and body size [F(1, 39) = 0.15, p = 0.70] or month and condition [F(1, 39) = 0.84, p = 0.48].
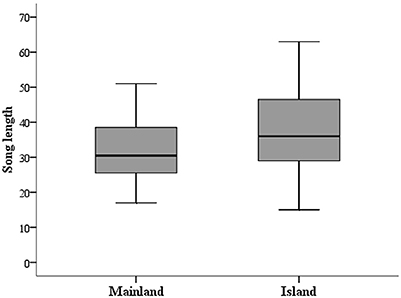
Female song length was neither related to her condition [Mainland: N = 13, Island: N = 9; GLMM: F(1, 17) = 1.93, p = 0.18] nor to her UV-Chroma [GLMM: F(1, 19) = 0.07, p = 0.79]. Also, the interaction of both variables turned out to be non-significant [GLMM: F(1, 18) = 0.06, p = 0.95]. Interestingly though, we found that study site predicted female song length as females from Kangaroo Island produced significantly longer songs compared to females from mainland populations [GLMM: F(1, 20) = 10.79, p < 0.01; Figure 1]. Also, we found a significant interaction effect of study site and female size on song length [GLMM: F(1, 20) = 5.66, p = 0.03]: Larger females produce longer songs compared to smaller ones, though this effect is only evident on Kangaroo Island (see Figure 2). We found no significant main effect of female size on song length [GLMM: F(1, 20) = 0.81, p = 0.38].
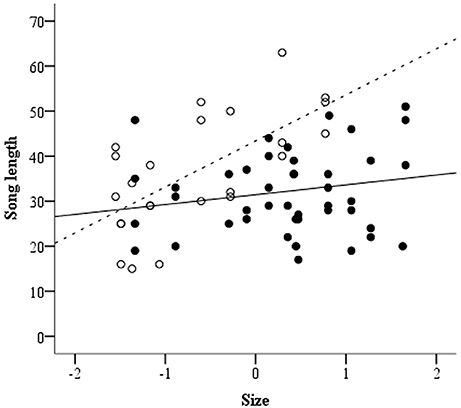
Figure 2. Relationship between female size and song length in mainland (black circles and solid line; R2 = 0.04) and island (white circles and dashed line; R2 = 0.44) populations.
In contrast to song length, song complexity did not differ significantly between study sites (mainland: N = 13, island: N = 9; GZLMM: β ± SE = −0.19 ± 0.11, z = −1.69, p = 0.09), though this effect was only marginally non-significant. Female size (GZLMM: β ± SE = −0.08 ± 0.05, z = 1.58, p = 0.11) and the interaction between female size and study site (GZLMM: β ± SE = 0.03 ± 0.13, z = 0.21, p = 0.83) turned out to have no significant relationship with song complexity. Also, female condition (GZLMM: β ± SE = −0.003 ± 0.01, z = −0.26, p = 0.79), UV-Chroma (GZLMM: β ± SE = −0.80 ± 1.04, z = −0.77, p = 0.44) and the interaction between UV-Chroma and study site (GZLMM: β ± SE = 1.44 ± 3.37, z = 0.42, p = 0.67) showed no significant relationship with song complexity.
Discussion
Our results show no relationship between plumage ornamentation and song characteristics in female Superb Fairy-wrens, but we revealed a positive relationship between the song length (total number of elements females produce per song) and body-size in females of the Kangaroo Island sub-species. Thus, female Superb Fairy-wrens that sing songs composed of more elements are bigger than females with shorter songs.
In our study populations, the average number of elements varies dramatically between females (between 16 and 50 elements per song). Some females produce more than twice the number of elements than others, which reveals strong individual differences in song strophe length. Consequently for fertile female Superb Fairy-wrens song length may possibly act as a signal for conspecifics to indicate quality. Our results also suggest that songs produced by females of the Kangaroo Island subspecies are significantly longer. These results are in line with previous findings by Kleindorfer et al. (2013), possibly indicating selection processes favoring the production of longer songs within the island population.
Hence, given that size can be an indicator of condition during early development, our results imply that song might act as an honest signal and underlies sexual selection processes in female Superb Fairy-wrens. The idea that female song signals individual quality is supported by earlier studies in Superb Fairy-wrens and New Zealand bellbirds (Anthornis melanura), indicating that female song performance (song rate and song complexity) predicts reproductive success (Cain et al., 2015; Brunton et al., 2016).
There are several explanations for why song length in female Superb Fairy-wrens could be an honest signal (Martin-Vivaldi et al., 1998; Farrell et al., 2012; Ferrer et al., 2015). First the production of longer songs is energetically demanding and requires certain physiological preconditions, since it forces females into a trade-off between allocating energy resources toward singing or other activities (Gil and Gahr, 2002). Secondly, the primary function of female song in Superb Fairy-wrens is suggested to be resource defense (Cooney and Cockburn, 1995; Cain and Langmore, 2015). In this context song length might be an indicator for the ability of an individual to defend resources. Finally, very recent findings (Kleindorfer et al., 2016), support the idea, that singing behavior in female Superb Fairy-wrens can also be costly in terms of increased nest-predation. Even though the study by Kleindorfer et al. (2016) refers to song rate rather than song length, one might expect that females producing longer songs may also face higher predation risk by exposing themselves toward predators.
Interestingly, the relationship between size and song length only applies to females from the Kangaroo Island subspecies, but not to females from the mainland populations. However, due to the low sample size, this result has to be treated with caution. A possible explanation for this result could be that Superb Fairy-wrens are in general considered to be long-lived and maintain long-term territories over several years. Stable territories like on the mainland may imply a reduced necessity of intense territorial behavior. In contrast some Superb Fairy-wren populations on Kangaroo Island have been affected by severe bushfires in 2007 (Peace et al., 2011). Within the last years the population started to recover and the number of breeding pairs is increasing in this region. One might assume that individuals face increasing competition from new intruders. Therefore, more competitive individuals, with the ability to maintain larger territories and therefore more resources, should be favored by selection processes. Given that song can be perceived over longer distance and indicates body-size, female song length might signal competitive abilities toward neighbors and intruders (Searcy et al., 2008). Therefore, singing behavior might primarily be of importance to continuously communicate dominance and prevent actual intrusion. Given that, due to natural reestablishment of breeding populations, Kangaroo Island birds might face more frequent encounters with intruders and investment into signals indicating quality might be beneficial to retain breeding sites. Furthermore, this idea is in line with previous findings from Cooney and Cockburn (1995), who demonstrated that female song-rate increased when territories were newly established.
Female song length might also serve as a quality indicator for male conspecifics (Amundsen, 2000). Even though clutch size might not be affected by body size, as females lay a maximum of three eggs per clutch in our population (own observation), choosing bigger females might provide other direct and indirect benefits to males. Size parameters can affect performance in foraging and territorial defense and, as previously mentioned, size can act as indicator for better condition during early development and might signal good genetic quality (Johnson, 1987; Amundsen et al., 1997; Amundsen, 2000). However, given that Superb Fairy-wrens are known to have the highest number of extra pair fertilizations within passerines (Double et al., 1997) and song acts as a signal over long distances, females might also signal quality to possible extra-pair mating partners. The recruitment of extra-pair fertilizations might in turn enhance female reproductive success by increasing genetic variability in the offspring (Andersson, 1994).
Our analyses further reveal that song complexity and song length are not correlated, raising the question of whether both song features signal different quality traits and carry multiple signaling functions. However, song complexity, which has been shown to be an important male song feature for many species (Gil and Gahr, 2002), seems to play only a minor role for female Superb Fairy-wrens. In our study, female song complexity does not reflect female condition or size, nor, in contrast to song length, varies significantly between populations, which has already been shown by previous studies (Dudaniec et al., 2011; Kleindorfer et al., 2013). Furthermore, female song complexity is not related to UV-reflectance of the tail feathers. Also the low variation in song complexity (between three and eight different elements) in comparison to the average number of elements between individual females points toward an inferior role in sexual selection. In previous studies it has been suggested that in some species male repertoire size (e.g., number of elements or songs males produce) does not predict pairing success and therefore plays a minor role in selection processes (Catchpole, 1986; Gil and Gahr, 2002; Byers and Kroodsma, 2009). This might also apply to female song complexity in Superb Fairy-wrens. Nevertheless, it has to be considered that female song complexity reflects quality parameters not recorded in this study.
Whereas a relationship between ornamental features and song performance has been found in interspecific comparisons for female songbirds (Garamszegi et al., 2007; Webb et al., 2016), our results indicate that there is no relationship between UV-Chroma and song features in female Superb Fairy-wrens. This indicates that both traits have evolved independently, rather than co-evolved.
However, it has to be considered that both studies did not examine relationships between song performance and plumage coloration within populations and Garamszegi et al. (2007) focused on carotenoid based plumage features (Garamszegi et al., 2007; Webb et al., 2016). Unlike carotenoid based coloration, UV-coloration is due to melanin based coloration and keratin structure (Prum, 2006). Since these two types of plumage ornaments underlie different physiological mechanisms, they may underlie different selection processes.
UV-Chroma is known to reflect condition of individuals, since the maintenance of UV-reflectance is time consuming and might further result in a trade-off between preening and e.g., parental activities or vigilance behavior (Redpath, 1988; Cucco and Malacarne, 1997; Shawkey et al., 2003). In this context, we expected a correlation between UV-Chroma and condition, but this particular prediction was not supported by our results. One possible explanation might be that the UV-coloration of the plumage is determined by the condition during molt, as shown in male Superb Fairy-wrens (Mulder and Magrath, 1994).
In summary, this is one of the first studies investigating multiple signals in a female songbird, suggesting that plumage features and song performance might underlie different selection processes. Our study revealed that song length is related to a trait reflecting quality and supports the idea of song as a sexually selected trait in female passerines (Cain et al., 2015; Kleindorfer et al., 2016). Our data provides new information on female song and plumage ornaments and more importantly, it extends our understanding of singing behavior in female songbirds.
Author Contributions
KM, HH, and MG designed the study; KM and CE conducted the field work and the data collection; KM, HH, KT, and CE performed the statistical analyses of the data; KM, HH, and MG wrote the manuscript with contributions from KT and CE.
Funding
The project was funded by the Nature Foundation of South Australia, and the ANZ Trustees Foundation and Holsworth Wildlife Research Endowment Grant. Furthermore, we would like to thank the University of Vienna (Research Scholarship of the University of Vienna), the ÖAD (Marietta-Blau Scholarship) and the Government of Lower Austria (NÖ-TOP Scholarships) for financial support.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Cleland Wildlife Park for access to the study site. We thank volunteers and students of the BirdLab at Flinders University for field assistance. We are grateful to Prof. Sonia Kleindorfer, who enabled us to conduct this study through her generous support, inspiring discussions and valuable comments on the manuscript. We kindly thank the anonymous referees for valuable comments on the manuscript, as well as Prof. Hans-Christoph Winkler, Dr. Diane Colombelli-Negrell, and Dr. Jeremy Robertson for discussions on the topic.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fevo.2016.00043
References
Amundsen, T. (2000). Why are female birds ornamented? Trends Ecol. Evol. 15, 149–155. doi: 10.1016/S0169-5347(99)01800-5
Amundsen, T., Forsgren, E., and Hansen, L. T. T. (1997). On the function of female ornaments: male Bluethroats prefer colourful females. Proc. R. Soc. B 264, 1579–1586. doi: 10.1098/rspb.1997.0220
Andersson, S., Pryke, S. R., Örnborg, J., Lawes, M. J., and Andersson, M. (2002). Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a Widowbird. Am. Nat. 160, 683–691. doi: 10.1086/342817
Badyaev, A. V., Hill, G. E., and Weckwort, B. V. (2002). Species divergence in sexually selected traits: increase in song elaboration is related to decrease in plumage ornamentation in finches. Evolution 56, 412–419. doi: 10.1111/j.0014-3820.2002.tb01350.x
Ball, G. F., and MacDougall-Shackleton, S. A. (2001). Sex differences in songbirds 25 years later: what have we learned and where do we go? Microsci. Res. Tech. 54, 327–334. doi: 10.1002/jemt.1146
Brunton, D. H., Roper, M. M., and Harmer, A. M. T. (2016). Female song rate and structure predict reproductive success in a socially monogamous bird. Front. Ecol. Evol. 4:13. doi: 10.3389/fevo.2016.00013
Buchanan, K. L., Leitner, S., Spencer, K. A., Goldsmith, A. R., and Catchpole, C. K. (2004). Developmental stress selectively affects the song control nucleus HVC in the Zebra finch. Proc. R. Soc. Lond. B 271, 2381–2386. doi: 10.1098/rspb.2004.2874
Burley, N. (1981). Mate choice by multiple criteria in a monogamous species. Am. Nat. 117, 515–528. doi: 10.1086/283732
Burley, N. (1986). Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 127, 415–445. doi: 10.1086/284493
Byers, B. E., and Kroodsma, D. E. (2009). Female mate choice and songbird song repertoires. Anim. Behav. 77, 13–22. doi: 10.1016/j.anbehav.2008.10.003
Cain, K. E., Cockburn, A., and Langmore, N. E. (2015). Female song rates in response to simulated intruder are positively related to reproductive success. Front. Ecol. Evol. 3:119. doi: 10.3389/fevo.2015.00119
Cain, K. E., and Langmore, N. E. (2015). Female and male song rates across breeding stage: testing for sexual and nonsexual functions of female song. Anim. Behav. 109, 65–71. doi: 10.1016/j.anbehav.2015.07.034
Cardoso, G. C., and Hu, Y. (2011). Birdsong performance and the evolution of simple (rather than elaborate) sexual signals. Am. Nat. 178, 679–686. doi: 10.1086/662160
Catchpole, C. K. (1986). Song repertoires and reproductive success in the great reed warbler Acrocephalus arundinaceus. Behav. Ecol. Sociobiol. 19, 439–445. doi: 10.1007/BF00300547
Chiver, I., Stutchbury, B. J. M., and Morton, E. S. (2008). Do male plumage and song characteristics influence female off-territory forays and paternity in the hooded warbler? Behav. Ecol. Sociobiol. 62, 1981–1990. doi: 10.1007/s00265-008-0629-x
Colombelli-Négrel, D., Robertson, J., and Kleindorfer, S. (2010). Nestling presence affects the anti-predator response of adult superb fairy-wrens (Malurus cyaneus). Acta Ethol. 13, 69–74. doi: 10.1007/s10211-010-0072-7
Cooney, R., and Cockburn, A. (1995). Territorial defence is the major function of female song in the superb fairy-wren, Malurus cyaneus. Anim. Behav. 49, 1635–1647. doi: 10.1016/0003-3472(95)90086-1
Cucco, M., and Malacarne, G. (1997). The effect of supplemental food on time budget and body condition in the Black Redstart Phoenicurus ochruros. Ardea 85, 211–221.
Dalziell, A. H., and Cockburn, A. (2008). Dawn song in superb fairy-wrens: a bird that seeks extrapair copulations during the C. Anim. Behav. 75, 489–500. doi: 10.1016/j.anbehav.2007.05.014
Dolnik, O., and Hoi, H. (2010). Honest signalling, dominance hierarchies and body condition in House Sparrows Passer domesticus (Aves: Passeriformes) during acute coccidiosis. Biol. J. Linn. Soc. 99, 718–726. doi: 10.1111/j.1095-8312.2010.01370.x
Double, M. C., Dawson, D., Burke, T., and Cockburn, A. (1997). Finding the fathers in the least faithful bird: a microsatellite-based genotyping system for the superb fairy-wren Malurus cyaneus. Mol. Ecol. 6, 691–693. doi: 10.1046/j.1365-294x.1997.00228.x
Doutrelant, C., Gregoire, A., Grnac, N., Gomez, D., Lambrechts, M. M., and Perret, P. (2008). Female coloration indicates female reproductive capacity in blue tits. J. Evol. Biol. 21, 226–233. doi: 10.1111/j.14209101.2007.01451.x
Dudaniec, R. Y., Schlotfeldt, B. E., Bertozzi, T., Donnellan, S. C., and Kleindorfer, S. (2011). Genetic and morphological divergence in island and mainland birds: informing conservation priorities. Biol. Conserv. 144, 2902–2912. doi: 10.1016/j.biocon.2011.08.007
Engqvist, L. (2005). The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim. Behav. 70, 967–971. doi: 10.1016/j.anbehav.2005.01.016
Evans, C., and Kleindorfer, S. (2016). Superb fairy-wren (Malurus cyaneus) sons daughters acquire song elements of mothers social fathers. Front. Ecol. Evol. 4:9. doi: 10.3389/fevo.2016.00009
Farrell, T. M., Weaver, K., An, Y. S., and MacDougall-Shackleton, S. A. (2012). Song bout length is indicative of spatial learning in European starlings. Behav. Ecol. 23, 101–111. doi: 10.1093/beheco/arr162
Ferrer, E. S., Garcia-Navas, V., Bueno-Enciso, J., Sanz, J. J., and Ortego, J. (2015). Multiple sexual ornaments signal heterozygosity in male blue tits. Biol. J. Linn. Soc. 115, 362–375. doi: 10.1111/bij.12513
Garamszegi, L. Z., Pavlova, D. Z., Eens, M., and Møller, A. P. (2007). The evolution of song in female birds in Europe. Behav. Ecol. 18, 86–96. doi: 10.1093/beheco/arl047
Gil, D., and Gahr, M. (2002). The honesty of bird song: multiple constraints for multiple traits. Trends Ecol. Evol. 17, 133–141. doi: 10.1016/S0169-5347(02)02410-2
Grafen, A. (1990). Biological signals as handicaps. J. Theor. Biol. 144, 517–546. doi: 10.1016/S0022-5193(05)80088-8
Grafen, A., and Hails, R. (2002). Modern Statistics for the Life Sciences. Oxford; New York, NY: Oxford University Press.
Griggio, M., Hoi, H., and Pilastro, A. (2010). Plumage maintenance affects ultraviolet colour and female preference in the Budgerigar. Behav. Process. 84, 739–744. doi: 10.1016/j.beproc.2010.05.003
Griggio, M., Serra, L., and Pilastro, A. (2011). The possible effect of dirtiness on structurally based ultraviolet plumage. Ital. J. Zool. 78, 90–95. doi: 10.1080/11250003.2010.504238
Hegyi, G., Garamszegi, L. Z., Eens, M., and Török, J. (2008). Female ornamentation and territorial conflicts in collared flycatchers (Ficedula albicollis). Naturwissenschaften 95, 993–996. doi: 10.1007/s00114-008-0408-6
Hegyi, G., Rosivall, B., Szöllősi, E., Hargitai, R., Eens, M., and Török, J. (2007). A role for female ornamentation in the facultatively polygynous mating system of collared flycatchers. Behav. Ecol. 18, 1116–1122. doi: 10.1093/beheco/arm085
Hill, G. E., and McGraw, K. J. (2006). Bird Coloration - Mechanisms and Measurements. Cambridge, MA; London: Harvard University Press.
Hoi, H., and Griggio, M. (2008). Dual utility of a melanin-based ornament in Bearded Tits. Ethology 114, 1094–1100. doi: 10.1111/j.1439-0310.2008.01566.x
Hoi, H., and Griggio, M. (2012). Bearded reedlings adjust their pair-bond behaviour in relation to the sex and attractiveness of unpaired conspecifics. PLoS ONE 7:e32806. doi: 10.1371/journal.pone.0032806
Johnsen, A., Delhey, K., Schlicht, E., Peters, A., and Kempenaers, B. (2005). Male sexual attractiveness and parental effort in blue tits: a test of the differential allocation hypothesis. Anim. Behav. 70, 877–888. doi: 10.1016/j.anbehav.2005.01.005
Johnson, K. (1987). Sexual selection in pinyon jays II: male choice and female-female competition. Anim. Behav. 36, 1048–1053. doi: 10.1016/S0003-3472(88)80064-2
Johnstone, R. A. (1995). Honest advertisment of multiple qualities using multiple signals. J. Theor. Biol. 177, 87–94. doi: 10.1016/S0022-5193(05)80006-2
Johnstone, R. A. (1996). Multiple displays in animal communication: ‘backup signals’ and ‘multiple messages’. Philos. Trans. R. Soc. B 351, 329–338. doi: 10.1098/rstb.1996.0026
Kapun, M., Darolová, A., Krištofik, J., Mahr, K., and Hoi, H. (2011). Distinct colour morphs in nestling European Bee-Eaters Merops apiaster: is there an adaptive value? J. Ornithol. 152, 1001–1005. doi: 10.1007/s10336-011-0688-z
Kleindorfer, S., Evans, C., and Mahr, K. (2016). Female in-nest chatter song increases predation. Biol. Lett. 12:20150513. doi: 10.1098/rsbl.2015.0513
Kleindorfer, S., Evans, C., Mihailova, M., Colombelli-Négrel, D., Hoi, H., Griggio, M., et al. (2013). When subspecies matter: resident superb fairy-wrens (Malurus cyaneus) distinguish the sex and subspecies of intruding birds. Emu 113, 259–269. doi: 10.1071/MU12066
Lande, R. (1980). Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305. doi: 10.2307/2407393
Langmore, N. E. (1997). Song switching in monandrous and polyandrous dunnocks, Prunella modularis. Anim. Behav. 53, 757–766. doi: 10.1006/anbe.1996.0312
Langmore, N. E. (1998). Functions of duet and solo songs of female birds. Trends Ecol. Evol. 13, 136–140. doi: 10.1016/S0169-5347(97)01241-X
Langmore, N. E., and Mulder, R. A. (1992). A novel context for bird song: predator calls prompt male singing in the kleptogamous superb fairy-wren, Malurus cyaneus. Ethology 90, 143–153. doi: 10.1111/j.1439-0310.1992.tb00828.x
Lozano, G. A. (2009). Multiple cues in mate selection: the sexual interference hypothesis. Biosci. Hypotheses 2, 37–42. doi: 10.1016/j.bihy.2008.09.001
Mahr, K., Griggio, M., Granatiero, M., and Hoi, H. (2012). Female attractiveness affects paternal investment: experimental evidence for male differential allocation in blue tits. Front. Zool. 9:14. doi: 10.1186/1742-9994-9-14
Marchetti, K. (1998). The evolution of multiple male traits in the yellow-browed leaf warbler. Anim. Behav. 55, 361–376. doi: 10.1006/anbe.1997.0586
Martin-Vivaldi, M., Palomino, J. J., and Soler, M. (1998). Song structure in the Hoopoe (Upupa epops) - strophe length reflects male condition. J. Ornithol. 139, 287–296. doi: 10.1007/BF01653339
Mason, N. A., Shultz, A. J., and Burns, K. J. (2014). Elaborate visual and acoustic signals evolve independently in a large, phenotypically diverse radiation of songbirds. Proc. R. Soc. B 281:20140967. doi: 10.1098/rspb.2014.0967
Møller, A. P., Saino, N., Taramino, G., Galeotti, P., and Ferrario, S. (1998). Paternity and multiple signaling: effects of a secondary sexual character and song on paternity in the Barn Swallow. Am. Nat. 151, 236–242. doi: 10.1086/286114
Moreno-Rueda, G., and Hoi, H. (2012). Female house sparrows prefer big males with a large white wing bar and fewer feather holes caused by chewing lice. Behav. Ecol. 23, 271–277. doi: 10.1093/beheco/arr182
Mulder, R. (1992). The Evolutionary Ecology of the Mating System of Superb Fairy Wrens. Ph.D. thesis, Australian National University, Canberra.
Mulder, R. A., and Magrath, M. J. L. (1994). Timing of prenuptial molt as a sexually selected indicator of male quality in superb fairy-wrens (Malurus cyaneus). Behav. Ecol. 5, 393–400. doi: 10.1093/beheco/5.4.393
Nowicki, S., Hasselquist, D., Bensch, S., and Peters, S. (2000). Nestling growth and song repertoire sire in great reed warblers: evidence for song learning as an indicator mechanism in mate choice. Proc. R. Soc. Lond. B 267, 2419–2424. doi: 10.1098/rspb.2000.1300
Nowicki, S., and Searcy, W. A. (2004). Song function and the evolution of female preferences. why birds sing, why brains matter. Ann. N.Y. Acad. Sci. 1016, 704–723. doi: 10.1196/annals.1298.012
Ödeen, A., Pruett-Jones, S., Driskell, A. C., Armenta, J. K., and Håstad, O. (2012). Multiple shifts between violet and ultraviolet vision in a family of passerine birds with associated changes in plumage coloration. Proc. R. Soc. B 279, 1269–1276. doi: 10.1098/rspb.2011.1777S
Örnborg, J., Andersson, S., Griffith, S. C., and Sheldon, B. C. (2002). Seasonal changes in a ultraviolet structural colour signal in blue tits, Parus caeruleus. Biol. J Linn. Soc. 76, 237–245. doi: 10.1046/j.1095-8312.2002.00061.x
Peace, M., Mattner, T., and Mills, G. (2011). “The Kangaroo Island bushfires of 2007. A meteorological case study and WRF-fire simulation,” in 19th International Congress on Modelling and Simulation (Perth, WA).
Prum, R. O. (2006). “Anatomy, physics and evolution of avian structure colors,” in Bird Coloration, eds G. E. Hill and K. J. McGraw (Cambridge: Harvard University Press), 295–353.
R Development Core Team (2011). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Redpath, S. (1988). Vigilance levels in preening Dunlin Calidris alpina. Ibis 130, 555–557. doi: 10.1111/j.1474-919X.1988.tb02723.x
Rivera-Gutierrez, H. F., Pinxten, R., and Eens, M. (2010). Multiple signals for multiple messages: great tit, Parus major, song signals age and survival. Anim. Behav. 80, 451–459. doi: 10.1016/j.anbehav.2010.06.002
Searcy, W. A., Anderson, R. C., and Nowicki, S. (2008). Is bird song a reliable signal of aggressive intent? A reply. Behav. Ecol. Sociobiol. 62, 1213–1216. doi: 10.1007/s00265-008-0569-5
Searcy, W. A., and Andersson, M. (1986). Sexual selection and the evolution of song. Annu. Rev. Ecol. Syst. 17, 507–533. doi: 10.1146/annurev.es.17.110186.002451
Shawkey, M. D., Estes, A. M., Siefferman, L. M., and Hill, G. E. (2003). Nanostructure predicts intraspecific variation in ultraviolet-blue plumage colour. Proc. R. Soc. Lond. B 270, 1455–1460. doi: 10.1098/rspb.2003.2390
Soma, M., and Garamszegi, L. Z. (2015). Evolution of courtship display in Estrildid finches: dance in relation to female song and plumage ornamentation. Front. Ecol. Evol. 3:4. doi: 10.3389/fevo.2015.00004
Spencer, K. A., Buchanan, K. L., Goldsmith, A. R., and Catchpole, C. K. (2003). Song as an honest signal of developmental stress in the Zebra finch (Taeniopygia guttata). Horm. Behav. 44, 132–139. doi: 10.1016/S0018-506X(03)00124-7
Taff, C. C., Steinberger, D., Clark, C., Belinsky, K., Sacks, H., Freeman-Gallant, C. R., et al. (2012). Multimodal sexual selection in a warbler: plumage and song are related to different fitness components. Anim. Behav. 84, 813–821. doi: 10.1016/j.anbehav.2012.07.002
Tobias, J. A., Montgomerie, R., and Lyon, B. E. (2012). The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Phil. Trans. R. Soc. B 367, 2274–2293. doi: 10.1098/rstb.2011.0280
Webb, W. H., Brunton, D. H., Aguirre, D., Thomas, D., Valcu, M., and Dale, J. (2016). Female song occurs in songbirds with more elaborate female coloration and reduced sexual dichromatism. Front. Ecol. Evol. 4:13. doi: 10.3389/fevo.2016.00022
Zampiga, E., Hoi, H., and Pilastro, A. (2004). Preening, plumage reflectance and female choice in budgerigars. Ethol. Ecol. Evol. 16, 339–349. doi: 10.1080/08927014.2004.9522625
Zuk, M., Ligon, J. D., and Thornhill, R. (1992). Effects of experimental manipulation of male secondary sex characters on female mate preference in red jungle fowl. Anim. Behav. 44, 999–1006. doi: 10.1016/S0003-3472(05)80312-4
Keywords: female, passerines, plumage coloration, song elaboration, multiple signals
Citation: Mahr K, Evans C, Thonhauser KE, Griggio M and Hoi H (2016) Multiple Ornaments—Multiple Signaling Functions? The Importance of Song and UV Plumage Coloration in Female Superb Fairy-wrens (Malurus cyaneus). Front. Ecol. Evol. 4:43. doi: 10.3389/fevo.2016.00043
Received: 02 November 2015; Accepted: 08 April 2016;
Published: 26 April 2016.
Edited by:
Michelle L. Hall, University of Melbourne, AustraliaReviewed by:
Masayo Soma, Hokkaido University, JapanAlexander Kirschel, University of Cyprus, Cyprus
Copyright © 2016 Mahr, Evans, Thonhauser, Griggio and Hoi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharina Mahr, katharina.mahr@vetmeduni.ac.at
 Katharina Mahr
Katharina Mahr Christine Evans
Christine Evans Kerstin E. Thonhauser
Kerstin E. Thonhauser Matteo Griggio
Matteo Griggio Herbert Hoi1
Herbert Hoi1