Productivity and Change in Fish and Squid in the Southern Ocean
- 1Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany
- 2Berlin Center for Genomics in Biodiversity Research, Berlin, Germany
- 3Department of Evolutionary Genetics, Leibniz Institute for Zoo and Wildlife Research, Berlin, Germany
- 4Laboratory of Biodiversity and Evolutionary Genomics, KU Leuven, Leuven, Belgium
- 5Australian Antarctic Division, Kingston, TAS, Australia
- 6Centre for Marine Socioecology, University of Tasmania, Hobart, TAS, Australia
- 7Institute for the Study of the Anthropic Impacts and the Sustainability in the Marine Environment (IAS), National Research Council of Italy (CNR), Genoa, Italy
- 8Oceans and Atmosphere, Commonwealth Scientific and Industrial Research Organisation (CSIRO), Hobart, TAS, Australia
- 9Environmental Studies Program, University of Colorado, Boulder, Boulder, CO, United States
- 10LOCEAN Laboratory, Sorbonne Universités (UPMC, University Paris 06)-CNRS-IRD-MNHN, Paris, France
- 11Institute of Neuroscience, University of Oregon, Eugene, OR, United States
- 12British Antarctic Survey, Cambridge, United Kingdom
- 13School of Biological Sciences, University of Bristol, Bristol, United Kingdom
- 14Southwest Fisheries Science Center, National Marine Fisheries Service, NOAA Fisheries, La Jolla, CA, United States
- 15UFR 918 Terre Environnement Biodiversité, Sorbonne Université, Paris, France
- 16Channel and North Sea Fisheries Research Unit, IFREMER, Boulogne-sur-Mer, France
- 17Royal Belgian Institute of Natural Sciences, Brussels, Belgium
- 18Universit libre de Bruxelles (ULB), Brussels, Belgium
- 19Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, TAS, Australia
- 20Department of Life Sciences, Marine and Environmental Sciences Centre, University of Coimbra, Coimbra, Portugal
Southern Ocean ecosystems are globally important and vulnerable to global drivers of change, yet they remain challenging to study. Fish and squid make up a significant portion of the biomass within the Southern Ocean, filling key roles in food webs from forage to mid-trophic species and top predators. They comprise a diverse array of species uniquely adapted to the extreme habitats of the region. Adaptations such as antifreeze glycoproteins, lipid-retention, extended larval phases, delayed senescence, and energy-conserving life strategies equip Antarctic fish and squid to withstand the dark winters and yearlong subzero temperatures experienced in much of the Southern Ocean. In addition to krill exploitation, the comparatively high commercial value of Antarctic fish, particularly the lucrative toothfish, drives fisheries interests, which has included illegal fishing. Uncertainty about the population dynamics of target species and ecosystem structure and function more broadly has necessitated a precautionary, ecosystem approach to managing these stocks and enabling the recovery of depleted species. Fisheries currently remain the major local driver of change in Southern Ocean fish productivity, but global climate change presents an even greater challenge to assessing future changes. Parts of the Southern Ocean are experiencing ocean-warming, such as the West Antarctic Peninsula, while other areas, such as the Ross Sea shelf, have undergone cooling in recent years. These trends are expected to result in a redistribution of species based on their tolerances to different temperature regimes. Climate variability may impair the migratory response of these species to environmental change, while imposing increased pressures on recruitment. Fisheries and climate change, coupled with related local and global drivers such as pollution and sea ice change, have the potential to produce synergistic impacts that compound the risks to Antarctic fish and squid species. The uncertainty surrounding how different species will respond to these challenges, given their varying life histories, environmental dependencies, and resiliencies, necessitates regular assessment to inform conservation and management decisions. Urgent attention is needed to determine whether the current management strategies are suitably precautionary to achieve conservation objectives in light of the impending changes to the ecosystem.
Introduction
The fish and squid species of the Southern Ocean are uniquely adapted to the extreme conditions of the waters surrounding Antarctica. These adaptations leave Southern Ocean fish and squid vulnerable to the environmental variability introduced by climate change and the potential for compounding impacts of existing drivers on diversity and biomass in the region. Hereafter, we collectively refer to these species as ‘fish’ unless there is a need to address species from other regions, or to explicitly differentiate between finfish and squid. In this paper, we assess the current state of knowledge for selected taxa, divided into seven groups by ecological function or habitat, as well as considering the utility of non-taxonomic groupings. We explore the interplay between hydrography and life history for their effects on population structures and health of species. We consider the ways in which data are collected on Antarctic fish, namely through the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) and from fisheries-independent sources. We assess challenges to the interpretation of these data with respect to observation bias and sampling. At its heart, this chapter presents an assessment of the impacts of drivers of change on Antarctic fish and squid abundance, considering the impacts of fisheries, climate change, pollution and pathogens. Finally, we outline prognoses for the future, including the identification of knowledge gaps and areas that will present challenges for researchers and managers.
Types of Fish and Squid in the Southern Ocean
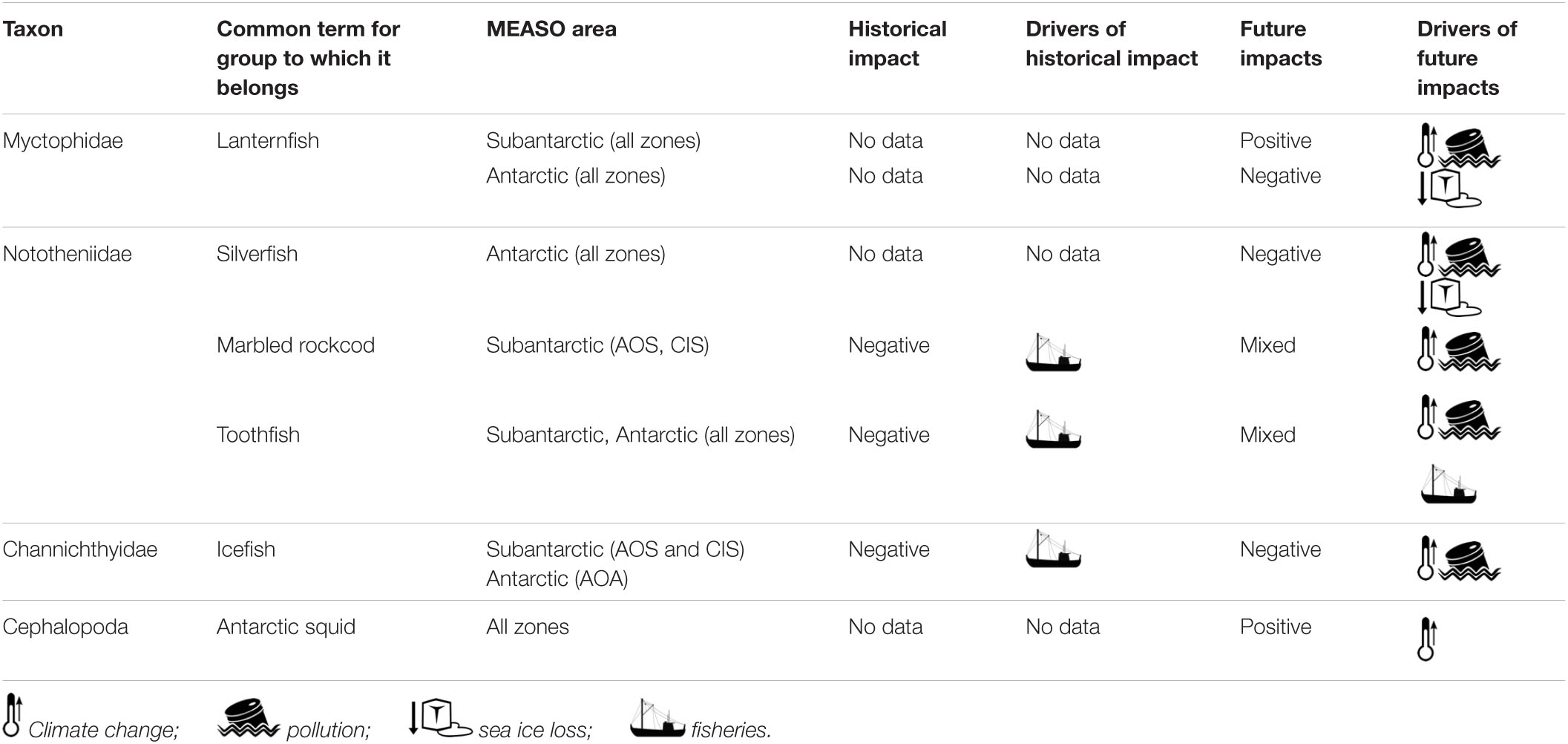
Fish and squid of the Southern Ocean are often considered with respect to groupings corresponding to ecological function and/or habitat. A typical distinction is between benthic and pelagic fish species (Table 1). The pelagic habitat can be further subdivided by depth and its corresponding light regimes. The Southern Ocean has very few epipelagic fish taxa, i.e., those which occupy the upper 200 m of the water column. The limited diversity of epipelagic species relates to the nature of the notothenioid fishes, the dominant clade in high-Antarctic waters in terms of diversity and abundance (Eastman, 2005). The notothenioid fishes diversified in an adaptive radiation from a benthic ancestor (Eastman, 1993). Adaptations such as reduced ossification and lipid sacs facilitated pelagization of some notothenioids despite their lack of a swimbladder (Devries and Eastman, 1978; Eastman and DeVries, 1981; Klingenberg and Ekau, 1996). Still, only nine notothenioid species are categorized as pelagic, including the most prominent pelagic Antarctic fish, Pleuragramma antarctica (Duhamel et al., 2014). The same authors list a total of 156 pelagic species in the Southern Ocean, 72 of which are recorded only occasionally south of the Sub-Tropical Front (STF). Unlike P. antarctica and a few cryopelagic notothenioids, the majority of pelagic species can be described as meso- or bathypelagic, i.e., dwelling in the twilight zone (∼200–1,000 m) or deeper. The most speciose family in this domain are the lanternfish (Myctophidae), but several others are regularly recorded (e.g., Bathylagidae, Stomiidae, Paralepididae, and Zoarcidae). Other species are typical deep-sea inhabitants that live beyond the Antarctic continental shelf in the vast deep-sea basins of the Southern Ocean. A total of 206 species, 9 of which are occasionally recorded south of the STF, are categorized as deep-sea fish (Duhamel et al., 2014). Common deep-sea representatives are snailfishes (Liparidae), eelpouts (Zoarcidae), grenadiers (Macrouridae), and some notothenioids (e.g., Bathydraco spp.). The toothfish species (Dissostichus eleginoides and Dissostichus mawsoni) traverse the boundaries of such a habitat-based classification, as they forage in both benthic, neritic as well as pelagic and deep-sea habitats (Hanchet et al., 2015; Troccoli et al., 2020). In addition, many fish species change habitat occupancy with ontogeny, such as a shift from pelagic larvae and juveniles to benthic adults.
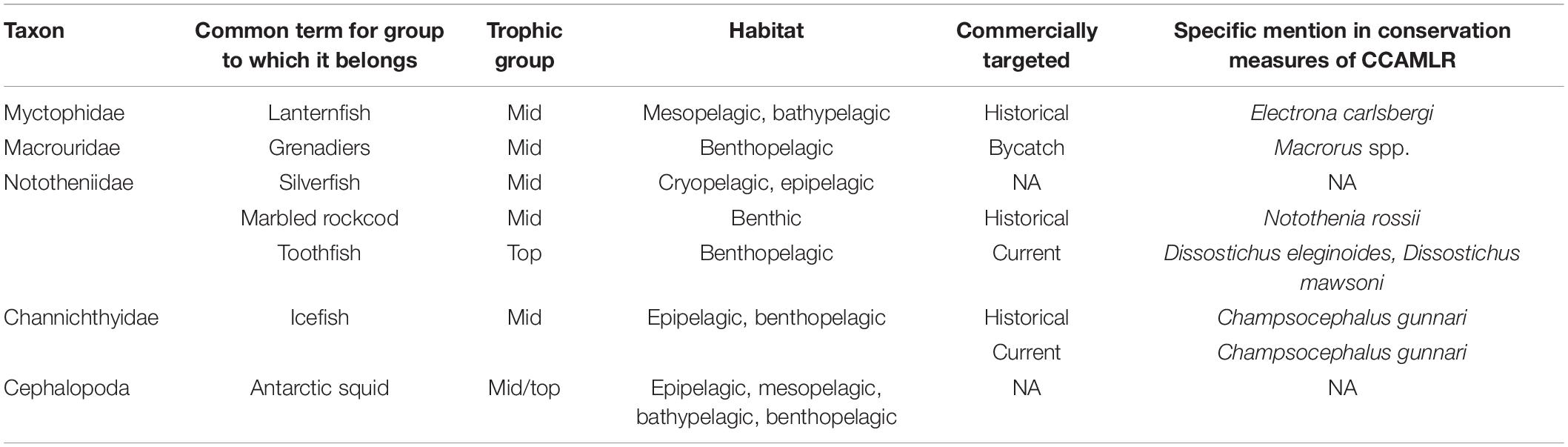
Table 1. Common taxa of fish and squid in the Southern Ocean, the types of habitats in which they reside, and the functional groupings to which they are often assigned.
The neritic zone, comprising the shallow (<200 m) waters of the Antarctic continental shelf as well as peri-insular subantarctic shelves and oceanic seamounts, is inhabited by 120 species, 3 of which have only occasionally been recorded south of the STF (Duhamel et al., 2014). Various notothenioids, mostly with benthic or epibenthic lifestyles, occur throughout the depth range of the neritic habitat (Eastman, 2017). Rays (Rajidae) and eel cods (Muraenolepididae) are abundant in some neritic habitats of the Southern Ocean as well. The ecology of neritic species has been investigated in diverse regions of the Southern Ocean. On the Antarctic shelf, species assemblages are related to the topography and disturbance of the environment. Projects from the Census of Antarctic Marine Life (Schiaparelli et al., 2013) revealed that there are clear differences between assemblages on banks, shelf breaks, inner shelf depressions, and outer shelf canyons (Causse et al., 2011). This has helped to draw fish ecoregions (Koubbi et al., 2010) that are probably explained by the capacity of neritic fish to deal with stressors in their environment, particularly habitat disturbance from iceberg scouring. Neritic fish assemblages in subantarctic areas are influenced by the geography of islands. Bays and fjords such as in South Georgia or Kerguelen are important spawning or nursery grounds for some species. The geography and island mass also influence the velocity of the Antarctic Circumpolar Current, creating retention areas which are very productive and essential for the early life stages of fishes (Koubbi et al., 2009). More details on the biogeographic and depth-related groupings of Southern Ocean fish can be found, e.g., in Duhamel et al. (2014) and Eastman (2017).
Regarding ecological function, or more specifically position in the food web, most Southern Ocean fish can be considered forage species that occupy a mid-trophic level. Plankton feeders like myctophids and Antarctic silverfish occur in vast numbers and are key food sources for various higher trophic level predators, including larger fishes, penguins, flying seabirds, and marine mammals (Vacchi et al., 2017). Many notothenioids and squid are mid-level consumers of other fish, crustaceans, and various other invertebrates, including cnidarians, salps, polychaetes, echiurans, sipunculids, priapulids, and molluscs (DeWitt et al., 1990; Xavier et al., 2018). Some nototheniids form large aggregations as well, at least historically, such as the marbled rockcod Notothenia rossii (Kock, 1992). While toothfishes (Dissostichus spp.) are preyed upon by seals and sperm and killer whales, they arguably are top predators of the marine realm themselves, with a role similar to that of sharks in other oceans, feeding on a variety of fish and squid (Fenaughty et al., 2003; Hanchet et al., 2015). Here, we review the productivity and change for selected groups of both forage species and top predators.
Research has focused mostly on the notothenioids because of their commercial relevance and fascinating evolutionary history (Kock, 2007; Matschiner et al., 2015). Recently, the importance of mesopelagic fish, particularly myctophids, has become widely-appreciated and attracted increased scientific attention (Hill et al., 2012; Taillebois et al., 2017; McCormack et al., 2020; Woods et al., 2020). Given the overall diversity of Southern Ocean fish, it seems clear that much remains to be learned about other species in addition to notothenioids and myctophids, but comparatively few studies have investigated these so far (see e.g., Todgham et al., 2007; Duhamel et al., 2010; Dettai et al., 2011; Moteki et al., 2011; McMillan et al., 2012; Pinkerton et al., 2013; Prirodina and Balushkin, 2015; Christiansen et al., 2018).
Life Histories, Functional Relationships and Population Ecology
Understanding life histories and geographical connectivity are essential to assess and model population and stock structure (Jones et al., 2007). The term ‘stock’ is typically used in fisheries contexts to refer to an independent, self-recruiting population (Reiss et al., 2009). Hereafter, we use the term population when discussing life histories and ecologies of fish. Population health is defined as the robustness of a population to variation in its environment, i.e., its ability to withstand die-off events, poor recruitment years, and climate change impacts (Young et al., 2018). Understanding population structure is key in assessing population health. Many approaches exist to ascertain population structure, including genetics, otolith analyses, parasitology, tagging, and age/growth analysis; integration of multiple techniques yields the most informative results (Paris et al., 2018).
We consider here three important aspects of life history and connectivity: (1) ontogenetic dispersal, (2) water column occupation, and (3) reproduction patterns and longevity. While many Antarctic fish occupy benthic niches as adults and are thus more restricted spatially, most have pelagic or cryopelagic larval phases that enable dispersal (Eastman, 2005). Inhabiting the water column provides these larvae with the opportunity to be transported over large distances depending on their exposure to prevailing current systems (Loeb et al., 1993; Matschiner et al., 2009; La Mesa et al., 2016). Furthermore, many fish and squid species, particularly those that account for large proportions of biomass within the Southern Ocean (e.g., Antarctic silverfish, myctophids), have wholly pelagic or mesopelagic life histories, and thus their life histories are necessarily more convoluted (Duhamel et al., 2014).
The prevailing hydrography in the Southern Ocean informs current hypotheses regarding population structure of many species of Antarctic fish (Ashford et al., 2017b). Driven by the westerlies, the ACC is the largest current system in the world, hovering around 60°S with varying widths and extents as it makes its way around the Antarctic continent (Rintoul and da Silva, 2019). The ACC has the potential to transport shelf species where it approaches the continental slope along the western Antarctic Peninsula and East Antarctica (Orsi et al., 1995). It forms portions of the Ross and Weddell Gyres, where it can transport fish from offshore seamounts toward the continental shelf (Rintoul and da Silva, 2019; Vernet et al., 2019). The Antarctic Slope Current (ASC) is measured from the Amundsen Sea to the tip of the Antarctic Peninsula, traveling in an anti-clockwise direction along the continental slope (Thompson et al., 2018). The ASC can promote regional transport between trough systems at the edge of the continental shelf, as well as facilitating circumpolar transport for shelf species (Ashford et al., 2017b; Caccavo et al., 2018). Finally, the buoyancy-forced Antarctic Coastal Current (AACC) hugs the coast, and is detected along all parts of the Antarctic continent to varying degrees depending on the season, given its relationship to meltwater runoff and wind stress (Moffat et al., 2008; Núñez-Riboni and Fahrbach, 2009; Kim et al., 2016). The AACC moves in an anti-clockwise direction and promotes local transport and inshore connectivity between trough heads (Ashford et al., 2017b; Caccavo et al., 2018).
Given the life history of Antarctic fish and the hydrography of the Southern Ocean, the null hypothesis for population structure for Antarctic fish is one of panmixia (Matschiner et al., 2009; Damerau et al., 2012). In many species, however, evidence for the contrary has been found. Recent work on the myctophid Electrona antarctica revealed a complex population structure influenced by the ACC and the ASC in East Antarctica characterized by divergent otolith nucleus chemistry, which reflects exposure to distinct early life environments (Zhu, 2018). Antarctic silverfish were found to have a largely contiguous population with evidence for high levels of gene flow between the Ross and Weddell Seas, as well as the Antarctic Peninsula (Zane et al., 2006; Caccavo et al., 2018, 2019). This connectivity was hypothesized to be supported by the ASC, as exceptions to this finding coincided with areas not reached by the near-circumpolar slope current (the western Antarctic Peninsula and the South Orkney Islands) (Agostini et al., 2015; Caccavo et al., 2018). Importantly, connectivity was found to vary over time in Antarctic silverfish, a phenomenon referred to as chaotic genetic patchiness (Agostini et al., 2015; Caccavo et al., 2018). Chaotic genetic patchiness has also been observed in icefish populations of Chaenocephalus aceratus at the South Shetland Islands (Papetti et al., 2007). Complex patterns of genetic connectivity were observed between populations at the South Shetland and South Orkney Islands related to the ACC in C. aceratus and Chionodraco rastrospinosus (Papetti et al., 2007, 2012). Work on Patagonian and Antarctic toothfish found mixed evidence of population heterogeneity on a circumpolar scale. Populations between the Indian, Pacific and Atlantic sectors of the Southern Ocean were significantly different using nuclear markers, while mitochondrial markers only differentiated the Pacific population (Toomey et al., 2016). Early genetics work in Antarctic toothfish with a small number of markers indicated conflicting results (Parker et al., 2002; Kuhn and Gaffney, 2008; Mugue et al., 2014), while otolith chemistry supported distinct populations between the Ross Sea and the Antarctic Peninsula (Ashford et al., 2012). Such variability in connectivity over time emphasizes stochasticity as an important aspect of population structure.
Forage Species
Myctophids
Myctophids (Family Myctophidae) are the most abundant and biomass dominant family of Southern Ocean ichthyofauna inhabiting the mesopelagic (200–1,000 m) and bathypelagic (>1,000 m) zones (Gjøsæter and Kawaguchi, 1980). Biomass estimates are likely to be an order of magnitude higher than the previously reported estimate of 212–396 million tonnes (Lubimova et al., 1987; Kaartvedt et al., 2012), with highest abundances at frontal zones (Pakhomov et al., 1996; Van de Putte et al., 2010; Collins et al., 2012). These small fish (<20 cm in length, Table 2) are relatively diverse, with 68 species recorded south of the Sub-Tropical Front (STF; Duhamel et al., 2014). Community composition is influenced by meso- and sub-mesoscale oceanographic features with a sharp transition in assemblages at the major frontal zones, the Polar Front, the Subantarctic Front and the Sub-Tropical Front (Koubbi et al., 2011b; Duhamel et al., 2014). The high association of lanternfish with certain pelagic ecoregions exposes them to impacts from major environmental changes in the subantarctic and subtropical zones, where a southward shift in frontal regions has been observed (Constable et al., 2014).
Table 2. Summary data for common taxa of fish and squid in the Southern Ocean on their life histories, population ecology, and stock structure.
Most myctophid species migrate vertically on a diel basis, feeding in surface waters at night and descending to mesopelagic depths during the daylight hours (Brierley, 2014; Duhamel et al., 2014). This behavior has implications for carbon sequestration and climate regulation, as organic carbon is actively transported from the surface to the deep ocean (Brierley, 2014; Irigoien et al., 2014). Myctophids are important mid-trophic level components of the pelagic food web, comprising a key link for the transfer of energy from primary consumers and omnivorous macro-zooplankton to a range of top predators (Murphy et al., 2012; Schaafsma et al., 2018). Importantly, their central position in the food web means that they exert considerable control on the ecosystem by top-down control on lower trophic levels, and on higher trophic levels via variations in their biomass (Griffiths et al., 2013).
In some regions and seasons, the energy pathway through myctophids is the most important in sustaining top-predator biomass (McCormack et al., 2020). It has been suggested that this pathway may become increasingly important and provide a potential buffer against reductions in Antarctic krill biomass (Murphy et al., 2007a; McCormack et al., 2020). Extensive study of the Scotia Sea ecosystem has allowed for the characterization of these alternative energy pathways in this region. Bioregionalization studies have identified a transition from a krill-dominated to a fish-dominated community in the Scotia Sea broadly partitioned by the Southern Antarctic Circumpolar Current Front (SACCF) (Stowasser et al., 2012; Ward et al., 2012). North of the SACCF, myctophids are key to the conversion of secondary productivity to King penguins (Bost et al., 1997, 2002; Olsson and North, 1997; Raclot et al., 1998; Cherel et al., 2010), Gentoo penguins (Xavier et al., 2017), albatrosses (Xavier et al., 2003), Antarctic fur seals (Croxall et al., 1999), and cephalopods (Rodhouse et al., 1992); while krill trophic pathways dominate in the south (Stowasser et al., 2012; Ward et al., 2012).
Similar trophic pathways have been identified in the southern Kerguelen Axis region in East Antarctica. In this region, myctophids are important prey items for Emperor penguins, Weddell seals, and southern elephant seals (McCormack et al., 2020). Here, the ecological role of myctophids varies latitudinally (McCormack et al., 2020). Latitudinal variation in diet and relative trophic position has been demonstrated for key myctophid species (Riaz et al., 2020; Woods et al., 2020). Euphausiids are the main prey item for myctophids residing in the pelagic zone south of the Southern Boundary of the Antarctic Circumpolar Current (SB-ACC). In contrast, myctophids residing north of the SACCF consume a more diverse range of prey items, including copepods, amphipods, fish, and gelatinous prey (Clarke et al., 2018; Riaz et al., 2020). Northern myctophids also occupy higher trophic levels compared to those in the south (Woods et al., 2020), indicating a more diverse range of trophic linkages.
Further north near the Kerguelen Islands, the pelagic ecosystem in the Polar Frontal Zone (PFZ) is void of Antarctic krill (Koubbi et al., 2011a). A high abundance and diversity of myctophids is characteristic of this ecosystem, in which 14 species coexist (Cherel et al., 2010). Their high abundance and availability in the water column emphasizes their importance to predators in the area, such as King penguins (Cherel et al., 2010; Duhamel et al., 2010) and breeding female southern elephant seals (Cherel et al., 2008). The isotopic signatures of the myctophid assemblage provides evidence of trophic partitioning, indicative of the coexistence of a concentrated number and diversity of species (Cherel et al., 2010). Species showed differences in either habitat usage or trophic level, which indicated latitudinal movements and a size-based trophic structure, with larger-sized species occupying higher trophic levels compared to smaller-sized species (Cherel et al., 2010). Studies undertaken in the Scotia Sea and in the Indian Ocean (Kerguelen Axis and Kerguelen Islands) have demonstrated the spatially variable trophic role of myctophids in the Southern Ocean ecosystem.
Antarctic Silverfish
The Antarctic silverfish (Pleuragramma antarctica) is a key species in the continental shelf ecosystems of the Southern Ocean (Vacchi et al., 2017). Although devoid of a swimbladder, silverfish are pelagic throughout their life history (Eastman and DeVries, 1989). Embryos develop under the solid fast ice in coastal areas among ice platelets (Vacchi et al., 2012; Guidetti et al., 2015). Larvae are found from 0 to 100 m and descend to deeper layers at the post-larval phase; post-larvae and juveniles up to 2+ years are found at depths up to 400 m (La Mesa et al., 2010) and adults are typically found at depths from 400 to 700 m (La Mesa and Eastman, 2012). There is also horizontal segregation of the early life stages (Koubbi et al., 2011b). Silverfish exhibit diel vertical migrations, moving to shallower depths at night and deeper during the day (Plötz et al., 2001; Fuiman et al., 2002; Robison, 2003; Lancraft et al., 2004) to avoid visual predators and to profit from the congregation of prey species such as Euphausia superba, which are nearer to the surface at night (Hopkins and Torres, 1988; Robison, 2003; Lancraft et al., 2004).
Antarctic silverfish are generalist feeders relying on various food items depending on life stage, location, and availability. Larvae feed on diatoms and small copepods (Koubbi et al., 2007; Granata et al., 2009). Other life stages predominantly feed on euphausiids, Euphausia crystallorophias in the continental shelf waters and E. superba near the continental slope and over deeper waters, and copepods, including Calanoides acutus, Calanus propinquus, Rhincalanus gigas, Metridia gerlachei, and Euchaeta antarctica, though other invertebrates and fish are also suitable prey items (Pinkerton, 2017; Tavernier and Giraldo, 2017; Carlig et al., 2019). Changes in habitat occupancy correlate with ontogenetic shifts in diet composition, such that silverfish occupy several trophic levels throughout their 12–14-year lifespan (Table 2) (Kellermann, 1987; Giraldo et al., 2011, 2015; Tavernier and Giraldo, 2017). Involved in the energy transfer from primary producers to large fish predators (Mintenbeck et al., 2012), in areas where krill is less abundant, the Antarctic silverfish is the main prey item for krill-dependent species, such as Adélie penguins (La Mesa et al., 2004).
Early life dependence on coastal fast ice, and later movement into deeper waters expose silverfish to hydrographic features along the continental shelf resulting in a complex life history which generates the conditions for a dynamic population structure and underlying patterns of connectivity (Ashford et al., 2017b). The silverfish life-history hypothesis incorporates these physical–biological interactions, predicting that cross-shelf circulation mediates retention within populations and exposure to along-shelf transport pathways (Agostini et al., 2015; Ashford et al., 2017b; Brooks et al., 2018b; Caccavo et al., 2018, 2019). Schools of adult silverfish have been observed moving inshore along the Antarctic Peninsula (Daniels and Lipps, 1982), and aggregations attributed to adult silverfish have been detected using bio-acoustic tools in the Ross Sea, supporting the hypothesis that silverfish are moving into Terra Nova Bay during winter to spawn (O’Driscoll et al., 2018b). Large amounts of eggs develop within the platelet ice layer under the fast ice in the western Ross Sea (Vacchi et al., 2012). Assemblages of silverfish larvae have been found in the summer polynya of the eastern Weddell Sea (Hubold, 1984), in waters along the Antarctic Peninsula (Kellermann, 1986), and in the Dumont d’Urville Sea (Koubbi et al., 2011b). Such observations suggest homing behavior in silverfish, wherein spawning migrations occur from open waters to coastal ice shelves where environmental conditions resemble those experienced during larval stages (Koubbi et al., 2011b; Ghigliotti et al., 2017). Such a migration is facilitated by the circulation within glacial trough systems across the continental shelf. Outflows aid in the dispersal of developing fish away from hatching sites, while inflows function to retain adults by transporting them back to spawning areas. The discovery of newly hatched larvae in trough systems along the Weddell Sea, Ross Sea, and Antarctic Peninsula (Hubold, 1984; Guidetti et al., 2015; La Mesa et al., 2015; Ashford et al., 2017b) implies this cycle of dispersion and retention occurs in multiple areas around Antarctica.
Icefishes
Icefishes, or Channichthyidae, are unique among vertebrates for their ‘white blood’ lacking hemoglobin. Icefishes are endemic to the Southern Ocean except for the pike icefish Champsocephalus esox, which occurs in southern Patagonia and around the Falkland Islands (Duhamel et al., 2014). Only a few icefish species were targeted by commercial fisheries starting in the 1970s, but following concerns of overexploitation and depletion of stocks, most fisheries were closed within a couple of decades (Kock, 1992; Kock and Jones, 2005). Today, only the mackerel icefish Champsocephalus gunnari fishery is still open (Table 1) and concentrates around South Georgia (CCAMLR Subarea 48.3) and Heard Island (CCAMLR Division 58.5.2) where the 2019/2020 catch limits were 3,225 and 527 tonnes, respectively (Table 3) (CCAMLR, 2019).
Icefish biology varies greatly across species (Table 2). Most icefishes are benthic and distributed on the continental shelf and slope from shallow depths down to 2,000 m (Duhamel et al., 2014; Eastman, 2017). There are a few species, however, that are benthopelagic and undertake diel migrations from the ocean floor to the water column for feeding (Kock, 2005a). Icefish reproduction does not differ from other notothenioids and is characterized by late sexual maturity, long gametogenesis time, high reproductive effort with large eggs, relatively low fecundity, and reproductive events occurring from late summer to early winter (Kock, 2005a, b; Militelli et al., 2015; Riginella et al., 2016; Le François et al., 2017; Mintenbeck, 2017; Novillo et al., 2019). Many icefishes display elaborate reproductive behavior involving nesting and egg guarding (Desvignes et al., 2019 for review), similar to many other notothenioid species (Jones and Near, 2012; La Mesa et al., 2021). In contrast, C. gunnari displays higher fecundity and smaller egg size than other icefishes, potentially compensating for a broadcast spawning strategy (Militelli et al., 2015).
Due to their unique phenotypic traits (Kock, 2005a, b; Braasch et al., 2015) icefishes have been extensively studied. Analysis of icefish skeletons has revealed the existence of developmental heterochronies (alterations in the timing and rate of bone development) that reflect paedomorphic characteristics (Voskoboinikova, 1997; Albertson et al., 2010; Eastman et al., 2014; Postlethwait et al., 2016). Studies of the population structure and connectivity for several icefish species inhabiting different parts of the Southern Ocean have investigated the hybridization potential between species (Papetti et al., 2009; Damerau et al., 2012; Young et al., 2015, 2018; Desvignes et al., 2019; Schiavon et al., 2021). Finally, the response of icefishes to environmental stressors such as temperature continues to be an active area of research (Table 3) (Beers and Sidell, 2011; Mueller et al., 2012; Joyce et al., 2018; O’Brien et al., 2018). Icefishes are also genetically and physiologically studied for being ‘evolutionary mutant models’ in regard to the absence of hemoglobin in their blood and the lack of cardiac myoglobin in some species (Near et al., 2006; Albertson et al., 2009; Desvignes et al., 2016; Harter et al., 2018; Bargelloni et al., 2019; Kim et al., 2019).
Notothenia rossii
Historically, one of the most important commercial fish species was the marbled notothen or marbled rockcod (N. rossii Richardson, 1844). This abundant demersal species reaches more than 50 cm in length and often occurs in dense shoals in subantarctic fjords and shelf waters (DeWitt et al., 1990; Kock and Jones, 2005). The adults spawn in specific areas at depths of 120–350 m, and the larvae and juveniles spend at least 6–8 months in the water column before settling in nearshore macroalgae habitats (DeWitt et al., 1990; Barrera-Oro et al., 2014). The biology of N. rossii has been widely studied, with recent research on diet (Barrera-Oro and Winter, 2008; Moreira et al., 2014; Barrera-Oro et al., 2019), reproduction and growth (Calì et al., 2017; Ferreira et al., 2017), buoyancy (Eastman et al., 2011), and population recovery and connectivity (Kock and Belchier, 2004; Marschoff et al., 2012; Young et al., 2012, 2015). In addition, the species is often used as a model for physiological changes under increasing temperature and/or acidification (Mark et al., 2012; Strobel et al., 2012, 2013; Machado et al., 2014; Miya et al., 2014; Forgati et al., 2017; Kandalski et al., 2018). Gaps in our knowledge of N. rossii remain regarding the impacts of climate change, current population status and dynamics, and their recovery from overfishing (but see Barrera-Oro et al., 2017; Young et al., 2018; Duhamel et al., 2019).
Squid
The abundance, biomass, ecology, and life history of Antarctic squid is still poorly known, though information is increasing. Squid are known to play an important role in the Southern Ocean as both prey and predators, with their distribution and trophic role in Antarctic marine ecosystems reviewed recently (Xavier et al., 2016, 2018). For example, the colossal squid Mesonychoteuthis hamiltoni is the world’s largest (heaviest) invertebrate and can prey on large fish (Rosa et al., 2017), and Moroteuthopsis longimana is a large squid that is highly abundant in the diet of numerous top predators in the Southern Ocean (Xavier and Cherel, 2021). Of the 54 cephalopod species found in Antarctic waters south of the Polar Front (PF), 20 species are from oegopsid squid (Xavier et al., 2018). Loliginid squid are absent in the region. The lifespan of Antarctic squid species is likely to be 1–2 years, exhibiting slightly slower growth and a longer life cycle than lower latitude squid. These adaptations allow Antarctic squid to withstand the low temperatures and highly seasonal primary and secondary production characteristic of the Southern Ocean (Table 2) (Collins and Rodhouse, 2006). The southern Kerguelen plateau region was recently suggested to be an important spawning area and nursery for several Antarctic squid species, such as M. longimana (Cherel and Weimerskirch, 1999; Lin et al., 2020). Antarctic squid are semelparous, i.e., they reproduce only once in their life (Collins and Rodhouse, 2006).
Currently, there are no commercial fisheries targeting squid species in the Southern Ocean. Historically, there was a fishery for the sevenstar flying squid Martialia hyadesi around South Georgia. This fishery was initiated during the 1996/1997 season, and had a catch limit of 2,500 tonnes in accordance with CCAMLR Conservation Measure 99/XV (CCAMLR, 1996) and subsequent annual measures. After a few years of relatively limited landings, the squid fishery closed during the 2009/2010 fishing season. Whether there is future interest in re-opening this fishery remains to be seen.
Most scientific information on Antarctic squid still comes from studies on the diet of their predators. Indeed, the importance of the squid M. hyadesi and Galiteuthis glacialis in the diet of albatrosses is directly related to their reproductive performance (Xavier et al., 2003; Mills et al., 2020). There are large data gaps for most species of Antarctic and subantarctic squid (areas not sampled from nets and where higher predator data are scarce or absent), so conclusions about their distribution remain tentative (Rodhouse et al., 2014a). Critically, data on large specimens and on the biology of species during the winter months remain underrepresented in observations, limiting our ability to determine seasonal changes in distribution patterns and spawning strategies (Lin et al., 2020). As a result, knowledge of the abundance, physiology, biology, ecology, and diet of Antarctic squid remain rather limited.
Top Predators
Toothfish
Antarctic toothfish
The Antarctic toothfish (Dissostichus mawsoni Norman, 1937) is a large predatory fish endemic to the Southern Ocean, and an important target of commercial fisheries. The species has a circumpolar distribution along the continental shelf and slope (Hanchet et al., 2015; Yates et al., 2019). Genetic studies indicate that habitat discontinuities, such as canyons and abyssal plains, may contribute to the development of genetically distinct populations at the circumpolar scale (Kuhn and Gaffney, 2008). Antarctic toothfish primarily lives in the frigid high Antarctic waters and have a suite of adaptations (e.g., Ciardiello et al., 1995; Metcalf et al., 1999; Kiss et al., 2004; Pointer et al., 2005), including high concentrations of antifreeze glycoproteins (AFGPs) in their blood (Haschemeyer et al., 1977).
Ontogenetic changes have been reported in the buoyancy of this species (Near et al., 2003), leading to the spatial segregation of length classes. Pelagic larval stages are found near the surface, while large adults are caught typically from 1,000 to 1,600 m, down to a maximum depth of 2,210 m (Hanchet et al., 2015). The occurrence of recently consumed large adult D. mawsoni in sperm whale stomachs collected over abyssal depth supports the hypothesis that D. mawsoni spend considerable time off the bottom in the pelagic zone (Yukhov, 1971). Data collected in tagging programs suggest that D. mawsoni are relatively sedentary, with most individuals exhibiting strong site fidelity and dispersing only short distances (less than 20–50 km) (Hanchet et al., 2008). Nevertheless, a small proportion of fish have been shown to move northward for long distances (between 200 and 2,300 km), possibly in association with reproduction (Parker and Mormede, 2015).
An opportunistic carnivore, Antarctic toothfish feeds primarily on fish, cephalopods and crustaceans; differences in diet have been found among size classes and geographical areas (Fenaughty et al., 2003; Stevens et al., 2014). While D. mawsoni are among the top fish predators in the Southern Ocean, they are also prey for large sea mammals, including sperm whales (Yukhov, 1971), Weddell seals (Kooyman, 1967; Calhaem and Christoffel, 1969; Testa et al., 1985; Castellini et al., 1992; Davis et al., 1999; Fuiman et al., 2002; Ainley and Siniff, 2009), and killer whales (Pitman and Ensor, 2003; Ainley and Ballard, 2012; Eisert et al., 2014). Despite this, the trophic level of D. mawsoni, derived from stable isotopes analyses is similar to that of killer whales and Weddell seals (Pinkerton and Bradford-Grieve, 2014).
Despite a reasonable understanding of the Antarctic toothfish biology and ecology, aspects of its life history are still a matter of debate and uncertainty (Yukhov, 1982; Hanchet et al., 2008; Ashford et al., 2012; Hanchet et al., 2015; Ainley et al., 2016). The number of populations of Antarctic toothfish remain unknown (Hanchet et al., 2015). Dissostichus mawsoni can live almost 40 years, grow relatively slowly (Hanchet et al., 2015), and come into maturity at 13 and 17 years of age for males and females, respectively (Table 2) (Parker and Grimes, 2010). However, the frequency of D. mawsoni spawning remains unknown, and the timing and location of spawning activity has only recently been documented in the northern Ross Sea region during the austral winter (Stevens et al., 2014; Parker et al., 2019). The effect of physical–biological interactions between life history and oceanographic features has been considered, but requires further research to better understand these dynamics (Ashford et al., 2017a).
Patagonian toothfish
The Patagonian toothfish (D. eleginoides Smitt, 1898) is a large, deep-water, slow-growing predator and scavenger of the family Nototheniidae, and an important commercially targeted species in the southern Atlantic, Indian, and Pacific Oceans, as well as in the Southern Ocean, where it has been found as far south as 75°S (Hanchet et al., 2004; Collins et al., 2010; Duhamel et al., 2014). Patagonian toothfish occur over a depth range from 50 to 2,500 m (Collins et al., 2010), with a circumpolar distribution spanning the Polar Front (PF) north to 35°S on the Patagonian Shelf (within the Atlantic), to 30°S off Chile (within the Pacific), and to 40°S within the SW Indian Ocean (López-Abellán, 2005; Collins et al., 2010).
Ontogenetic segregation has been observed in D. eleginoides, with specimens’ length strongly correlating with increasing depth. Juveniles and sub-adults are found in shallow water (150–400 m) until 7–8 years old (Laptikhovsky and Brickle, 2005), before moving down the slope to deeper water (Laptikhovsky et al., 2006; Collins et al., 2010). Off South Georgia, mature female D. eleginoides were found deeper on the slope than smaller, mature, male toothfish; both sexes converge at intermediate depths to spawn (Heaps et al., 1999). The species shows strong site fidelity, with a mean annual movement rate of 10 km (Agnew and Clark, 2006). Population genetics studies have shown that oceanographic and geographic discontinuities drive population differentiation in this species (Smith and McVeagh, 2000; Appleyard et al., 2004; Rogers et al., 2006; Toomey et al., 2016). The existence of a large population along the South American continental shelf is hypothesized, driven by the continuity of the deep-sea habitat and the biological features of the species (Canales-Aguirre et al., 2018). The Patagonian toothfish is an opportunistic carnivore, showing ontogenetic shifts in diet associated with down-slope migration and a general switch to scavenging as size increases (Collins et al., 2010; Roberts et al., 2011). Juvenile populations inhabiting shelf areas mainly prey on small demersal notothenioid fish, supplemented with krill, squid or pelagic fish depending on availability; adults on the slope prey on large fishes such as macrourids, morids, and rajids; in deeper waters, specimens consume mainly shrimps, squid, and scavenged prey (Pilling et al., 2001; Roberts et al., 2011).
Patagonian toothfish can live more than 50 years (Horn, 2002) and mature between the ages of 6 and 10 for males and 10 and 13 for females (Table 2) (Everson and Murray, 1999). However, gaps remain in the knowledge of the life cycle of Patagonian toothfish, specifically regarding their reproductive biology (Heaps et al., 1999; Arana, 2009). On the Patagonian shelf, toothfish are known to spawn on the Burdwood Bank in two spawning peaks, a first small peak in May and a second main peak in August (Laptikhovsky et al., 2006). At South Georgia, the spawning period has historically broadly been recognized as April to September; however, long-term assessment of spawning dynamics, spanning almost two decades, showed a progressive shift of peak spawning in females toward the end of July (Brigden et al., 2017). Current knowledge suggests that not all mature females spawn every year, and skip spawning has been hypothesized for the species (Everson and Murray, 1999; Arana, 2009; Boucher, 2018), leading to uncertainties in the estimation of spawning biomass and other population indicators.
Data
Inherent biases exist in Southern Ocean sampling and fisheries-dependent data for most Antarctic fish and squid species. The remote and extreme nature of the Southern Ocean makes it a difficult place to conduct systematic research on abundance, biomass, and productivity. Most data collected and used in management is fisheries-dependent, coming from fisheries observers who report data from commercial fishing vessels1. Fisheries are limited by gear and the environment, e.g., they can only fish as deep as their gear allows, and will be constrained by ice. Gear selectivity can bias the sample catch by selecting for specific age groups, e.g., longline gear tends to select larger individuals (Ashford et al., 2005). The CCAMLR observer program demands 100% observer coverage on toothfish fishing vessels, which ensures consistent observation; however, the observers do not always follow a rigorous (e.g., random) sampling design, instead favoring opportunistic sampling2. In some regions, such as the subantarctic waters around the Heard and McDonald Islands, the fishing industry works with the Australian government to perform an annual random stratified survey, which provides valuable fisheries-independent data (Constable and Welsford, 2011; Brooks et al., 2019). However, in more remote areas like the Ross Sea, fishery-dependent data are the most readily-available data source and can still be used to draw important conclusions regarding Antarctic toothfish life history and management.
With the exception of some localized nearshore work focused on toothfish, icefish and Antarctic silverfish (e.g., Fuiman et al., 2002; O’Driscoll et al., 2018a, b; Parker et al., 2019), sampling is conducted almost exclusively in the summertime and in ice-free areas. Vessels tend to avoid areas where summer sea ice concentration remains too high, and instead aggregate mostly around research stations and along the logistical routes toward them (Griffiths et al., 2014). Benthic sampling is focused on the shelf zone and rarely goes deeper than 1,000 m. Pelagic sampling often focuses on the pelagic zone (<200 m) and neglects the mesopelagic and deeper zones. This can be understood in a context of optimization of ship time and the use of specific sampling protocols (Griffiths et al., 2014).
Data on fish and squid in the Southern Ocean are therefore both patchy and spatially and temporally biased, limited mainly to small-scale studies. Nonetheless, computational methods allow extrapolation to less well-sampled areas (Koubbi et al., 2011a; Xavier et al., 2016). For example, species distribution modeling was used to couple fishery catch and effort data with environmental factors to develop a distribution model for Antarctic toothfish (Yates et al., 2019).
Fisheries Data
CCAMLR makes catch and fishing effort statistics from 1970 to the present for all fisheries in CCAMLR’s Convention Area publicly available in an annual Statistical Bulletin3. These statistics are reported as green weight (i.e., weight before processing) in tonnes, and based on monthly catch and effort data for reporting areas of CCAMLR Subarea and Division (Figure 1). These are derived from haul-by-haul catch and effort data reported to the CCAMLR Secretariat by Member countries, although the first two decades of fisheries were mostly reported in the aggregate form by reporting areas. The Statistical Bulletin also includes trade statistics (landings and exports) of toothfish and planimetric seabed areas by selected depth intervals in the Convention Area. In addition to fisheries data published in the Statistical Bulletin, CCAMLR receives data from scientific observers onboard fishing vessels, research survey data, and from the CCAMLR Ecosystem Monitoring Program (CEMP) on dependent and related species, as well as receiving information on compliance activities.
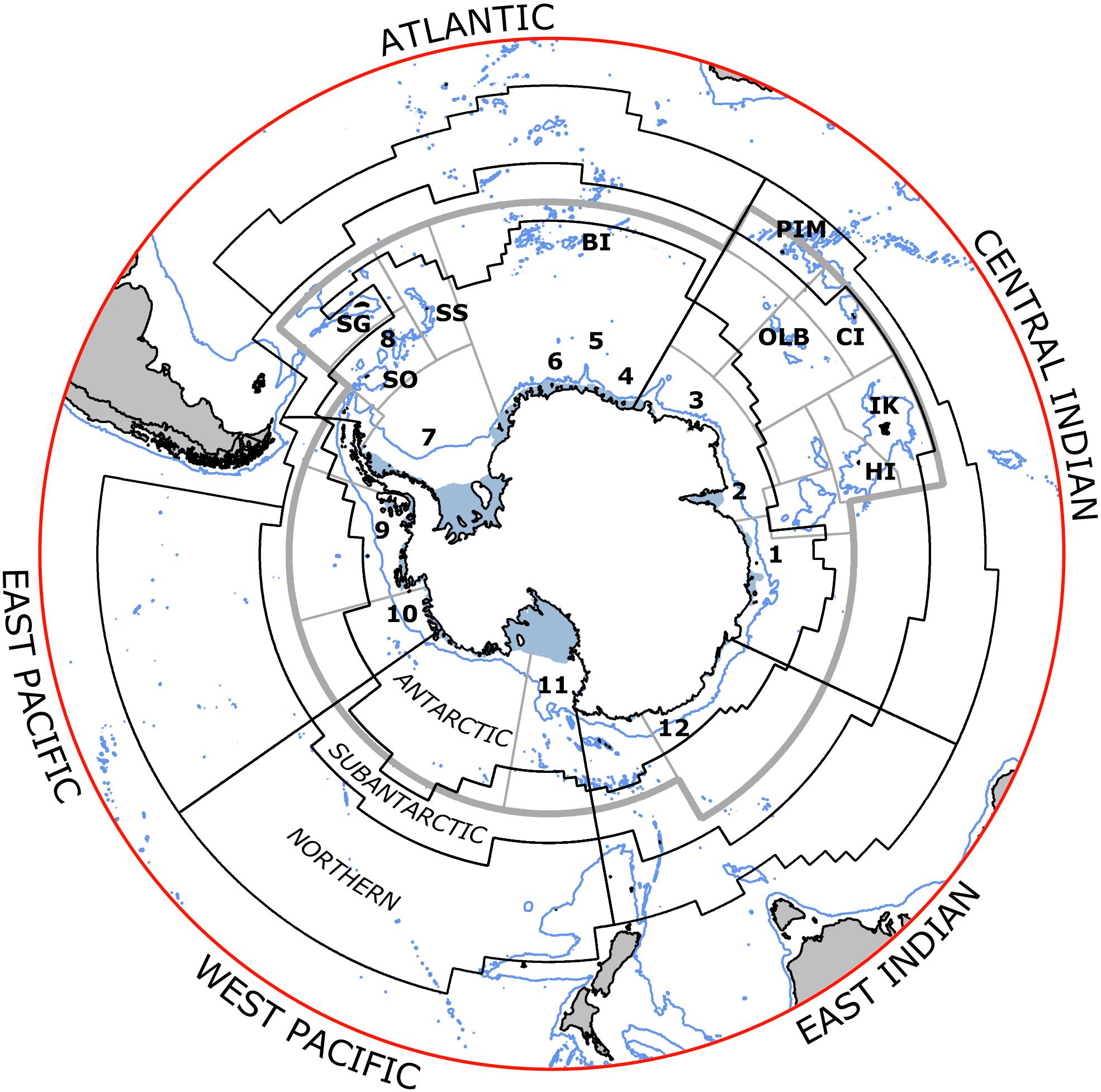
Figure 1. Areas for assessing status and trends of fish and squid in the Marine Ecosystem Assessment for the Southern Ocean (black lines). Labels indicate the sectors (first two letters) and zones (last letter) for each area. Sectors are Atlantic (AO), Central Indian (CI), East Indian (EI), West Pacific (WP), East Pacific (EA). Zones are Antarctic (A), Subantarctic (S), Northern (N) (zonal boundaries are the Southern ACC Front between A and S, and Subantarctic Front between S and N, and Sub-Tropical Front in the North). Seas are marked in the Antarctic Zone as (1) Davis, (2) Cooperation, (3) Cosmonaut, (4) Riiser-Larsen, (5) Haakon VII, (6) Lazarev, (7) Weddell, (8) Scotia, (9) Bellingshausen, (10) Amundsen, (11) Ross, (12) Dumont D’Urville. Islands in the CCAMLR area include Heard (HI), Isle Kerguelen (IK), Crozet (CI), Prince Edward-Marion (PEM), Bouvet (BI), South Sandwich (SS), South Georgia (SG), South Orkney (SO), South Shetland (SSh); and Ob and Lena Banks (OLB). Gray lines indicate the CCAMLR reporting areas (Subareas and Divisions).
All high-resolution fishery and research data not in the Statistical Bulletin are not available to those outside of CCAMLR due to commercial confidentiality concerns by fishing Members. These data may only be released with permission from CCAMLR Members, according to the Rules for Access and Use of CCAMLR Data4.
Active Acoustic Data
Studying fish ecology in the ocean’s mesopelagic realm is challenging because rapid light attenuation limits visual observations, and net sampling can only ever sample a small portion of the community (Warren, 2012). In contrast, active acoustics facilitate the collection of relatively large spatial and temporal data sets, which at frequencies of 38 kHz and below can sample throughout the mesopelagic zone (Kloser et al., 2009). While acoustic data can be collected from a range of platforms (see Benoit-Bird and Lawson, 2016), hull-mounted echosounders are the platform most regularly deployed in the Southern Ocean, and hold the potential to build a comprehensive region-wide spatial data set.
Active acoustics are widely used to monitor commercially important fish stocks, including Antarctic krill. However, there has been a recent increase in the use of active acoustics to study mesopelagic fish globally and in the Southern Ocean, where acoustic backscatter is used as a proxy for fish biomass (Saunders et al., 2013; Irigoien et al., 2014; Moteki et al., 2017; Proud et al., 2017). Acoustic data have provided insight into diel vertical migration behavior, and the relationships between environmental variables and the horizontal and vertical distribution of acoustic scattering layers (Béhagle et al., 2016; Escobar-Flores et al., 2018). Multi-frequency acoustic data have also been used to investigate the schooling behavior of mesopelagic fish around South Georgia (Fielding et al., 2012; Saunders et al., 2013).
While all notothenoids lack a swimbladder, making them weak acoustic targets, there have been investigations into the potential use of marine acoustics to study the notothenioids Antarctic silverfish and Antarctic toothfish. Due to their biological features, lack of swimbladder, and occurrence of few dense structures, the Antarctic silverfish are poor reflectors of sound. A reliable calculation of the species target strength has been obtained by ex situ experiments and in situ analyses (reviewed in O’Driscoll et al., 2017), paving the way for the use of marine acoustics to estimate abundances of this key mid-trophic species. Estimates of the acoustic target strength of the Antarctic toothfish has been performed from in situ observations (O’Driscoll et al., 2018a). Compared to conventional fishery-based methods, acoustics can be operated from a diverse range of platforms, representing a promising means to obtain high spatial and temporal resolution data at an affordable cost. Moorings, normally deployed for the study of currents and environmental conditions, might represent suitable platforms for acoustic instrumentation, allowing observations also in periods of the year when vessels cannot operate due to the sea-ice cover. At Terra Nova Bay, echosounder deployment on moorings allowed the observation of reflections consistent with adult silverfish in September, providing complementary information in support of the hypothesis of coastal migration of fish for spawning during that period (O’Driscoll et al., 2018b).
However, to meaningfully interpret patterns in acoustic data and estimate fish abundance and biomass, we require knowledge of the taxa present and their unique scattering properties or ‘target strength’ (Simmonds and MacLennan, 2005; Davison, 2011). Within the Southern Ocean there is a notable decline in acoustic backscatter toward the pole (Proud et al., 2017; Dornan et al., 2019), which parallels a change in the mesopelagic fish community (Escobar-Flores et al., 2018; Dornan et al., 2019). However, the biomass dominant mesopelagic fish species in colder polar waters tend to lose the highly reflective gas-component in their swimbladders, making them weak acoustic targets and potentially masking high polar fish biomass (Dornan et al., 2019). Another confounding factor is that of target identification (Demer, 2004). Net sampling is routinely used to ground-truth acoustic data; however, net avoidance behavior (Kaartvedt et al., 2012) and backscatter from gas-bearing siphonophores, which are poorly sampled by nets, have the potential to bias biomass estimates if they are not accounted for (Kloser et al., 2016; Proud et al., 2019).
Because of the uncertainties associated with net and acoustic sampling, and the lack of target strength models, estimates of mesopelagic fish biomass have large uncertainties. However, the international community is increasingly recognizing the value of standardizing methods of acoustic data collection and processing, and investing in the development of new tools and approaches to enable robust estimation of mesopelagic biomass (Newman et al., 2019). This was evidenced by the recent MEsopelagic Southern Ocean Prey and Predators (MESOPP) project (2016–2019; Lehodey, 2019). As highlighted by MESOPP and the Southern Ocean Observing System (SOOS) (Lehodey, 2019; Newman et al., 2019), better coupling of observational data and ecosystem models has strong potential for helping resolve uncertainties in acoustic and net observations. Similarly, combining acoustic and net observation data with observations from new platforms such as optical profilers and animal-borne sensors is likely to lead to a greatly improved ability to estimate mesopelagic biomass in the near future (Kloser et al., 2016; Newman et al., 2019).
Groundfish Trawl Surveys
Bottom trawl surveys for groundfish (demersal finfish) have been undertaken in the Southern Ocean since the mid-1960s (Shust, 1998), though results of these early surveys have only appeared in published literature since the late 1970s (Kock, 1992). Most Antarctic demersal fish surveys are undertaken on shelf areas <500 m, and the results are used to characterize abundance, distribution, and demography of fish stocks, as well as to assess changes in the composition and abundance of fish populations over time. In addition, trawl surveys have been used to collect specimens for a variety of purposes, including genetic, physiological, ecological, and process studies.
Net avoidance has been reported for a number of mesopelagic fish species (Collins et al., 2012; Kaartvedt et al., 2012), and is likely common in other fish and squid species as well. The implications of this avoidance are difficult to quantify with traditional methods, and it is likely that better resolved biomass estimates of mesopelagic fishes and squid will depend upon emerging technologies such as codend-less nets towed at higher speeds and equipped with high-speed cameras, optical profiling, and animal-borne acoustic tags (Newman et al., 2019).
Data collected from trawl surveys is primarily used to generate scientific advice for fishery management and conservation under the auspices of CCAMLR. Most surveys are fishery independent, and have been conducted across most archipelagoes of the Southern Ocean. Most trawl surveys are multi-species, though the objective is usually to provide information on currently harvested species (e.g., C. gunnari and Dissostichus spp.) for stock assessments. The primary regions of the Southern Ocean regularly or periodically surveyed are South Georgia (Reid et al., 2007; Clarke et al., 2008), Kerguelen (Duhamel et al., 2019), Heard and MacDonald Islands (Constable and Welsford, 2011; Ziegler and Welsford, 2019), the South Shetland Islands, and the South Orkney Islands (Kock and Jones, 2005; Arana et al., 2020).
Mark-Recapture (Tagging) Programs
There has been an ongoing fish tagging program for toothfish (Dissostichus spp.) in the Southern Ocean administered by the CCAMLR Secretariat for almost two decades. This tagging program is primarily fisheries-based and uses tag and recapture data to estimate abundance of toothfish, which is then directly incorporated into stock assessment models (Hillary et al., 2006; Candy and Constable, 2008; Mormede et al., 2014). Additionally, this information has been used to characterize movement and growth of fish (Williams and Tuck, 2002; Marlow and Agnew, 2003).
Toothfish tagging and reporting of recaptures is compulsory by all fishing vessels targeting toothfish or undertaking research fishing for toothfish in the Southern Ocean. As of the 2019/2020 fishing season, approximately 285,000 toothfish have been tagged, and 29,000 have been recaptured across all regions of the Southern Ocean. More information on the CCAMLR tagging program can be found at https://www.ccamlr.org/en/science/ccamlr-tagging-programme.
Drivers of Change and Their Impacts
The Southern Ocean has experienced rapid climate-related change over recent decades including increases in sea surface temperatures and regional reductions in sea ice extent (Table 3) (William, 1997; Curran et al., 2003; Meredith and King, 2005; Morley et al., 2020 this volume). Climate models suggest that further warming and sea ice loss is likely during the current century (Hill et al., 2013; Cavanagh et al., 2017), which may have a negative impact on oceanic biota and the local ecosystems (Murphy et al., 2007a; Flores et al., 2012; Constable et al., 2014; Atkinson et al., 2019). In this section, we first consider the major environmental impacts of climate change on different fish and squid groups, followed by an exploration of other types of drivers of change (fisheres, pollution, and pathogens), and their potential impacts.
Effects of Climate Change
The effects of climate change on fish are not well studied. Fishes of the Southern Ocean are expected to be affected by increasing temperatures because of their special adaptations to colder temperatures (e.g., the presence of antifreeze proteins in their blood) (Constable et al., 2014). In this respect, any life stage of fish inhabiting shallower waters could become vulnerable. Icefish in the Subantarctic Zone may be especially vulnerable in the shallow waters adjacent to islands (Constable et al., 2014; Reid, 2019). For deeper water species, such as toothfish, the prognosis is less clear. One study indicated the potential for Antarctic toothfish to become extinct, due to its restricted range and affinity for below freezing temperatures (Cheung et al., 2008). However, that study did not include data for D. mawsoni from the Ross Sea, where it has large abundances.
Recently, productivity of Patagonian toothfish on the Kerguelen Plateau has been related to oceanographic conditions, where prey availability may decrease because of increased variability in these conditions in coming years (Subramaniam et al., 2020). This has also been suggested for icefish populations that prey on krill (Reid, 2019), for which the area of greatest productivity is expected to move south (Johnston et al., under review).
In contrast to these results, climate change may favor the relative importance of the energy pathway through fish, possibly resulting in increased productivities of fish predators, such as toothfish (Trebilco et al., 2020). This is predicated on changes in productivity (see Pinkerton et al., 2021 this volume) increasing the relative importance of zooplankton that preyed on by mesopelagic fish. Productivity of mesopelagic fish may also be altered by changing sea ice conditions affecting the life cycles of mesopelagic fish in the Antarctic Zone (Moteki et al., 2017). These hypotheses need to be tested in stock dynamic models embedded in food web models coupled to biogeochemical and Earth system models.
Myctophids
Changes to the Southern Ocean are expected to impact the ecology and behavior of Southern Ocean myctophids, but there is presently insufficient baseline information to detect, monitor, or predict the consequences of this change on these fish. However, there is evidence to suggest that sustained ocean-warming will impact the broad-scale distribution patterns and abundance of myctophids in the region.
A recent study has predicted that most species will undergo a major poleward shift in their distribution patterns under realistic scenarios of ocean-warming in the Southern Ocean (Freer et al., 2019). As the ocean warms in the coming decades, predominantly temperate and subantarctic species are predicted to gain suitable habitat as they extend to higher latitudes, whilst more high-latitude Antarctic species with restricted thermal ranges are likely to lose habitat, and are thus the group most vulnerable to climate change. Saunders and Tarling (2018) further reported evidence for a major biogeographic shift in body size patterns under sustained ocean-warming, with smaller sized myctophid species and life stages increasing their southernmost ranges to waters at higher latitudes, possibly displacing those that reside in these regions ordinarily. Increases in ocean temperature are also likely to alter the vertical distribution and behavior patterns of Southern Ocean myctophids, as the vertical distribution patterns of several myctophid species, particularly the more temperate and subantarctic types, appear to be temperature dependent (Kozlov et al., 1991; Duhamel et al., 2000; Collins et al., 2008, 2012). However, the exact vertical migration behavior of all myctophid species currently remains unresolved, so it remains unclear how they will be impacted and what will be the implications for food web and ecosystem dynamics. The overall effects of such shifts in distribution patterns and community structure would be a reduction in the size spectra of myctophids in the Southern Ocean and a reduction in myctophid fish biomass (Dornan et al., 2019), which could have implications for trophic interactions and thus the wider Southern Ocean ecosystem (Murphy et al., 2007b; Saunders et al., 2019; Trebilco et al., 2020).
A further plausible consequence of ocean-warming on Southern Ocean myctophids is that their abundance may be negatively impacted by changes in food web structure (Murphy et al., 2007b). Although the Southern Ocean food web is predominantly centered on Antarctic krill (Murphy et al., 2007b), myctophids are an integral part of the system, being both prey for many higher predators and major consumers of zooplankton and krill (Saunders et al., 2019). Under realistic scenarios of regional ocean-warming and projected redistribution and/or declines in krill biomass (Atkinson et al., 2004, 2019; Flores et al., 2012), the role of myctophids in Southern Ocean food webs may become increasingly important as higher predators turn more to myctophids as alternative food sources (Murphy et al., 2007b; Trebilco et al., 2020). Increased predation pressure on myctophids is therefore likely to lead to substantial reductions in the abundance of all species in the region, as well as possible adverse impacts on their population dynamics and changes in their behavior. Populations of the larger, krill-consuming myctophids, may also be impacted concurrently by sustained reductions of krill in their diet (Saunders et al., 2019). The extent to which myctophids can prevail and support the Southern Ocean ecosystem against such change is currently unknown and is a critical knowledge gap that requires urgent attention.
Squid
Antarctic squid are likely to be influenced by climate change, including by changes in mesoscale oceanography, position of the oceanic fronts (and associated productivity), sea ice extent, and ocean acidification (Rodhouse, 2013; Xavier et al., 2018). A recent long-term study showed that the habitat of Taonius notalia, Gonatus antarcticus, G. glacialis, and Histioteuthis atlantica had changed over the last 50 years despite their trophic levels remaining similar (Abreu et al., 2020).
As with squid and other cephalopods elsewhere, Antarctic squid have a unique set of biological traits, including rapid growth, short lifespans (in comparison with other marine organisms, such as fish), and life-history plasticity, which may allow them to adapt well to climate change (Rodhouse, 2013; Rodhouse et al., 2014b; Doubleday et al., 2016). Indeed, increasing temperature is thought to accelerate the life cycles of squid (and other cephalopods), particularly those species with high thermal ranges (e.g., species distributed in more than one water mass), improving their reproductive success (Doubleday et al., 2016; Xavier et al., 2016; Queirós et al., 2020). Moreover, as there are no fisheries established for Antarctic squid, they do not experience pressure from fishing, while their natural predators, such as the Antarctic and Patagonian toothfish, do.
Species Targeted by Fisheries
History of Finfish Fisheries
The first commercial fishing for Antarctic fish commenced during the 1969 – 1970 austral summer around South Georgia, followed by Kerguelen in 1970 – 1971 by Soviet trawlers (Kock et al., 1985; Kock, 1992). These catches were primarily groundfish, dominated in the early years by Notothenia rossii (Figure 2). Commercial fishing expanded to the southern Scotia Arc region and Antarctic Peninsula in 1977 – 1978, targeting N. rossii and Champsocephalus gunnari (Kock and Jones, 2005), as well as at Ob and Lena Banks in 1978 – 1979. This coincided with an expansion to high-Antarctic coastal areas when Polish and East German vessels fished for Chaenodraco wilsoni off the Antarctic Peninsula. A short-lived pelagic fishery for the myctophid Electrona carlsbergi was initiated in 1987 – 1988 off South Georgia. The first commercial fishing trials for Patagonian toothfish D. eleginoides in the Southern Ocean took place off South Georgia in 1978 – 1979, followed by Kerguelen in 1983 – 1984, and a further expansion at South Georgia in 1988 – 1989. The fishery for Antarctic toothfish D. mawsoni was initiated in the Ross Sea in 1997 – 1998.
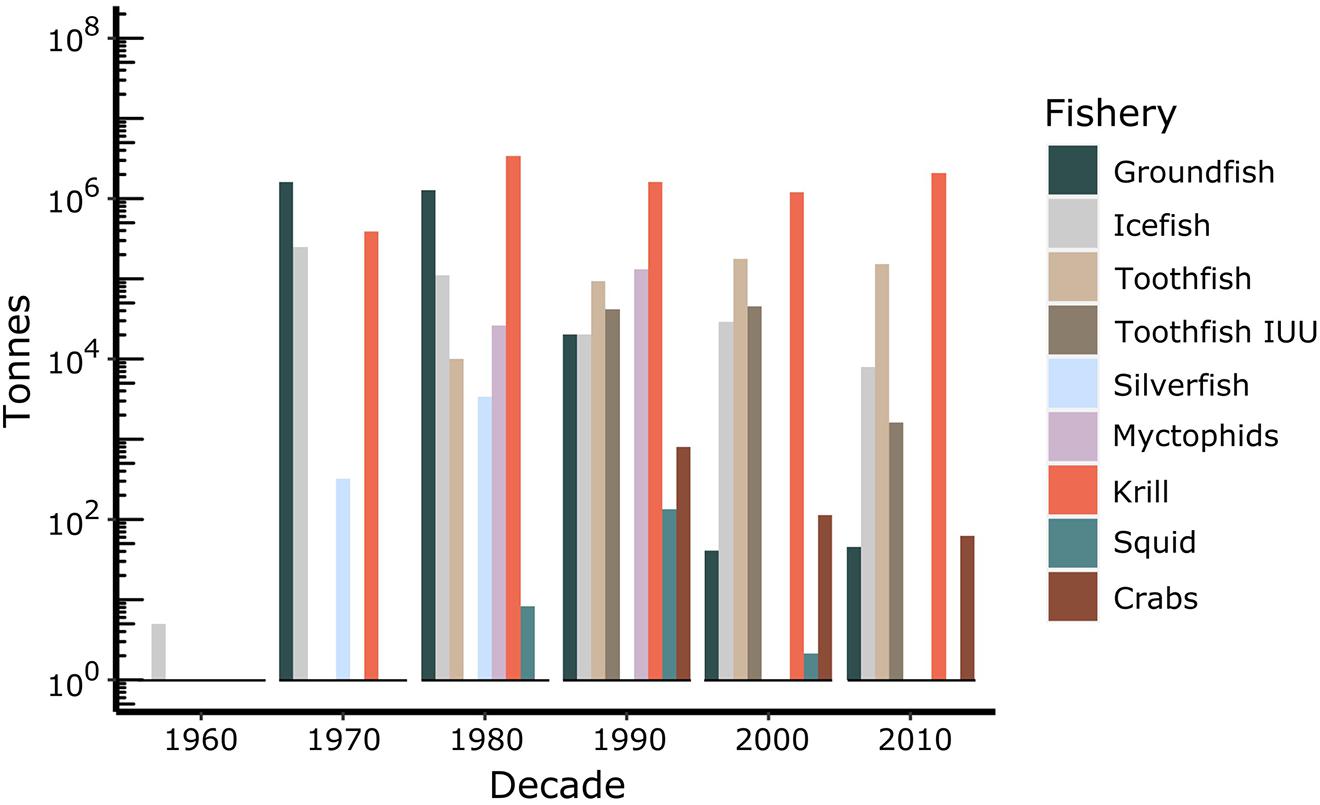
Figure 2. Total biomass (tonnes) caught in the different fisheries undertaken in the CCAMLR Area from 1969 to 2019. Source: CCAMLR Statistical Bulletin 2019 (https://www.ccamlr.org/en/publications/statistical-bulletin), except for catches of toothfish taken in illegal, unregulated or unreported (IUU) activities, which were derived from the CCAMLR Fishery Reports for toothfish (http://fisheryreports.ccamlr.org/).
To date, there have been seven species of Antarctic fish specifically targeted by fishing vessels at various times and in various locations around the Southern Ocean: C. gunnari, C. wilsoni, D. eleginoides, E. carlsbergi, Lepidonotothen squamifrons, N. rossii, Patagonotothen guntheri (Kock, 1992), and D. mawsoni. C. gunnari, L. squamifrons, and N.rossii were heavily depleted in the subantarctic, and some have yet to recover (e.g., N. rossii) (Kock, 2007; Marschoff et al., 2012). Antarctic finfish fisheries are now dominated by D. eleginoides and D. mawsoni, along with small fisheries for C. gunnari exclusively on shelf areas of CCAMLR Subarea 48.3 and Division 58.5.1.
CCAMLR first met in 1982 and inherited a number of depleted fisheries from the activities in the 1970s. Its precautionary approach was established in 1991 for krill and later applied to toothfish and icefish fisheries, including exploratory fisheries (Constable et al., 2000). This approach is intended to take account of parameter uncertainty and environmental stochasticity, although the degree to which this has been achieved has been debated (e.g., Abrams et al., 2016). A challenge to the sustainability of toothfish fisheries was the emergence of illegal, unregulated and unreported (IUU) fishing in the CCAMLR Area in the 1990s and 2000s, though levels of IUU fishing have decreased over the past decade (Figure 2) (Miller, 2004; Österblom et al., 2015).
Toothfish fisheries are now present in CCAMLR Subareas 48.3, Divisions 58.5.1 and 58.5.2 (D. eleginoides), and Subareas 88.1 and 88.2 (D. mawsoni), along with smaller established fisheries for D. eleginoides in Subarea 48.4 (South Sandwich Islands), Subarea 58.6 (Prince Edward and Marion Islands), and Subarea 58.7 (Crozet Island). There are also small exploratory fisheries for D. eleginoides in parts of Subarea 48.6 (near Bouvet), Division 58.4.4 (Ob and Lena banks) and 58.4.3a (Elan Bank). Additional to the fisheries in the Ross Sea, exploratory fisheries for D. mawsoni are currently undertaken in the southern regions of Subarea 48.6, as well as the coastal and seamount high Antarctic regions of Subarea 88.2 (western Amundsen Sea), and Divisions 58.4.1 and 58.4.2 (coastal East Antarctica). In recent years, purported research fishing for D. mawsoni has been undertaken in Subarea 48.1 (Antarctic Peninsula), and Subarea 88.3 (southern Antarctic Peninsula/eastern Amundsen Sea).
Pressures on Stocks From Fishing
Pressures on stocks from fishing in MEASO areas were determined using catch data from the CCAMLR Statistical Bulletin 2019 (see Grant et al., under review, for more). These data were allocated to MEASO areas according to evidence for fishing depths and locations in reports of the Scientific Committee, CCAMLR Conservation Measures, and descriptions in the scientific literature on the fishing operations, Kock (1992) in particular. Available fishing area in the depth ranges of the fisheries was used to spread the catch uniformly across the identified reporting area of operation. Seabed areas were calculated from the GEBCO 2014 grid at 4 km2 resolution5. Catches were spread uniformly across the area identified from those sources for an individual catch based on country, depth range for a given target fishery and gear type, and, where reports allowed it, year. Figure 3 shows the total catches of target species in the CCAMLR area along with time-series of those catches in the MEASO assessment areas.
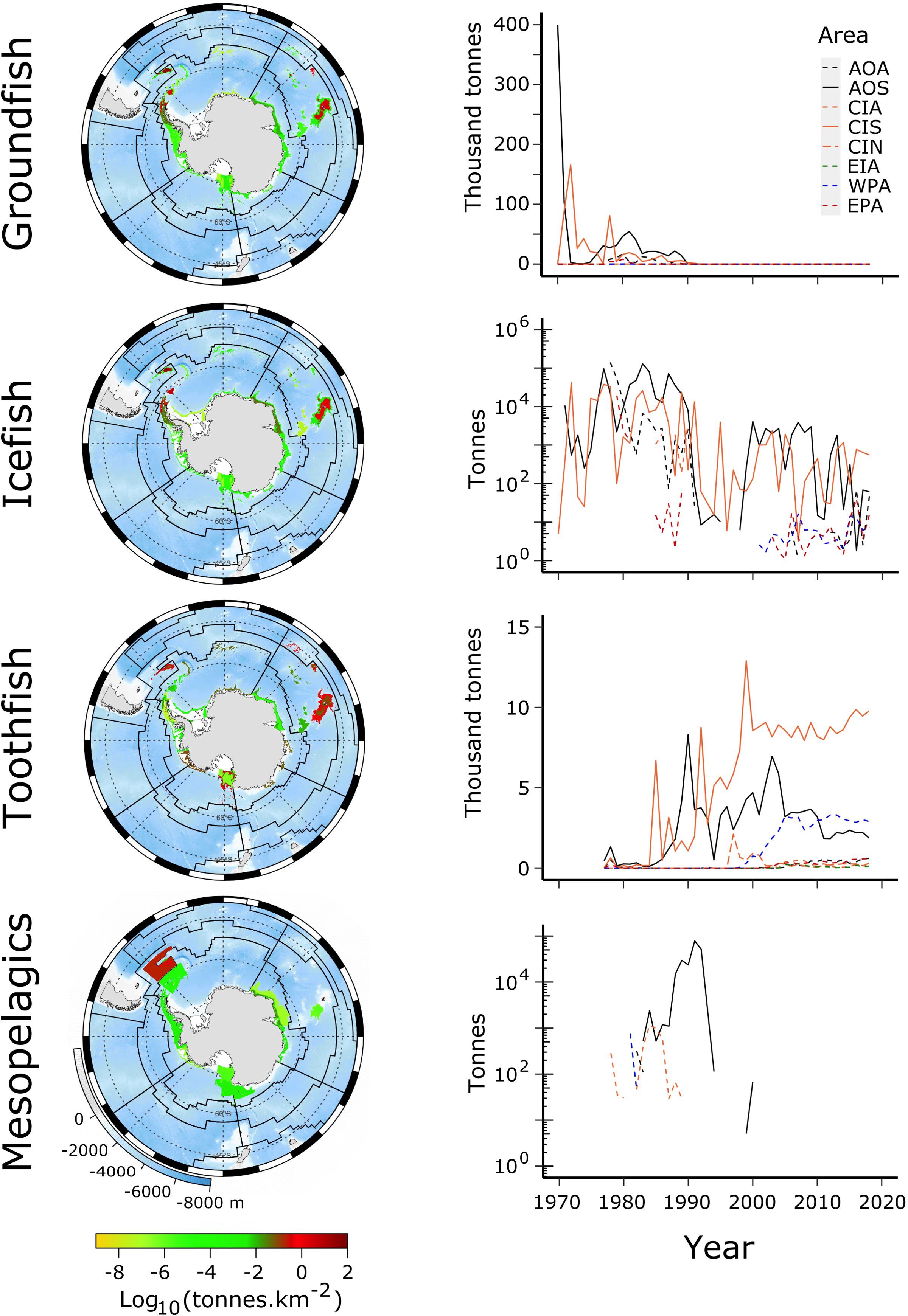
Figure 3. Catches of groundfish, toothfish, icefish, and mesopelagic fish from 1970 to 2018, derived from the CCAMLR Statistical Bulletin 2019. (Left column) Total catch over this period as log10 (catch density in tonnes.km–2) (see color ramp at bottom). Background is ocean depth in meters (bottom left legend on bottom map). Black lines show boundaries of MEASO areas as depicted in Figure 1. (Right column) Time-series of catches of the fish group in each of the MEASO areas according to the line types in the legend in the top right panel. Legend labels indicate the MEASO sectors (first two letters) and zones (last letter) for each area. Sectors are Atlantic (AO), Central Indian (CI), East Indian (EI), West Pacific (WP), East Pacific (EA). Zones are Antarctic (A), Subantarctic (S), Northern (N). Y-axis is log10 for Icefish and Mesopelagics but labeled with corresponding tonnes. Gray lines show a graticule (see Grant et al., under review).
Until CCAMLR established limits to catches of target species, the annual catches in some areas were two orders of magnitude greater than what is now considered sustainable. Groundfish, including N. rossii, experienced the greatest catches and are yet to recover (Kock, 2007; Marschoff et al., 2012). The groundfish fishery had its highest catches in the Subantarctic Zone of the Atlantic and Central Indian Sectors during the 1970s and 1980s, ending in the early 1990s.
Icefish were the next largest fishery to groundfish. The icefish fishery in the Subantarctic Zone (South Georgia in the Atlantic Sector and the northern Kerguelen Plateau in the Central Indian Sector) was concentrated on C. gunnari, a bentho-pelagic species that is important prey for seals. Annual catches are now less than two orders of magnitude than the fishery in the 1970s and 1980s. Although catches are now considered sustainable (Marine Stewardship Council6, 7), it is unknown whether stocks will ever reach the abundances observed in the early fishery.
In the Antarctic Zone, C. gunnari was exploited in the Atlantic Sector around the South Orkney and east of the South Shetland Islands for only a few years before stocks became depleted. Other icefishes were exploited in bottom trawl activities in the early decades but in much lower abundances.
The distribution of each species of toothfish, D. mawsoni and D. eleginoides, are mostly constrained to the Antarctic Zone and north of that zone, respectively. There is some crossover in range at the South Sandwich Islands and Bouvet Island in the Atlantic Sector and on the southern Kerguelen Plateau in the Central Indian Sector. The fisheries for D. mawsoni have all been longline fisheries. Dissostichus eleginoides has been exploited using trawl, longlines, and pots. Longline fisheries take older mature fish whereas trawl fisheries concentrate on juvenile fish in the shallower shelf areas (see Text Footnote 1).
Over the time-series of these fisheries, annual catches are considered sustainable using the CCAMLR Decision Rule for toothfish8, 9, 10, 11,12.
Some questions have been raised about the long-term sustainability of the fishery for D. mawsoni, particularly in relation to structural uncertainty in the model of the dynamics of this stock and in the role that this species may play as prey in the food web of the Ross Sea (Abrams et al., 2016; but see Pinkerton and Bradford-Grieve, 2014; Hanchet et al., 2015). Such evaluations would help resolve different perspectives on what is required to successfully manage the stocks under CCAMLR. Furthermore, the vast majority of analyses used in management are not available in the public domain (e.g., papers from CCAMLR working group meetings), despite this being a recommendation of the most recent CCAMLR performance review13. Thus, independent analyses of CCAMLR data and fisheries management have proved difficult (Abrams et al., 2016; Ainley et al., 2016).
Pelagic and mesopelagic fish have been of interest to commercial fishers since the 1980s. Myctophids were an important fishery in the late 1980s in the Subantarctic Zone of the Atlantic Sector. In the Antarctic Zone, there has been interest from time to time in the Antarctic silverfish P. antarctica. However, a fishery for this species has never developed. Should mesopelagic species be of interest in the future, it would be expected that such species will be managed in a similar manner to Antarctic krill.
Although krill fisheries are not assessed here (see Johnston et al., under review), they catch larval fish and may be contributing to the lack of recovery of some over-exploited stocks (Nicol and Foster, 2016).
Pollution
Climate change is expected to increase the levels of pollutants at the poles. Warming air temperatures increase the levels of persistent organic pollutants (POPs) available for atmospheric transport, while ocean-warming could lead to an increased release of POPs that have been trapped in the pack ice over time (Nash, 2011; Hellmer et al., 2012). Local anthropogenic activities such as industry, fishing, tourism, and research activities contribute to the progressive, point-source contamination of the Antarctic with POPs and microplastics (UNEP, 2011; Waller et al., 2017).
There is little information on plastics in the diet of fish and squid from Antarctic waters. To date, there have been no reports of plastic in the diet of Antarctic squid, however, two microplastic items (acrylic resin) were recovered from the gastrointestinal tract of a single Antarctic toothfish D. mawsoni from the Banzare Banks (Cannon et al., 2016). The same study also sampled Gymnoscopelus nicholsi from the Southern Ocean but found no evidence of plastic in the diet of this species. Similarly, Waluda (unpublished data) found no plastic in the gastrointestinal tract of eight species of fish (n = 142 individuals including C. gunnari, E. carlsbergi, L. nudifrons, Lepidonotothen larseni, Parachaenichthys georgianus, Psilodraco breviceps, and Trematomus hansoni) caught during a groundfish survey around South Georgia in 2011.
While no studies exist for the impact of pollutants on early life stages of Antarctic fish, there is growing evidence that environmental pollutants such as polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), and dioxins have adverse effects on embryonic and early larval development in fish (Nahrgang et al., 2016). The sensitivity of early life stages to environmental changes might be enhanced due to stressor interactions. Indeed, in the Antarctic dragonfish Gymnodraco acuticeps, combined exposure to ocean acidification and warming caused alterations in metabolism and developmental rate in embryonic stages (Flynn et al., 2015).
Mercury is considered to be one the most dangerous substances exhibiting toxicological characteristics, which raises environmental concerns. In marine ecosystems mercury can easily accumulate along food webs, ultimately concentrating in top predators in its different chemical forms (Wiener et al., 2007; Bargagli, 2008; Tavares et al., 2013). Some mercury uptake can occur from sea water through the gills, but mercury in prey is likely the main intake pathway (Lacoue-Labarthe et al., 2009). Squid, which link primary consumers and top predators, may play an important role as vectors of mercury bioaccumulation. The main comprehensive source of information on Hg bioaccumulation in Southern Ocean squid is the recent work by Seco et al. (2020), which documents the concentrations of total, and proportions of organic, mercury in the tissues of 8 squid species caught around South Georgia between 2007 and 2017. While several studies have shown that Patagonian toothfish D. eleginoides have elevated mercury levels (Méndez et al., 2001; McArthur et al., 2003; Guynn and Peterson, 2008), Hanchet et al. (2012), Son et al. (2014), and Yoon et al. (2018) reported lower levels of mercury concentrations for the Antarctic toothfish D. mawsoni, usually not exceeding the 0.5 mg kg–1 food safety threshold.
Major gaps remain in our knowledge of the impacts of pollution on fish and squid in the Southern Ocean, and importantly, the extent to which these impacts may have population level effects (Seco et al., 2021). Studies that build on in situ work identifying the physiological mechanisms of pollution impacts will be critical to build hypotheses that identify vulnerable species and populations.
Pathogens
Many Antarctic fish species are heavily parasitized, especially in their liver and guts, by various helminth worms as well as other groups such as acanthocephalans, hirudinea, and copepods (Oguz et al., 2015; Gordeev and Sokolov, 2017; Klimpel et al., 2017; Münster et al., 2017; Kuhn et al., 2018; Muñoz and Rebolledo, 2019). Fish parasite community assemblages help to elucidate the ecology of their hosts, their role in the food web, and in the life cycle and transfer of parasites across species in the Southern Ocean (Kloser et al., 1992; Palm et al., 2007; Klimpel et al., 2010; Mattiucci et al., 2015; Kuhn et al., 2018). While all affected fish show various levels of chronic inflammation at infection sites, only Antarctic icefishes, which tend to be the most infected group, showed a significantly negative correlation between parasitic load and condition index (Santoro et al., 2013).
While no Antarctic fish diseases have been attributed to bacteria, X-cell disease caused by Alveolata protists (Freeman et al., 2017) and characterized by tumor-like growths (xenomas) on fish gills was reported in a few Antarctic notothenioid species (Franklin and Davison, 1988; Bucke, 1992; Davison, 1997; Evans and Tupmongkol, 2014). X-cell disease negatively affects condition index (Davison, 1997) and is likely to impair the capacity of the gills to extract oxygen from seawater. Affected fish displayed signs of chronic hypertension and extended recovery time from exercise, potentially altering their capacity to swim and thus forage (Davison and Franklin, 2003).
Viruses in Antarctic fish are poorly studied compared to other Antarctic animals (Smeele et al., 2018). There are only two reports on the identification of polyomaviruses (circular double stranded DNA viruses) in two fish species in the Ross Sea (Buck et al., 2016; Van Doorslaer et al., 2018).
Under global climate change, modifications in environmental conditions such as sea water temperature, acidity, salinity, ice coverage, and sediment input can have important consequences on pathogen abundance, distribution, and assemblage in the Southern Ocean (Danovaro et al., 2011; Klimpel and Palm, 2011; Palm, 2011). At the opposite end of the host-pathogen interaction, the same environmental stressors may push fish and squid past a point from which the parasitic load can start impacting the overall health of the host, making viral outbreaks more likely to occur. Both situations can potentially lead to dramatic effects on the fitness and the survival of the fish host.
Concluding Remarks
Globally, regions of the Antarctic are the fastest changing in the world, yet the impacts of these changes on marine ecosystems, including fish, remain unknown and highly complex. Circumpolar frontal systems seem to be in the process of changing their position, though the mechanisms behind these changes – and how they will impact fish and squid – remain uncertain. CCAMLR and its scientific community have recognized the critical need to manage for environmental uncertainty (an essential part of the CAMLR Convention mandate), including climate change (Rayfuse, 2018). For example, CCAMLR added climate change as an agenda item in 2008 (CCAMLR, 2007, 2008). In 2009, CCAMLR adopted resolution 30 on Climate Change, which recognizes “that global climate change is one of the greatest challenges facing the Southern Ocean…” and urged CCAMLR to consider climate change impacts in its management (CCAMLR, 2009). Yet, little progress in managing fish with climate change considerations has been made in the decade since the adoption of Resolution 30. The proposed Climate Change Response Work Plan proposed in 2017 will be important in adjusting fisheries to be sustainable in the changing Southern Ocean ecosystem (Brooks et al., 2018a).
What happens in Antarctica does not stay in Antarctica – water masses of the Southern Ocean and their dependent ecosystems are intricately connected to the global oceans (see Murphy et al., under review). Addressing the gaps in our knowledge of fish and squid ecology and adapting management strategies to an ever-changing Southern Ocean will require a global perspective: consideration of geopolitics, and collaboration with adjacent management bodies. In turn, understanding the impacts of climate change and fisheries on fish and squid ecology in the extreme environments of the Southern Ocean will ultimately inform how these same drivers will impact fish and squid globally.
Author Contributions
JC, HC, AC, LG, and RT conceptualized the manuscript and developed the author team. JC managed the author team, including author recruitment, solicitation of contributions, as well as manuscript streamlining and editing. HC wrote the introduction to Section “Types of Fish and Squid in the Southern Ocean.” JC wrote Section “Life Histories, Functional Relationships and Population Ecology.” BW, CC, and AV wrote Section “Forage Species.” LG wrote Section “Antarctic Silverfish.” TDe wrote Sections “Icefishes” and “Pathogens.” HC wrote Section “Notothenia rossii”. JX wrote Sections “Forage Species” and “Drivers of Change and Their Impacts.” CB and LG wrote both parts of Section “Toothfish.” CB, AV, RT, and AW wrote the introduction to Section “Data.” CB and CJ wrote Section “Fisheries Data.” TDo wrote Section “Active Acoustic Data.” AC and CJ wrote Sections “Groundfish Trawl Surveys” and “Mark-Recapture (Tagging) Programs.” JC and AC wrote the introduction to Section “Drivers of Change and Their Impacts.” AC wrote Sections “Effects of Climate Change” and “Pressures on Stocks from Fishing.” CC and RS wrote Section “Drivers of Change and Their Impacts.” CB, CJ, and AC wrote Section “History of Finfish Fisheries.” AS, MV, CW, and JX wrote Section “Pollution.” CB, JC, and AC wrote Section “Concluding Remarks.” JC and AC created Tables 1–3. AC created Figures 1–3. All authors reviewed and approved the final version of the manuscript.
Funding
JC acknowledges support from the Alexander von HumboldtFoundation in the form of a Humboldt Research Fellowship forPostdoctoral Researchers. TDe acknowledges funding from NSFOPP-1543383 and OPP-1947040. TDo acknowledges funding from NERCNE/L002434/1 (NERC GW4+ Doctoral Training Partnership award). LG andMV acknowledge funding from PNRA 2015/B1.02 [DISMAS – Biological andecological information on the Antarctic toothfish (Dissostichus mawsoni) in the Ross Sea], PNRA 2013/AZ1.18 (RAISE – Integrate Research on Antarctic Silverfish Ecology in the Ross Sea) and PNRA 2016/AZ1.19 (PILOT – Pieces in place for a research and monitoring program targeting the two key fish species of the establishing Ross Sea MPA). AV was funded by the Belgian Science Policy Office (BELSPO, contract n° FR/36/AN1/AntaBIS) in the Framework of EU-Lifewatch.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is a core contribution to the first Marine Ecosystem Assessment for the Southern Ocean (MEASO) of IMBeR’s program ICED. We thank the MEASO Support Group and Steering Committee for their assistance during the various stages of the MEASO development process, as well as the stakeholders who provided feedback during the MEASO consultation process, including: SOOS, PEW, WWF, CEP, and SC-CAMLR. Additional thanks are due to WWF, PEW, and COLTO who provided financial support for the MEASO project.
Footnotes
- ^ https://www.ccamlr.org/en/fisheries/fisheries
- ^ https://www.ccamlr.org/en/science/observer-sampling-requirements-dissostichus-spp
- ^ https://www.ccamlr.org/en/publications/statistical-bulletin
- ^ https://www.ccamlr.org/en/document/publications/rules-access-and-use-ccamlr-data
- ^ https://www.gebco.net/news_and_media/gebco_2014_grid.html
- ^ https://fisheries.msc.org/en/fisheries/south-georgia-icefish-pelagic-trawl/
- ^ https://fisheries.msc.org/en/fisheries/australia-mackerel-icefish/
- ^ https://fisheries.msc.org/en/fisheries/south-georgia-patagonian-toothfish-longline/@@view
- ^ https://fisheries.msc.org/en/fisheries/australian-heard-island-and-mcdonald-islands-toothfish-icefish-fisheries/@@view
- ^ https://fisheries.msc.org/en/fisheries/macquarie-island-mi-toothfish/@@view
- ^ https://fisheries.msc.org/en/fisheries/sarpc-toothfish/@@view
- ^ https://fisheries.msc.org/en/fisheries/ross-sea-toothfish-longline/@@view
- ^ https://www.ccamlr.org/en/organisation/second-ccamlr-performance-review
References
Abrams, P. A., Ainley, D. G., Blight, L. K., Dayton, P. K., Eastman, J. T., and Jacquet, J. L. (2016). Necessary elements of precautionary management: implications for the Antarctic toothfish. Fish Fish. 17, 1152–1174. doi: 10.1111/faf.12162
Abreu, J., Phillips, R. A., Ceia, F. R., Ireland, L., Paiva, V. H., and Xavier, J. C. (2020). Long-term changes in habitat and trophic level of Southern Ocean squid in relation to environmental conditions. Sci. Rep. 10:15215.
Agnew, D., and Clark, J. M. (2006). A study of Patagonian toothfish (Dissostichus eleginoides) post-tagging survivorship in Subarea 48.3. CCAMLR Sci. 13, 279–289.
Agostini, C., Patarnello, T., Ashford, J. R., Torres, J. J., Zane, L., and Papetti, C. (2015). Genetic differentiation in the ice-dependent fish Pleuragramma antarctica along the Antarctic Peninsula. J. Biogeogr. 42, 1103–1113. doi: 10.1111/jbi.12497
Ainley, D., and Ballard, G. (2012). Trophic Interactions and the decrease in Killer Whale (Orcinus orca) prevalence with reduced availability of large fish in the southern Ross Sea. Aquat. Mammals 38, 153–160. doi: 10.1578/am.38.2.2012.153
Ainley, D. G., Eastman, J. T., and Brooks, C. M. (2016). Comments on “The Antarctic toothfish (Dissostichus mawsoni): biology, ecology, and life history in the Ross Sea region,” by S. Hanchet et al. Hydrobiologia 771, 1–7. doi: 10.1007/s10750-015-2607-4
Ainley, D. G., and Siniff, D. B. (2009). The importance of antarctic toothfish as prey of Weddell seals in the ross sea. Antarct. Sci. 21, 317–327. doi: 10.1017/S0954102009001953
Albertson, R. C., Cresko, W., Detrich, H. W. III, and Postlethwait, J. H. (2009). Evolutionary mutant models for human disease. Trends Genet. 25, 74–81. doi: 10.1016/j.tig.2008.11.006
Albertson, R. C., Yan, Y. L., Titus, T. A., Pisano, E., Vacchi, M., Yelick, P. C., et al. (2010). Molecular pedomorphism underlies craniofacial skeletal evolution in Antarctic notothenioid fishes. BMC Evol. Biol. 10:4. doi: 10.1186/1471-2148-10-4
Appleyard, S., Williams, R., and Ward, R. (2004). Population genetic structure of Patagonian toothfish in the West Indian Ocean sector of the Southern Ocean. CCAMLR Sci. 11, 21–32.
Arana, P. (2009). Reproductive aspects of the Patagonian toothfish (Dissostichus eleginoides) off southern Chile. Latin Am. J. Aquat. Res. 37, 381–393. doi: 10.3856/vol37-issue3-fulltext-9
Arana, P. M., Jones, C. D., Alegría, N. A., Sarralde, R., and Rolleri, R. (2020). Antarctic demersal finfish around the Elephant and the South Orkney islands: distribution, abundance and biological characteristics. Latin Am. J. Aquat. Res. 48, 304–322. doi: 10.3856/vol48-issue2-fulltext-2469
Ashford, J., Dinniman, M., and Brooks, C. (2017a). Physical–biological interactions influencing large toothfish over the Ross Sea shelf. Antarctic Sci. 29, 1–8. doi: 10.1017/S0954102017000359
Ashford, J., Dinniman, M., Brooks, C., Andrews, A. H., Hofmann, E., Cailliet, G., et al. (2012). Does large-scale ocean circulation structure life history connectivity in Antarctic toothfish (Dissostichus mawsoni)? Can. J. Fish. Aquat. Sci. 69, 1903–1919. doi: 10.1139/f2012-111
Ashford, J., Zane, L., Torres, J. J., La Mesa, M., and Simms, A. R. (2017b). “Population structure and life history connectivity of Antarctic Silverfish (Pleuragramma antarctica) in the Southern ocean ecosystem,” in The Antarctic Silverfish: A Keystone Species in a Changing Ecosystem, eds M. Vacchi, E. Pisano, and L. Ghigliotti (Cham: Springer International Publishing), 193–234. doi: 10.1007/978-3-319-55893-6_10
Ashford, J. R., Jones, C. M., Hofmann, E., Everson, I., Moreno, C., Duhamel, G., et al. (2005). Can otolith elemental signatures record the capture site of Patagonian toothfish (Dissostichus eleginoides), a fully marine fish in the Southern Ocean? Can. J. Fish. Aquat. Sci. 62, 2832–2840. doi: 10.1139/f05-191
Atkinson, A., Hill, S. L., Pakhomov, E. A., Siegel, V., Reiss, C. S., Loeb, V. J., et al. (2019). Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat. Clim. Change 9, 142–147. doi: 10.1038/s41558-018-0370-z
Atkinson, A., Siegel, V., Pakhomov, E., and Rothery, P. (2004). Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432, 100–103. doi: 10.1038/nature02996
Bargagli, R. (2008). Environmental contamination in Antarctic ecosystems. Sci. Total Environ. 400, 212–226. doi: 10.1016/j.scitotenv.2008.06.062
Bargelloni, L., Babbucci, M., Ferraresso, S., Papetti, C., Vitulo, N., Carraro, R., et al. (2019). Draft genome assembly and transcriptome data of the icefish Chionodraco myersi reveal the key role of mitochondria for a life without hemoglobin at subzero temperatures. Commun. Biol. 2, 1–11.
Barrera-Oro, E., La Mesa, M., and Moreira, E. (2014). Early life history timings in marbled rockcod (Notothenia rossii) fingerlings from the South Shetland Islands as revealed by otolith microincrement. Polar Biol. 37, 1099–1109. doi: 10.1007/s00300-014-1503-0
Barrera-Oro, E., Marschoff, E., and Ainley, D. (2017). Changing status of three notothenioid fish at the South Shetland Islands (1983–2016) after impacts of the 1970–80s commercial fishery. Polar Biol. 40, 2047–2054. doi: 10.1007/s00300-017-2125-0
Barrera-Oro, E., Moreira, E., Seefeldt, M. A., Francione, M. V., and Quartino, M. L. (2019). The importance of macroalgae and associated amphipods in the selective benthic feeding of sister rockcod species Notothenia rossii and N. coriiceps (Nototheniidae) in West Antarctica. Polar Biol. 42, 317–334. doi: 10.1007/s00300-018-2424-0
Barrera-Oro, E. R., and Winter, D. J. (2008). Age composition and feeding ecology of early juvenile Notothenia rossii (Pisces, Nototheniidae) at Potter Cove, South Shetland Islands, Antarctica. Antarctic Sci. 20:339. doi: 10.1017/s0954102008000953
Beers, J. M., and Sidell, B. D. (2011). Thermal tolerance of Antarctic notothenioid fishes correlates with level of circulating hemoglobin. Physiol. Biochem. Zool. 84, 353–362. doi: 10.1086/660191
Béhagle, N., Cotté, C., Ryan, T. E., Gauthier, O., Roudaut, G., Brehmer, P., et al. (2016). Acoustic micronektonic distribution is structured by macroscale oceanographic processes across 20–50 S latitudes in the South-Western Indian Ocean. Deep Sea Res. Part I Oceanogr. Res. Papers 110, 20–32. doi: 10.1016/j.dsr.2015.12.007
Benoit-Bird, K. J., and Lawson, G. L. (2016). Ecological insights from pelagic habitats acquired using active acoustic techniques. Annu. Rev. Mar. Sci. 8, 463–490. doi: 10.1146/annurev-marine-122414-034001
Bost, C., Georges, J., Guinet, C., Cherel, Y., Pütz, K., Charrassin, J., et al. (1997). Foraging habitat and food intake of satellite-tracked king penguins during the austral summer at Crozet Archipelago. Mar. Ecol. Prog. Ser. 150, 21–33. doi: 10.3354/meps150021
Bost, C.-A., Zorn, T., Le Maho, Y., and Duhamel, G. (2002). Feeding of diving predators and diel vertical migration of prey: king penguins1 diet versus trawl sampling at Kerguelen Islands. Mar. Ecol. Prog. Ser. 227, 51–61. doi: 10.3354/meps227051
Boucher, E. (2018). Disentangling reproductive biology of the Patagonian toothfish Dissostichus eleginoides: skipped vs obligatory annual spawning, foraging migration vs residential life style. Environ. Biol. Fishes 101, 1343–1356. doi: 10.1007/s10641-018-0781-8
Braasch, I., Peterson, S. M., Desvignes, T., McCluskey, B. M., Batzel, P., and Postlethwait, J. H. (2015). A new model army: emerging fish models to study the genomics of vertebrate Evo-Devo. J. Exp. Zool. Part B Mol. Dev. Evol. 324, 316–341. doi: 10.1002/jez.b.22589
Brigden, K., Marshall, C., Scott, B., Young, E., and Brickle, P. (2017). Interannual variability in reproductive traits of the Patagonian toothfish Dissostichus eleginoides around the sub-Antarctic island of South Georgia. J. Fish Biol. 91, 278–301. doi: 10.1111/jfb.13344
Brooks, C. M., Ainley, D. G., Abrams, P. A., Dayton, P. K., Hofman, R. J., Jacquet, J., et al. (2018a). Antarctic fisheries: factor climate change into their management. Nature 558, 177–180. doi: 10.1038/d41586-018-05372-x
Brooks, C. M., Caccavo, J. A., Ashford, J., Dunbar, R., Goetz, K., La Mesa, M., et al. (2018b). Early life history connectivity of Antarctic silverfish (Pleuragramma antarctica) in the Ross Sea. Fish. Oceanogr. 27, 274–287. doi: 10.1111/fog.12251
Brooks, C. M., Epstein, G., and Ban, N. C. (2019). Managing marine protected areas in remote areas: the case of the subantarctic heard and McDonald Islands. Front. Mar. Sci. 6:631. doi: 10.3389/fmars.2019.00631
Buck, C. B., Van Doorslaer, K., Peretti, A., Geoghegan, E. M., Tisza, M. J., An, P., et al. (2016). The ancient evolutionary history of polyomaviruses. PLoS Pathog. 12:e1005574. doi: 10.1371/journal.ppat.1005574
Bucke, D. (1992). ‘X-cell’ lesions in Notothenia (Lepidonotothen) squamifrons Gunther. Bull. Eur. Assoc. Fish Pathol. 12:83.
Caccavo, J. A., Ashford, J. R., Ryan, S., Papetti, C., Schröder, M., and Zane, L. (2019). Spatial structuring and life history connectivity of Antarctic silverfish along the southern continental shelf of the Weddell Sea. Mar. Ecol. Prog. Ser. 624, 195–212. doi: 10.3354/meps13017
Caccavo, J. A., Papetti, C., Wetjen, M., Knust, R., Ashford, J. R., and Zane, L. (2018). Along-shelf connectivity and circumpolar gene flow in Antarctic silverfish (Pleuragramma antarctica). Sci. Rep. 8:17856. doi: 10.1038/s41598-018-36030-x
Calhaem, I., and Christoffel, D. (1969). Some observations of the feeding habits of a Weddell seal, and measurements of its prey, Dissostichus mawsoni, at McMurdo Sound, Antarctica. N. Ze. J. Mar. Freshw. Res. 3, 181–190. doi: 10.1080/00288330.1969.9515287
Calì, F., Riginella, E., La Mesa, M., and Mazzoldi, C. (2017). Life history traits of Notothenia rossii and N. coriiceps along the southern Scotia Arc. Polar Biol. 40, 1409–1423. doi: 10.1007/s00300-016-2066-z
Canales-Aguirre, C. B., Ferrada-Fuentes, S., Galleguillos, R., Oyarzun, F. X., and Hernández, C. E. (2018). Population genetic structure of Patagonian toothfish (Dissostichus eleginoides) in the Southeast Pacific and Southwest Atlantic Ocean. PeerJ 6:e4173. doi: 10.7717/peerj.4173
Candy, S., and Constable, A. (2008). An integrated stock assessment for the Patagonian toothfish (Dissostichus eleginoides) for the Heard and McDonald Islands using CASAL. CCAMLR Sci. 15, 1–34.
Cannon, S. M., Lavers, J. L., and Figueiredo, B. (2016). Plastic ingestion by fish in the Southern Hemisphere: a baseline study and review of methods. Mar. Pollut. Bull. 107, 286–291. doi: 10.1016/j.marpolbul.2016.03.057
Carlig, E., Di Blasi, D., Ghigliotti, L., Pisano, E., Koubbi, P., and Vacchi, M. (2019). Diversified feeding strategies of Pleuragramma antarctica (Nototheniidae) in the Southern Ocean. Polar Biol. 42, 2045–2054. doi: 10.1007/s00300-019-02579-0
Castellini, M., Davis, R., and Kooymann, G. (1992). Diving behavior and ecology of the Weddell seal: annual cycles. Bull. Scripps Inst. Oceanogr. 28, 1–54.
Causse, R., Ozouf-Costaz, C., Koubbi, P., Lamy, D., Eléaume, M., Dettaï, A., et al. (2011). Demersal ichthyofaunal shelf communities from the Dumont d’Urville Sea (East Antarctica). Polar Sci. 5, 272–285. doi: 10.1016/j.polar.2011.03.004
Cavanagh, R. D., Murphy, E. J., Bracegirdle, T. J., Turner, J., Knowland, C. A., Corney, S. P., et al. (2017). A synergistic approach for evaluating climate model output for ecological applications. Front. Mar. Sci. 4:308. doi: 10.3389/fmars.2017.00308
CCAMLR (2007). Report of the Twenty-Sixth Meeting of the Commission. CCAMLR Meeting Reports. Hobart: CCAMLR.
CCAMLR (2008). Report of the Twenty-Seventh Meeting of the Commission in CCAMLR Meeting Reports. Hobart: CCAMLR.
CCAMLR (2009). “Resolution 30 about climate change,” in Proceedings of the Summary of Conservation Measures and Resolutions in Force 2008/09, Hobart.
Cherel, Y., Ducatez, S., Fontaine, C., Richard, P., and Guinet, C. (2008). Stable isotopes reveal the trophic position and mesopelagic fish diet of female southern elephant seals breeding on the Kerguelen Islands. Mar. Ecol. Prog. Ser. 370, 239–247. doi: 10.3354/meps07673
Cherel, Y., Fontaine, C., Richard, P., and Labatc, J.-P. (2010). Isotopic niches and trophic levels of myctophid fishes and their predators in the Southern Ocean. Limnol. Oceanogr. 55, 324–332. doi: 10.4319/lo.2010.55.1.0324
Cherel, Y., and Weimerskirch, H. (1999). Spawning cycle of onychoteuthid squids in the southern Indian Ocean: new information from seabird predators. Mar. Ecol. Prog. Ser. 188, 93–104. doi: 10.3354/meps188093
Cheung, W., Lam, V., and Pauly, D. (2008). Modelling Present and Climate-Shifted Distribution of Marine Fishes and Invertebrates. Rome: FAO.
Christiansen, H., Dettai, A., Heindler, F. M., Collins, M. A., Duhamel, G., Hautecoeur, M., et al. (2018). Diversity of mesopelagic fishes in the Southern Ocean-A phylogeographic perspective using DNA barcoding. Front. Ecol. Evol. 6:120. doi: 10.3389/fevo.2018.00120
Ciardiello, M. A., Camardella, L., and di Prisco, G. (1995). Glucose-6-phosphate dehydrogenase from the blood cells of two Antarctic teleosts: correlation with cold adaptation. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1250, 76–82. doi: 10.1016/0167-4838(95)00046-w
Clarke, L. J., Trebilco, R., Walters, A., Polanowski, A. M., and Deagle, B. E. (2018). DNA-based diet analysis of mesopelagic fish from the southern Kerguelen Axis. Deep Sea Res. Part II Top. Stud. Oceanogr. 2, 1–23.
Clarke, S., Reid, W. D., Collins, M. A., and Belchier, M. (2008). Biology and distribution of South Georgia icefish (Pseudochaenichthys georgianus) around South Georgia and Shag Rocks. Antarctic Sci. 20:343. doi: 10.1017/s0954102008000990
Collins, M. A., Brickle, P., Brown, J., and Belchier, M. (2010). The Patagonian toothfish: biology, ecology and fishery. Adv. Mar. Biol. 58, 227–300.
Collins, M. A., and Rodhouse, P. G. (2006). Southern ocean cephalopods. Adv. Mar. Biol. 50, 191–265. doi: 10.1016/s0065-2881(05)50003-8
Collins, M. A., Stowasser, G., Fielding, S., Shreeve, R., Xavier, J. C., Venables, H. J., et al. (2012). Latitudinal and bathymetric patterns in the distribution and abundance of mesopelagic fish in the Scotia Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 59, 189–198. doi: 10.1016/j.dsr2.2011.07.003
Collins, M. A., Xavier, J. C., Johnston, N. M., North, A. W., Enderlein, P., Tarling, G. A., et al. (2008). Patterns in the distribution of myctophid fish in the northern Scotia Sea ecosystem. Polar Biol. 31, 837–851. doi: 10.1007/s00300-008-0423-2
Constable, A. J., de la Mare, W. K., Agnew, D. J., Everson, I., and Miller, D. (2000). Managing fisheries to conserve the Antarctic marine ecosystem: practical implementation of the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR). ICES J. Mar. Sci. 57, 778–791. doi: 10.1006/jmsc.2000.0725
Constable, A. J., Melbourne-Thomas, J., Corney, S. P., Arrigo, K. R., Barbraud, C., Barnes, D. K., et al. (2014). Climate change and Southern Ocean ecosystems I: how changes in physical habitats directly affect marine biota. Glob. Change Biol. 20, 3004–3025.
Constable, A. J., and Welsford, D. (2011). “Developing a precautionary, ecosystem approach to managing fisheries and other marine activities at Heard Island and McDonald Islands in the Indian Sector of the Southern Ocean,” in The Kerguelen Plateau: Marine Ecosystem and Fisheries, eds G. Duhamel and D. Welsford (Kingston, TAS: Société française d’ichtyologie).
Croxall, J., Reid, K., and Prince, P. (1999). Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Mar. Ecol. Prog. Ser. 177, 115–131. doi: 10.3354/meps177115
Curran, M. A., van Ommen, T. D., Morgan, V. I., Phillips, K. L., and Palmer, A. S. (2003). Ice core evidence for Antarctic sea ice decline since the 1950s. Science 302, 1203–1206. doi: 10.1126/science.1087888
Damerau, M., Matschiner, M., Salzburger, W., and Hanel, R. (2012). Comparative population genetics of seven notothenioid fish species reveals high levels of gene flow along ocean currents in the southern Scotia Arc. Antarctica. Polar Biol. 35, 1073–1086. doi: 10.1007/s00300-012-1155-x
Daniels, R. A., and Lipps, J. H. (1982). Distribution and ecology of fishes of the antarctic Peninsula. J. Biogeogr. 9, 1–9. doi: 10.2307/2844726
Danovaro, R., Corinaldesi, C., Dell’Anno, A., Fuhrman, J. A., Middelburg, J. J., Noble, R. T., et al. (2011). Marine viruses and global climate change. FEMS Microbiol. Rev. 35, 993–1034.
Davis, R., Fuiman, L., Williams, T., Collier, S., Hagey, W., Kanatous, S., et al. (1999). Hunting behavior of a marine mammal beneath the Antarctic fast ice. Science 283, 993–996. doi: 10.1126/science.283.5404.993
Davison, P. (2011). The specific gravity of mesopelagic fish from the northeastern Pacific Ocean and its implications for acoustic backscatter. ICES J. Mar. Sci. 68, 2064–2074. doi: 10.1093/icesjms/fsr140
Davison, W. (1997). X-cell gill disease in Pagothenia borchgrevinki from McMurdo Sound. Antarctica. Polar Biol. 19, 17–23. doi: 10.1007/s003000050211
Davison, W., and Franklin, C. (2003). Hypertension in Pagothenia borchgrevinki caused by X-cell disease. J. Fish Biol. 63, 129–136. doi: 10.1046/j.1095-8649.2003.00135.x
Demer, D. A. (2004). An estimate of error for the CCAMLR 2000 survey estimate of krill biomass. Deep Sea Res. Part II Top. Stud. Oceanogr. 51, 1237–1251. doi: 10.1016/s0967-0645(04)00077-3
Desvignes, T., Detrich, H. W. III, and Postlethwait, J. H. (2016). Genomic conservation of erythropoietic microRNAs (erythromiRs) in white-blooded Antarctic icefish. Mar. Genomics 30, 27–34. doi: 10.1016/j.margen.2016.04.013
Desvignes, T., Le François, N., Goetz, L., Smith, S., Shusdock, K., Parker, S., et al. (2019). Intergeneric hybrids inform reproductive isolating barriers in the Antarctic icefish radiation. Sci. Rep. 9:5989. doi: 10.1038/s41598-019-42354-z
Dettai, A., Lautredou, A.-C., Bonillo, C., Goimbault, E., Busson, F., Causse, R., et al. (2011). The actinopterygian diversity of the CEAMARC cruises: barcoding and molecular taxonomy as a multi-level tool for new findings. Deep Sea Res. Part II Top. Stud. Oceanogr. 58, 250–263. doi: 10.1016/j.dsr2.2010.05.021
Devries, A. L., and Eastman, J. T. (1978). Lipid sacs as a buoyancy adaptation in an Antarctic fish. Nature 271, 352–353. doi: 10.1038/271352a0
DeWitt, H., Heemstra, P. C., and Gon, O. (1990). ““Nototheniidae,”,” in Fishes of the Southern Ocean, eds O. Gon and P. C. Heemstra (Grahamstown: J.L.B. Smith Institute of Ichthyology).
Dornan, T., Fielding, S., Saunders, R. A., and Genner, M. J. (2019). Swimbladder morphology masks Southern Ocean mesopelagic fish biomass. Proc. R. Soc. B 286:20190353. doi: 10.1098/rspb.2019.0353
Doubleday, Z. A., Prowse, T. A., Arkhipkin, A., Pierce, G. J., Semmens, J., Steer, M., et al. (2016). Global proliferation of cephalopods. Curr. Biol. 26, R406–R407.
Duhamel, G., Hautecoeur, M., Dettai, A., Causse, R., Pruvost, P., Busson, F., et al. (2010). Liparids From the Eastern Sector of Southern Ocean and First Information From Molecular Studies. Paris: Muséum national d’histoire naturelle.
Duhamel, G., Hulley, P.-A., Causse, R., Koubbi, P., Vacchi, M., Pruvost, P., et al. (2014). “Biogeographic patterns of fish,” in Biogeographic Atlas of the Southern Ocean, eds C. De Broyer, P. Koubbi, H. J. Griffiths, B. Raymond, B. Danis, B. David, et al. (Cambridge: Scientific Committee on Antarctic Research), 328–362.
Duhamel, G., Koubbi, P., and Ravier, C. (2000). Day and night mesopelagic fish assemblages off the Kerguelen Islands (Southern Ocean). Polar Biol. 23, 106–112. doi: 10.1007/s003000050015
Duhamel, G., Péron, C., Sinègre, R., Chazeau, C., and Gasco, N. (2019). “Important readjustments in the biomass and distribution of groundfish species in the northern part of the Kerguelen plateau and skiff bank,” in Proceedings of the Second Symposium on The Kerguelen Plateau: Marine Ecosystem and Fisheries, eds D. Welsford, J. Dell, and G. Duhamel (Hobart, TAS: CCAMLR), 135–184.
Eastman, J. T. (1993). Antarctic Fish Biology: Evolution in a Unique Environment. San Diego, CA: Academic Press.
Eastman, J. T. (2005). The nature of the diversity of Antarctic fishes. Polar Biol. 28, 93–107. doi: 10.1007/s00300-004-0667-4
Eastman, J. T. (2017). Bathymetric distributions of notothenioid fishes. Polar Biol. 40, 1–19. doi: 10.1007/s00300-017-2128-x
Eastman, J. T., Barrera-Oro, E., and Moreira, E. (2011). Adaptive radiation at a low taxonomic level: divergence in buoyancy of the ecologically similar Antarctic fish Notothenia coriiceps and N. rossii. Mar. Ecol. Prog. Ser. 438, 195–206. doi: 10.3354/meps09287
Eastman, J. T., and DeVries, A. L. (1981). Buoyancy adaptations in a swim-bladderless Antarctic fish. J. Morphol. 167, 91–102. doi: 10.1002/jmor.1051670108
Eastman, J. T., and DeVries, A. L. (1989). Ultrastructure of the lipid sac wall in the Antarctic notothenioid fish Pleuragramma antarcticum. Polar Biol. 9, 333–335. doi: 10.1007/BF00287433
Eastman, J. T., Witmer, L. M., Ridgely, R. C., and Kuhn, K. L. (2014). Divergence in skeletal mass and bone morphology in antarctic notothenioid fishes. J. Morphol. 275, 841–861. doi: 10.1002/jmor.20258
Eisert, R., Pinkerton, M., Torres, L., Currey, R., Ensor, P., Ovsyanikova, E., et al. (2014). Update on the Top Predator Alliance Project, 2013–14 Season: killer Whales. Document SC-CAMLR-WG-EMM-14/52. Hobart, TAS: CCAMLR.
Escobar-Flores, P. C., Driscoll, R. L., and Montgomery, J. C. (2018). Spatial and temporal distribution patterns of acoustic backscatter in the New Zealand sector of the Southern Ocean. Mar. Ecol. Prog. Ser. 592, 19–35. doi: 10.3354/meps12489
Evans, C. W., and Tupmongkol, K. (2014). X-cell disease in Antarctic fishes. Polar Biol. 37, 1261–1269. doi: 10.1007/s00300-014-1518-6
Everson, I., and Murray, A. (1999). Size at sexual maturity of Patagonian toothfish. CCAMLR Sci. 6, 37–46.
Fenaughty, J. M., Stevens, D. W., and Hanchet, S. M. (2003). Diet of the Antarctic toothfish (Dissostichus mawsoni) from the Ross Sea, Antarctica (Subarea 88.1). CCAMLR Sci. 10, 113–123.
Ferreira, M. F., Varela, M. L., Nostro, F. L., Ansaldo, M., and Genovese, G. (2017). Reproductive aspects of Notothenia rossii and N. coriiceps (Perciformes, Nototheniidae) at Potter Cove, 25 de Mayo (King George) Island during austral summer. Polar Biol. 40, 1–11. doi: 10.1007/s00300-016-1918-x
Fielding, S., Watkins, J. L., Collins, M. A., Enderlein, P., and Venables, H. J. (2012). Acoustic determination of the distribution of fish and krill across the Scotia Sea in spring 2006, summer 2008 and autumn 2009. Deep Sea Res. Part II Top. Stud. Oceanogr. 59, 173–188. doi: 10.1016/j.dsr2.2011.08.002
Flores, H., Atkinson, A., Kawaguchi, S., Krafft, B., Milinevsky, G., Nicol, S., et al. (2012). Impact of climate change on Antarctic krill. Mar. Ecol. Prog. Ser. 458, 1.
Flynn, E. E., Bjelde, B. E., Miller, N. A., and Todgham, A. E. (2015). Ocean acidification exerts negative effects during warming conditions in a developing Antarctic fish. Conserv. Physiol. 3:cov033. doi: 10.1093/conphys/cov033
Forgati, M., Kandalski, P. K., Herrerias, T., Zaleski, T., Machado, C., Souza, M. R. D. P., et al. (2017). Effects of heat stress on the renal and branchial carbohydrate metabolism and antioxidant system of Antarctic fish. J. Comp. Physiol. B 187, 1137–1154. doi: 10.1007/s00360-017-1088-3
Franklin, C., and Davison, W. (1988). X-cells in the gills of an Antarctic teleost, Pagothenia borchgrevinki. J. Fish Biol. 32, 341–353. doi: 10.1111/j.1095-8649.1988.tb05372.x
Freeman, M. A., Fuss, J., Kristmundsson, A., Bjorbækmo, M. F., Mangot, J.-F., Del Campo, J., et al. (2017). X-cells are globally distributed, genetically divergent fish parasites related to perkinsids and dinoflagellates. Curr. Biol. 27, 1645–1651.3.
Freer, J. J., Tarling, G. A., Collins, M. A., Partridge, J. C., and Genner, M. J. (2019). Predicting future distributions of lanternfish, a significant ecological resource within the Southern Ocean. Divers. Distrib. 25, 1259–1272. doi: 10.1111/ddi.12934
Fuiman, L. A., Davis, R. W., and Williams, T. M. (2002). Behavior of midwater fishes under the Antarctic ice: observations by a predator. Mar. Biol. 140, 815–822. doi: 10.1007/s00227-001-0752-y
Ghigliotti, L., Ferrando, S., Carlig, E., Di Blasi, D., Gallus, L., Pisano, E., et al. (2017). Reproductive features of the Antarctic silverfish (Pleuragramma antarctica) from the western Ross Sea. Polar Biol. 40, 199–211. doi: 10.1007/s00300-016-1945-7
Giraldo, C., Cherel, Y., Vallet, C., Mayzaud, P., Tavernier, E., Moteki, M., et al. (2011). Ontogenic changes in the feeding ecology of the early life stages of the Antarctic silverfish (Pleuragramma antarcticum) documented by stable isotopes and diet analysis in the Dumont d’Urville Sea (East Antarctica). Polar Sci. 5, 252–263. doi: 10.1016/j.polar.2011.04.004
Giraldo, C., Mayzaud, P., Tavernier, E., Boutoute, M., Penot, F., and Koubbi, P. (2015). Lipid dynamics and trophic patterns in Pleuragramma antarctica life stages. Antarctic Sci. 27, 429–438. doi: 10.1017/S0954102015000036
Gjøsæter, J., and Kawaguchi, K. (1980). A Review of the World Resources of Mesopelagic fish. Rome: FAO.
Gordeev, I., and Sokolov, S. (2017). Helminths and the feeding habits of the marbled moray cod Muraenolepis marmorata Günther, 1880 (Gadiformes, Muraenolepididae) in the Ross Sea (Southern Ocean). Polar Biol. 40, 1311–1318. doi: 10.1007/s00300-016-2055-2
Granata, A., Zagami, G., Vacchi, M., and Guglielmo, L. (2009). Summer and spring trophic niche of larval and juvenile Pleuragramma antarcticum in the Western Ross Sea. Antarctica. Polar Biol. 32, 369–382. doi: 10.1007/s00300-008-0551-8
Griffiths, H. J., Van de Putte, A. P., and Danis, B. (2014). “Data distribution: patterns and implications,” in Biogeographic Atlas of the Southern Ocean, eds C. De Broyer, P. Koubbi, H. J. Griffiths, B. Danis, B. David, and S. Grant (Cambridge: Scientific Committee on Antarctic Research), 16–26.
Griffiths, S. P., Olson, R. J., and Watters, G. M. (2013). Complex wasp-waist regulation of pelagic ecosystems in the Pacific Ocean. Rev. Fish Biol. Fish. 23, 459–475. doi: 10.1007/s11160-012-9301-7
Guidetti, P., Ghigliotti, L., and Vacchi, M. (2015). Insights into spatial distribution patterns of early stages of the Antarctic silverfish, Pleuragramma antarctica, in the platelet ice of Terra Nova Bay, Antarctica. Polar Biol. 38, 333–342. doi: 10.1007/s00300-014-1589-4
Guynn, K. D., and Peterson, M. S. (2008). Mercury concentrations in the Patagonian toothfish, Dissostichus eleginoides Smitt 1898, among three distinct stocks. Polar Biol. 31, 269–274. doi: 10.1007/s00300-007-0354-3
Hanchet, S., Dunn, A., Parker, S., Horn, P., Stevens, D., and Mormede, S. (2015). The Antarctic toothfish (Dissostichus mawsoni): biology, ecology, and life history in the Ross Sea region. Hydrobiologia 761, 397–414. doi: 10.1007/s10750-015-2435-6
Hanchet, S., Stevenson, M., Phillips, N., and Horn, P. (2004). A Characterisation of the Toothfish Fishery in Subareas 88.1 and 88.2 From 1997/98 to 2003/04. Document WGFSA04/20. Hobart: CCAMLR.
Hanchet, S. M., Rickard, G. J., Fenaughty, J. M., Dunn, A., and Williams, M. J. H. (2008). A hypothetical life cycle for Antarctic toothfish (Dissostichus mawsoni) in the Ross Sea region. CCAMLR Sci. 15, 35–53.
Hanchet, S. M., Tracey, D., Dunn, A., Horn, P., and Smith, N. (2012). Mercury concentrations of two toothfish and three of its prey species from the Pacific sector of the Antarctic. Antarctic Sci. 24:34. doi: 10.1017/s0954102011000654
Harter, T. S., Sackville, M. A., Wilson, J. M., Metzger, D. C., Egginton, S., Esbaugh, A. J., et al. (2018). A solution to Nature’s haemoglobin knockout: a plasma-accessible carbonic anhydrase catalyses CO2 excretion in Antarctic icefish gills. J. Exp. Biol. 221(Pt 22), jeb190918.
Haschemeyer, A., Guschlbauer, W., and Devries, A. L. (1977). Water binding by antifreeze glycoproteins from Antarctic fish. Nature 269, 87–88. doi: 10.1038/269087a0
Heaps, L., Jones, C., Watson, A., Berkieta, K., and Pearce, J. (1999). Depth distribution and spawning pattern of Dissostichus eleginoides at South Georgia. CCAMLR Sci. 6, 19–36.
Hellmer, H. H., Kauker, F., Timmermann, R., Determann, J., and Rae, J. (2012). Twenty-first-century warming of a large Antarctic ice-shelf cavity by a redirected coastal current. Nature 485, 225–228. doi: 10.1038/nature11064
Hill, S. L., Keeble, K., Atkinson, A., and Murphy, E. J. (2012). A foodweb model to explore uncertainties in the South Georgia shelf pelagic ecosystem. Deep Sea Res. Part II Top. Stud. Oceanogr. 5, 237–252. doi: 10.1016/j.dsr2.2011.09.001
Hill, S. L., Phillips, T., and Atkinson, A. (2013). Potential climate change effects on the habitat of Antarctic krill in the Weddell quadrant of the Southern Ocean. PLoS One 8:e72246. doi: 10.1371/journal.pone.0072246
Hillary, R., Kirkwood, G., and Agnew, D. (2006). An assessment of toothfish in Subarea 48.3 using CASAL. CCAMLR Sci. 13, 65–95.
Hopkins, T. L., and Torres, J. J. (1988). The zooplankton community in the vicinity of the ice edge, western Weddell Sea, March 1986. Polar Biol. 9, 79–87. doi: 10.1007/BF00442033
Horn, P. L. (2002). Age and growth of Patagonian toothfish (Dissostichus eleginoides) and Antarctic toothfish (D. mawsoni) in waters from the New Zealand subantarctic to the Ross Sea, Antarctica. Fish. Res. 56, 275–287. doi: 10.1016/S0165-7836(01)00325-3
Hubold, G. (1984). Spatial distribution of Pleuragramma antarcticum (Pisces: Nototheniidae) near the Filchner- and Larsen ice shelves (Weddell Sea/Antarctica). Polar Biol. 3, 231–236. doi: 10.1007/BF00292628
Irigoien, X., Klevjer, T. A., Røstad, A., Martinez, U., Boyra, G., Acuña, J. L., et al. (2014). Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 5, 1–10.
Jones, C., and Near, T. (2012). The reproductive behaviour of Pogonophryne scotti confirms widespread egg-guarding parental care among Antarctic notothenioids. J. Fish Biol. 80, 2629–2635. doi: 10.1111/j.1095-8649.2012.03282.x
Jones, G. P., Srinivasan, M., and Almany, G. R. (2007). Population connectivity and conservation of marine biodiversity. Oceanography 20, 100–111. doi: 10.5670/oceanog.2007.33
Joyce, W., Egginton, S., Farrell, A. P., Crockett, E. L., O’Brien, K. M., and Axelsson, M. (2018). Exploring nature’s natural knockouts: in vivo cardiorespiratory performance of Antarctic fishes during acute warming. J. Exp. Biol. 221(Pt 15):jeb183160.
Kaartvedt, S., Staby, A., and Aksnes, D. L. (2012). Efficient trawl avoidance by mesopelagic fishes causes large underestimation of their biomass. Mar. Ecol. Prog. Ser. 456, 1–6. doi: 10.3354/meps09785
Kandalski, P. K., de Souza, M. R. D. P., Herrerias, T., Machado, C., Zaleski, T., Forgati, M., et al. (2018). Effects of short-term thermal stress on the plasma biochemical profiles of two Antarctic nototheniid species. Rev. Fish Biol. Fish. 28, 925–940. doi: 10.1007/s11160-018-9535-0
Kellermann, A. (1986). Geographical distribution and abundance of postlarval and juvenile Pleuragramma antarcticum (Pisces, Notothenioidei) off the Antarctic Peninsula. Polar Biol. 6, 111–119. doi: 10.1007/BF00258262
Kellermann, A. (1987). Food and feeding ecology of postlarval and juvenile Pleuragramma antarcticum (Pisces; Notothenioidei) in the seasonal pack ice zone off the Antarctic peninsula. Polar Biol. 7, 307–315. doi: 10.1007/BF00443949
Kim, B.-M., Amores, A., Kang, S., Ahn, D.-H., Kim, J.-H., Kim, I.-C., et al. (2019). Antarctic blackfin icefish genome reveals adaptations to extreme environments. Nat. Ecol. Evol. 3, 469–478. doi: 10.1038/s41559-019-0812-7
Kim, C. S., Kim, T. W., Cho, K. H., Ha, H. K., Lee, S., Kim, H. C., et al. (2016). Variability of the Antarctic Coastal Current in the Amundsen Sea. Estuar. Coast. Shelf Sci. 181, 123–133. doi: 10.1016/j.ecss.2016.08.004
Kiss, A. J., Mirarefi, A. Y., Ramakrishnan, S., Zukoski, C. F., DeVries, A. L., and Cheng, C.-H. C. (2004). Cold-stable eye lens crystallins of the Antarctic nototheniid toothfish Dissostichus mawsoni Norman. J. Exp. Biol. 207, 4633–4649. doi: 10.1242/jeb.01312
Klimpel, S., Busch, M. W., Kuhn, T., Rohde, A., and Palm, H. W. (2010). The Anisakis simplex complex off the South Shetland Islands (Antarctica): endemic populations versus introduction through migratory hosts. Mar. Ecol. Prog. Ser. 403, 1–11. doi: 10.3354/meps08501
Klimpel, S., Kuhn, T., and Mehlhorn, H. (2017). Biodiversity and Evolution of Parasitic Life in the Southern Ocean. Berlin: Springer.
Klimpel, S., and Palm, H. W. (2011). “Anisakid nematode (Ascaridoidea) life cycles and distribution: increasing zoonotic potential in the time of climate change?,” in Progress in Parasitology, ed. H. Mehlhorn (Berlin: Springer), 201–222. doi: 10.1007/978-3-642-21396-0_11
Klingenberg, C. P., and Ekau, W. (1996). A combined morphometric and phylogenetic analysis of an ecomorphological trend: pelagization in Antarctic fishes (Perciformes: Nototheniidae). Biol. J. Linnean Soc. 59, 143–177. doi: 10.1111/j.1095-8312.1996.tb01459.x
Kloser, H., Plotz, J., Palm, H., Bartsch, A., and Hubold, G. (1992). Adjustment of anisakid nematode life cycles to the high Antarctic food web as shown by Contracaecum radiatum and C. osculatum in the Weddell Sea. Antarctic Sci. 4, 171–178. doi: 10.1017/s0954102092000269
Kloser, R. J., Ryan, T. E., Keith, G., and Gershwin, L. (2016). Deep-scattering layer, gas-bladder density, and size estimates using a two-frequency acoustic and optical probe. ICES J. Mar. Sci. 73, 2037–2048. doi: 10.1093/icesjms/fsv257
Kloser, R. J., Ryan, T. E., Young, J. W., and Lewis, M. E. (2009). Acoustic observations of micronekton fish on the scale of an ocean basin: potential and challenges. ICES J. Mar. Sci. 66, 998–1006. doi: 10.1093/icesjms/fsp077
Kock, K., and Belchier, M. (2004). Is the attempt to estimate the biomass of Antarctic fish from a multi-species survey appropriate for all targeted species? Notothenia rossii in the Atlantic Ocean sector–revisited. CCAMLR Sci. 11, 141–153. doi: 10.1007/bf00287000
Kock, K.-H. (2005a). Antarctic icefishes (Channichthyidae): a unique family of fishes. A review, Part I. Polar Biol. 28, 862–895. doi: 10.1007/s00300-005-0019-z
Kock, K.-H. (2005b). Antarctic icefishes (Channichthyidae): a unique family of fishes. A review, Part II. Polar Biol. 28, 897–909. doi: 10.1007/s00300-005-0020-6
Kock, K. H. (2007). Antarctic marine living resources - exploitation and its management in the Southern Ocean. Antarctic Sci. 19, 231–238. doi: 10.1017/s0954102007000302
Kock, K.-H., Hureau, J. C., and Duhamel, G. (1985). Biology and Status of Exploited Antarctic fish Stocks: A Review. Cambridge: Scott Polar Research Institute.
Kock, K. H., and Jones, C. D. (2005). Fish stocks in the southern Scotia Arc region - a review and prospects for future research. Rev. Fish. Sci. 13, 75–108. doi: 10.1080/10641260590953900
Kooyman, G. L. (1967). An analysis of some behavioral and physiological characteristics related to diving in the Weddell seal. Antarct. Res. Ser. 11, 227–261. doi: 10.1029/ar011p0227
Koubbi, P., Duhamel, G., Hecq, J.-H., Beans, C., Loots, C., Pruvost, P., et al. (2009). Ichthyoplankton in the neritic and coastal zone of Antarctica and Subantarctic islands: a review. J. Mar. Syst. 78, 547–556. doi: 10.1016/j.jmarsys.2008.12.024
Koubbi, P., Moteki, M., Duhamel, G., Goarant, A., Hulley, P.-A., O’driscoll, R., et al. (2011a). Ecoregionalization of myctophid fish in the Indian sector of the Southern Ocean: results from generalized dissimilarity models. Deep Sea Res. Part II Top. Stud. Oceanogr. 58, 170–180. doi: 10.1016/j.dsr2.2010.09.007
Koubbi, P., O’Brien, C., Loots, C., Giraldo, C., Smith, M., Tavernier, E., et al. (2011b). Spatial distribution and inter-annual variations in the size frequency distribution and abundances of Pleuragramma antarcticum larvae in the Dumont d’Urville Sea from 2004 to 2010. Polar Sci. 5, 225–238. doi: 10.1016/j.polar.2011.02.003
Koubbi, P., Ozouf-Costaz, C., Goarant, A., Moteki, M., Hulley, P., Romain, C., et al. (2010). Estimating the biodiversity of the East Antarctic shelf and oceanic zone for ecoregionalisation: Example of the ichthyofauna of the CEAMARC (Collaborative East Antarctic Marine Census) CAML surveys. Polar Sci. 4, 115–133. doi: 10.1016/j.polar.2010.04.012
Koubbi, P., Vallet, C., Razouls, S., Grioche, A., Hilde, D., Courcot, L., et al. (2007). Condition and diet of larval Pleuragramma antarcticum (Nototheniidae) from Terre Adélie (Antarctica) during summer. Cybium 31, 67–76.
Kozlov, A., Shust, K., and Zemsky, A. (1991). Seasonal and inter-annual variability in the distribution of Electrona carlsbergi in the Southern Polar Front area (the area to the north of South Georgia is used as an example). CCAMLR Sel. Sci. Pap. 7, 337–368.
Kuhn, K. L., and Gaffney, P. M. (2008). Population subdivision in the Antarctic toothfish (Dissostichus mawsoni) revealed by mitochondrial and nuclear single nucleotide polymorphisms (SNPs). Antarctic Sci. 20, 327–338. doi: 10.1017/S0954102008000965
Kuhn, T., Zizka, V. M., Münster, J., Klapper, R., Mattiucci, S., Kochmann, J., et al. (2018). Lighten up the dark: metazoan parasites as indicators for the ecology of Antarctic crocodile icefish (Channichthyidae) from the north-west Antarctic Peninsula. PeerJ 6:e4638. doi: 10.7717/peerj.4638
La Mesa, M., Catalano, B., Russo, A., Greco, S., Vacchi, M., and Azzali, M. (2010). Influence of environmental conditions on spatial distribution and abundance of early life stages of antarctic silverfish, Pleuragramma antarcticum (Nototheniidae), in the Ross Sea. Antarctic Sci. 22, 243–254. doi: 10.1017/S0954102009990721
La Mesa, M., and Eastman, J. T. (2012). Antarctic silverfish: life strategies of a key species in the high-Antarctic ecosystem. Fish Fish. 13, 241–266. doi: 10.1111/j.1467-2979.2011.00427.x
La Mesa, M., Eastman, J. T., and Vacchi, M. (2004). The role of notothenioid fish in the food web of the Ross Sea shelf waters: a review. Polar Biol. 27, 321–338. doi: 10.1007/s00300-004-0599-z
La Mesa, M., La Mesa, G., Catalano, B., and Jones, C. D. (2016). Spatial distribution pattern and physical – biological interactions in the larval notothenioid fish assemblages from the Bransfield Strait and adjacent waters. Fish. Oceanogr. 25, 624–636. doi: 10.1111/fog.12178
La Mesa, M., Llompart, F., Riginella, E., and Eastman, J. T. (2021). Parental care and reproductive strategies in notothenioid fishes. Fish Fish. 22, 356–376. doi: 10.1111/faf.12523
La Mesa, M., Piñones, A., Catalano, B., and Ashford, J. (2015). Predicting early life connectivity of Antarctic silverfish, an important forage species along the Antarctic Peninsula. Fish. Oceanogr. 24, 150–161. doi: 10.1111/fog.12096
Lacoue-Labarthe, T., Warnau, M., Oberhänsli, F., Teyssié, J.-L., and Bustamante, P. (2009). Bioaccumulation of inorganic Hg by the juvenile cuttlefish Sepia officinalis exposed to 203Hg radiolabelled seawater and food. Aquat. Biol. 6, 91–98. doi: 10.3354/ab00172
Lancraft, T. M., Reisenbichler, K. R., Robison, B. H., Hopkins, T. L., and Torres, J. J. (2004). A krill-dominated micronekton and macrozooplankton community in Croker Passage, Antarctica with an estimate of fish predation. Deep Sea Res. Part II Top. Stud. Oceanogr. 51, 2247–2260. doi: 10.1016/j.dsr2.2004.07.004
Laptikhovsky, V., Arkhipkin, A., and Brickle, P. (2006). Distribution and reproduction of the Patagonian toothfish Dissostichus eleginoides Smitt around the Falkland Islands. J. Fish Biol. 68, 849–861. doi: 10.1111/j.0022-1112.2006.00973.x
Laptikhovsky, V., and Brickle, P. (2005). The Patagonian toothfish fishery in Falkland Islands’ waters. Fish. Res. 74, 11–23. doi: 10.1016/j.fishres.2005.04.006
Le François, N. R., Sheehan, E., Desvignes, T., Belzile, C., Postlethwait, J. H., and Detrich, H. W. (2017). Characterization and husbandry of wild broodstock of the blackfin icefish Chaenocephalus aceratus (Lönnberg 1906) from the Palmer Archipelago (Southern Ocean) for breeding purposes. Polar Biol. 40, 2499–2516. doi: 10.1007/s00300-017-2161-9
Lehodey, P. (2019). “Report of the 3rd MESOPP Workshop: designing the needs for the implementation of a global coupled acoustic-based observation-modelling system,” in Proceedings of the Third MESOPP Workshop 9 - 11 Oct. 2018, Falmouth.
Lin, D., Walters, A., Bestley, S., Zhu, G., Chen, X., and Trebilco, R. (2020). Distribution of larval and juvenile pelagic squids in the Kerguelen axis region: oceanographic influence on size structure and evidence of spawning locations. Deep Sea Res. Part II Top. Stud. Oceanogr. 174:104615. doi: 10.1016/j.dsr2.2019.07.003
Loeb, V. J., Kellermann, A. K., Koubbi, P., North, A. W., and White, M. G. (1993). Antarctic larval fish assemblages: a review. Bull. Mar. Sci. 53, 416–449.
López-Abellán, L. (2005). Patagonian toothfish in international waters of the southwest Indian Ocean (Statistical Area 51). CCAMLR Sci. J. Sci. Commit. 12, 207–214.
Lubimova, T., Shust, K., and Popkov, V. (1987). Specific Features in the Ecology of Southern Ocean Mesopelagic Fish of the Family Myctophidae. Moscow: Nauka Press, 320–337.
Machado, C., Zaleski, T., Rodrigues, E., dos Santos, Carvalho, C., Cadena, S. M. S. C., et al. (2014). Effect of temperature acclimation on the liver antioxidant defence system of the Antarctic nototheniids Notothenia coriiceps and Notothenia rossii. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 172, 21–28. doi: 10.1016/j.cbpb.2014.02.003
Mark, F. C., Lucassen, M., Strobel, A., Barrera-Oro, E., Koschnick, N., Zane, L., et al. (2012). Mitochondrial function in antarctic nototheniids with ND6 translocation. PLoS One 7:e31860. doi: 10.1371/journal.pone.0031860
Marlow, T., and Agnew, D. (2003). Movement and growth of tagged Dissostichus eleginoides around South Georgia and Shag Rocks (Subarea 48.3). CCAMLR Sci. 10, 101–111.
Marschoff, E. R., Barrera-Oro, E. R., Alescio, N. S., and Ainley, D. G. (2012). Slow recovery of previously depleted demersal fish at the South Shetland Islands, 1983–2010. Fish. Res. 125, 206–213. doi: 10.1016/j.fishres.2012.02.017
Matschiner, M., Colombo, M., Damerau, M., Ceballos, S., Hanel, R., and Salzburger, W. (2015). “The adaptive radiation of notothenioid fishes in the waters of Antarctica,” in Extremophile Fishes, eds R. Riesch, M. Tobler, and M. Plath (Berlin: Springer), 35–57. doi: 10.1007/978-3-319-13362-1_3
Matschiner, M., Hanel, R., and Salzburger, W. (2009). Gene flow by larval dispersal in the Antarctic notothenioid fish Gobionotothen gibberifrons. Mol. Ecol. 18, 2574–2587. doi: 10.1111/j.1365-294X.2009.04220.x
Mattiucci, S., Cipriani, P., Paoletti, M., Nardi, V., Santoro, M., Bellisario, B., et al. (2015). Temporal stability of parasite distribution and genetic variability values of Contracaecum osculatum sp. D and C. osculatum sp. E (Nematoda: Anisakidae) from fish of the Ross Sea (Antarctica). Int. J. Parasitol. Parasit. Wildlife 4, 356–367. doi: 10.1016/j.ijppaw.2015.10.004
McArthur, T., Butler, E. C., and Jackson, G. D. (2003). Mercury in the marine food chain in the Southern Ocean at Macquarie Island: an analysis of a top predator, Patagonian toothfish (Dissostichus eleginoides) and a mid-trophic species, the warty squid (Moroteuthis ingens). Polar Biol. 27, 1–5. doi: 10.1007/s00300-003-0560-6
McCormack, S. A., Melbourne-Thomas, J., Trebilco, R., Blanchard, J. L., and Constable, A. (2020). Alternative energy pathways in Southern Ocean food webs: Insights from a balanced model of Prydz Bay, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 174:104613. doi: 10.1016/j.dsr2.2019.07.001
McMillan, P., Iwamoto, T., Stewart, A., and Smith, P. J. (2012). A new species of grenadier, genus Macrourus (Teleostei, Gadiformes, Macrouridae) from the southern hemisphere and a revision of the genus. Zootaxa 3165, 1–24. doi: 10.11646/zootaxa.3165.1.1
Méndez, E., Giudice, H., Pereira, A., Inocente, G., and Medina, D. (2001). Preliminary report on the total mercury content of Patagonian toothfish (Dissostichos eleginoides). J. Food Compos. Anal. 14, 547–549. doi: 10.1006/jfca.2001.1006
Meredith, M. P., and King, J. C. (2005). Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 32:L19604.
Metcalf, V. J., Brennan, S. O., and George, P. M. (1999). The Antarctic toothfish (Dissostichus mawsoni) lacks plasma albumin and utilises high density lipoprotein as its major palmitate binding protein. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 124, 147–155. doi: 10.1016/s0305-0491(99)00051-6
Militelli, M. I., Macchi, G. J., and Rodrigues, K. A. (2015). Maturity and fecundity of Champsocephalus gunnari, Chaenocephalus aceratus and Pseudochaenichthys georgianus in South Georgia and Shag Rocks Islands. Polar Sci. 9, 258–266. doi: 10.1016/j.polar.2015.03.004
Miller, D. G. M. (2004). “Patagonian Toothfish - the storm gathers,” in Fish Piracy: Combating Illegal, Unreported and Unregulated Fishing, ed. K. Gray (Bhubaneswar: IMIS), 105–145.
Mills, W. F., Xavier, J. C., Bearhop, S., Cherel, Y., Votier, S. C., Waluda, C. M., et al. (2020). Long-term trends in albatross diets in relation to prey availability and breeding success. Mar. Biol. 167, 1–12.
Mintenbeck, K. (2017). Impacts of climate change on the Southern Ocean. Climate Change Impacts Fish. Aquacult. Glob. Anal. 2, 663–701. doi: 10.1002/9781119154051.ch20
Mintenbeck, K., Barrera-Oro, E. R., Brey, T., Jacob, U., Knust, R., Mark, F. C., et al. (2012). Impact of climate change on fishes in complex antarctic ecosystems. Adv. Ecol. Res. 46, 351–426. doi: 10.1016/b978-0-12-396992-7.00006-x
Miya, T., Gon, O., Mwale, M., and Cheng, C.-H. C. (2014). The effect of habitat temperature on serum antifreeze glycoprotein (AFGP) activity in Notothenia rossii (Pisces: Nototheniidae) in the Southern Ocean. Polar Biol. 37, 367–373. doi: 10.1007/s00300-013-1437-y
Moffat, C., Beardsley, R. C., Owens, B., and van Lipzig, N. (2008). A first description of the Antarctic Peninsula coastal current. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 277–293. doi: 10.1016/j.dsr2.2007.10.003
Moreira, E., Juáres, M., and Barrera-Oro, E. (2014). Dietary overlap among early juvenile stages in an Antarctic notothenioid fish assemblage at Potter Cove, South Shetland Islands. Polar Biol. 37, 1507–1515. doi: 10.1007/s00300-014-1545-3
Morley, S. A., Abele, D., Barnes, D. K. A., Cárdenas, C. A., Cotté, C., Gutt, J., et al. (2020). Global drivers on southern ocean ecosystems: changing physical environments and anthropogenic pressures in an earth system. Front. Mar. Sci. 7:1097. doi: 10.3389/fmars.2020.547188
Mormede, S., Dunn, A., and Hanchet, S. M. (2014). A stock assessment model of Antarctic toothfish incorporating multi-year mark-recapture data. CCAMLR Sci. 21, 39–62.
Moteki, M., Fujii, K., Amakasu, K., Shimada, K., Tanimura, A., and Odate, T. (2017). Distributions of larval and juvenile/adult stages of the Antarctic myctophid fish, Electrona antarctica, off Wilkes Land in East Antarctica. Polar Sci. 12, 99–108. doi: 10.1016/j.polar.2017.02.004
Moteki, M., Koubbi, P., Pruvost, P., Tavernier, E., and Hulley, P.-A. (2011). Spatial distribution of pelagic fish off Adélie and George V Land, East Antarctica in the austral summer 2008. Polar Sci. 5, 211–224. doi: 10.1016/j.polar.2011.04.001
Mueller, I. A., Devor, D. P., Grim, J. M., Beers, J. M., Crockett, E. L., and O’Brien, K. M. (2012). Exposure to critical thermal maxima increases oxidative stress in hearts of white-but not red-blooded Antarctic notothenioid fishes. J. Exp. Biol. 215, 3655–3664. doi: 10.1242/jeb.071811
Mugue, N. S., Petrov, A. F., Zelenina, D. A., Gordeev, I. I., and Sergeev, A. A. (2014). Low genetic diversity and temporal stability in the Antarctic toothfish (Dissostichus mawsoni) from near-continental seas of Antarctica. CCAMLR Sci. 21, 1–10.
Muñoz, G., and Rebolledo, M. (2019). Comparison of the parasite community of two notothens, Notothenia rossii and N. coriiceps (Pisces: Nototheniidae), from King George Island, Antarctica. J. Helminthol. 93, 732–737. doi: 10.1017/s0022149x18000858
Münster, J., Kochmann, J., Grigat, J., Klimpel, S., and Kuhn, T. (2017). Parasite fauna of the Antarctic dragonfish Parachaenichthys charcoti (Perciformes: Bathydraconidae) and closely related Bathydraconidae from the Antarctic Peninsula, Southern Ocean. Parasit. Vect. 10, 1–9.
Murphy, E., Cavanagh, R., Hofmann, E., Hill, S., Constable, A., Costa, D., et al. (2012). Developing integrated models of Southern Ocean food webs: including ecological complexity, accounting for uncertainty and the importance of scale. Prog. Oceanogr. 102, 74–92. doi: 10.1016/j.pocean.2012.03.006
Murphy, E. J., Trathan, P. N., Watkins, J. L., Reid, K., Meredith, M. P., Forcada, J., et al. (2007a). Climatically driven fluctuations in Southern Ocean ecosystems. Proc. R. Soc. B Biol. Sci. 274, 3057–3067. doi: 10.1098/rspb.2007.1180
Murphy, E. J., Watkins, J. L., Trathan, P. N., Reid, K., Meredith, M. P., Thorpe, S. E., et al. (2007b). Spatial and temporal operation of the Scotia Sea ecosystem: a review of large-scale links in a krill centred food web. Philos. Trans. R. Soc. B Biol. Sci. 362, 113–148. doi: 10.1098/rstb.2006.1957
Nahrgang, J., Dubourg, P., Frantzen, M., Storch, D., Dahlke, F., and Meador, J. P. (2016). Early life stages of an arctic keystone species (Boreogadus saida) show high sensitivity to a water-soluble fraction of crude oil. Environ. Pollut. 218, 605–614. doi: 10.1016/j.envpol.2016.07.044
Nash, S. B. (2011). Persistent organic pollutants in Antarctica: current and future research priorities. J. Environ. Monitor. 13, 497–504. doi: 10.1039/c0em00230e
Near, T. J., Parker, S. K., and Detrich, H. W. III (2006). A genomic fossil reveals key steps in hemoglobin loss by the antarctic icefishes. Mol. Biol. Evol. 23, 2008–2016. doi: 10.1093/molbev/msl071
Near, T. J., Russo, S. E., Jones, C. D., and DeVries, A. L. (2003). Ontogenetic shift in buoyancy and habitat in the Antarctic toothfish, Dissostichus mawsoni (Perciformes: Nototheniidae). Polar Biol. 26, 124–128. doi: 10.1007/s00300-002-0459-7
Newman, L., Heil, P., Trebilco, R., Katsumata, K., Constable, A., van Wijk, E., et al. (2019). Delivering sustained, coordinated, and integrated observations of the Southern Ocean for global impact. Front. Mar. Sci. 6:433. doi: 10.3389/fmars.2019.00433
Nicol, S., and Foster, J. (2016). “The fishery for Antarctic krill: its current status and management regime,” in Biology and Ecology of Antarctic krill, ed. V. Siegel (Cham: Springer), 387–421. doi: 10.1007/978-3-319-29279-3_11
Novillo, M., Moreira, E., Macchi, G., and Barrera-Oro, E. (2019). Reproductive effort in Chaenocephalus aceratus validated by gonadal histology: inshore sites serve as spawning grounds for some notothenioids. Polar Biol. 42, 1959–1972. doi: 10.1007/s00300-019-02571-8
Núñez-Riboni, I., and Fahrbach, E. (2009). Seasonal variability of the Antarctic coastal current and its driving mechanisms in the Weddell Sea. Deep Sea Res. Part I Oceanogr. Res. Papers 56, 1927–1941. doi: 10.1016/j.dsr.2009.06.005
O’Brien, K. M., Rix, A. S., Egginton, S., Farrell, A. P., Crockett, E. L., Schlauch, K., et al. (2018). Cardiac mitochondrial metabolism may contribute to differences in thermal tolerance of red-and white-blooded Antarctic notothenioid fishes. J. Exp. Biol. 221(Pt 15), jeb177816.
O’Driscoll, R. L., Canese, S., Ladroit, Y., Parker, S. J., Ghigliotti, L., Mormede, S., et al. (2018a). First in situ estimates of acoustic target strength of Antarctic toothfish (Dissostichus mawsoni). Fish. Res. 206, 79–84. doi: 10.1016/j.fishres.2018.05.008
O’Driscoll, R. L., Ladroit, Y., Parker, S. J., Vacchi, M., Canese, S., Ghigliotti, L., et al. (2018b). Acoustic deployments reveal Antarctic silverfish under ice in the Ross Sea. Antarctic Sci. 30, 345–353. doi: 10.1017/s0954102018000366
O’Driscoll, R. L., Leonori, I., De Felice, A., and Macaulay, G. J. (2017). “Acoustic methods of monitoring antarctic silverfish distribution and abundance,” in The Antarctic Silverfish: A Keystone Species in a Changing Ecosystem, eds M. Vacchi, E. Pisano, and L. Ghigliotti (Cham: Springer International Publishing), 237–252. doi: 10.1007/978-3-319-55893-6_11
Oguz, M. C., Tepe, Y., Belk, M. C., Heckmann, R. A., Aslan, B., Gürgen, M., et al. (2015). Metazoan parasites of Antarctic fishes. Türkiye Parazitolojii Dergisi 39:174. doi: 10.5152/tpd.2015.3661
Olsson, O., and North, A. (1997). Diet of the king penguin Aptenodytes patagonicus during three summers at South Georgia. Ibis 139, 504–512. doi: 10.1111/j.1474-919x.1997.tb04666.x
Orsi, A. H., Whitworth, T. III, and Nowlin, W. D. Jr. (1995). On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Res. Part I 42, 641–673. doi: 10.1016/0967-0637(95)00021-W
Österblom, H., Bodin, O., Rashid Sumaila, U., and Press, A. J. (2015). Reducing illegal fishing in the Southern Ocean: a global effort. Solutions 4, 72–79.
Pakhomov, E., Perissinotto, R., and McQuaid, C. (1996). Prey composition and daily rations of myctophid fishes in the Southern Ocean. Mar. Ecol. Prog. Ser. 134, 1–14. doi: 10.3354/meps134001
Palm, H. W. (2011). “Fish parasites as biological indicators in a changing world: can we monitor environmental impact and climate change?,” in Progress in Parasitology, ed. H. Mehlhom (Berlin: Springer), 223–250. doi: 10.1007/978-3-642-21396-0_12
Palm, H. W., Klimpel, S., and Walter, T. (2007). Demersal fish parasite fauna around the South Shetland Islands: high species richness and low host specificity in deep Antarctic waters. Polar Biol. 30, 1513–1522. doi: 10.1007/s00300-007-0312-0
Papetti, C., Pujolar, J. M., Mezzavilla, M., La Mesa, M., Rock, J., Zane, L., et al. (2012). Population genetic structure and gene flow patterns between populations of the Antarctic icefish Chionodraco rastrospinosus. J. Biogeogr. 39, 1361–1372. doi: 10.1111/j.1365-2699.2011.02682.x
Papetti, C., Susana, E., La Mesa, M., Kock, K. H., Patarnello, T., and Zane, L. (2007). Microsatellite analysis reveals genetic differentiation between year-classes in the icefish Chaenocephalus aceratus at South Shetlands and Elephant Island. Polar Biol. 30, 1605–1613. doi: 10.1007/s00300-007-0325-8
Papetti, C., Susana, E., Patarnello, T., and Zane, L. (2009). Spatial and temporal boundaries to gene flow between Chaenocephalus aceratus populations at South Orkney and South Shetlands. Mar. Ecol. Prog. Ser. 376, 269–281. doi: 10.3354/meps07831
Paris, J. R., Sherman, K. D., Bell, E., Boulenger, C., Delord, C., El-Mahdi, M. B. M., et al. (2018). Understanding and managing fish populations: keeping the toolbox fit for purpose. J. Fish Biol. 92, 727–751. doi: 10.1111/jfb.13549
Parker, R. W., Paige, K. N., and DeVries, A. L. (2002). Genetic variation among populations of the Antarctic toothfish: evolutionary insights and implications for conservation. Polar Biol. 25, 256–261. doi: 10.1007/s00300-001-0333-z
Parker, S., and Grimes, P. (2010). Length and age at spawning of Antarctic toothfish (Dissostichus mawsoni) in the Ross Sea. CCAMLR Sci. 17, 53–73.
Parker, S. J., and Mormede, S. (2015). “Descriptive analysis of the toothfish (Dissostichus spp.) tagging programme in Subareas 88.1 & 88.2 for the years 2000-01 to 2014-15,” in Proceedings of the CCAMLR Scientific Papers Working Group on Fish Stock Assessment WG-FSA-15/37, New Zealand.
Parker, S. J., Stevens, D. W., Ghigliotti, L., La Mesa, M., Di Blasi, D., and Vacchi, M. (2019). Winter spawning of Antarctic toothfish Dissostichus mawsoni in the Ross Sea region. Antarctic Sci. 31, 243–253. doi: 10.1017/S0954102019000282
Pilling, G., Purves, M., Daw, T., Agnew, D., and Xavier, J. (2001). The stomach contents of Patagonian toothfish around South Georgia (South Atlantic). J. Fish Biol. 59, 1370–1384. doi: 10.1111/j.1095-8649.2001.tb00198.x
Pinkerton, M., McMillan, P., Forman, J., Marriott, P., Horn, P., Bury, S., et al. (2013). Distribution, morphology and ecology of Macrourus whitsoni and M. caml (Gadiformes, Macrouridae) in the Ross Sea region. CCAMLR Sci. 20, 37–61.
Pinkerton, M. H. (2017). “Diet and trophic ecology of adult Antarctic Silverfish (Pleuragramma antarctica),” in The Antarctic Silverfish: a Keystone Species in a Changing Ecosystem, eds M. Vacchi, E. Pisano, and L. Ghigliotti (Cham: Springer International Publishing), 93–111. doi: 10.1007/978-3-319-55893-6_5
Pinkerton, M. H., Boyd, P., Deppeler, S., Hayward, A., Höfer, J., and Moreau, S. (2021). Evidence for the impact of climate change on primary producers in the Southern Ocean. Front. Mar. Sci. 9:592027. doi: 10.3389/fevo.2021.592027
Pinkerton, M. H., and Bradford-Grieve, J. M. (2014). Characterizing foodweb structure to identify potential ecosystem effects of fishing in the Ross Sea. Antarctica. ICES J. Mar. Sci. 71, 1542–1553. doi: 10.1093/icesjms/fst230
Pitman, R. L., and Ensor, P. (2003). Three forms of killer whales (Orcinus orca) in Antarctic waters. J. Cetacean Res. Manag. 5, 131–140.
Plötz, J., Bornemann, H., Knust, R., Schröder, A., and Bester, M. (2001). Foraging behaviour of Weddell seals, and its ecological implications. Polar Biol. 24, 901–909. doi: 10.1007/s003000100297
Pointer, M. A., Cheng, C.-H. C., Bowmaker, J. K., Parry, J. W., Soto, N., Jeffery, G., et al. (2005). Adaptations to an extreme environment: retinal organisation and spectral properties of photoreceptors in Antarctic notothenioid fish. J. Exp. Biol. 208, 2363–2376. doi: 10.1242/jeb.01647
Postlethwait, J. H., Yan, Y. L., Desvignes, T., Allard, C., Titus, T., Le François, N. R., et al. (2016). Embryogenesis and early skeletogenesis in the antarctic bullhead notothen, Notothenia coriiceps. Dev. Dyn. 245, 1066–1080. doi: 10.1002/dvdy.24437
Prirodina, V., and Balushkin, A. (2015). First record of Muraenolepis evseenkoi in the Weddell Sea and identification key to the eel cods (Muraenolepididae) of continental seas of Antarctica. J. Ichthyol. 55, 778–782. doi: 10.1134/s0032945215060168
Proud, R., Cox, M. J., and Brierley, A. S. (2017). Biogeography of the global ocean’s mesopelagic zone. Curr. Biol. 27, 113–119. doi: 10.1016/j.cub.2016.11.003
Proud, R., Handegard, N. O., Kloser, R. J., Cox, M. J., and Brierley, A. S. (2019). From siphonophores to deep scattering layers: uncertainty ranges for the estimation of global mesopelagic fish biomass. ICES J. Mar. Sci. 76, 718–733. doi: 10.1093/icesjms/fsy037
Queirós, J. P., Hilário, J. A., Thompson, D., Ceia, F. R., Elliott, G., Walker, K., et al. (2020). From hot to cold waters: new insights in the habitat and trophic ecology of Southern Ocean squids during their life cycle. Mar. Ecol. Prog. Ser. 659, 113–126. doi: 10.3354/meps13551
Raclot, T., Groscolas, R., and Cherel, Y. (1998). Fatty acid evidence for the importance of myctophid fishes in the diet of king penguins. Aptenodytes patagonicus. Mar. Biol. 132, 523–533. doi: 10.1007/s002270050418
Rayfuse, R. (2018). Climate change and Antarctic fisheries: ecosystem management in CCAMLR. Ecol. LQ 45, 53.
Reid, K. (2019). “Climate change impacts, vulnerabilities and adaptations: Southern Ocean marine fisheries,” in Impacts of Climate Change on Fisheries and Aquaculture (Rome: FAO), 363.
Reid, W. D., Clarke, S., Collins, M. A., and Belchier, M. (2007). Distribution and ecology of Chaenocephalus aceratus (Channichthyidae) around South Georgia and Shag Rocks (Southern Ocean). Polar Biol. 30, 1523–1533. doi: 10.1007/s00300-007-0313-z
Reiss, H., Hoarau, G., Dickey-Collas, M., and Wolff, W. J. (2009). Genetic population structure of marine fish: mismatch between biological and fisheries management units. Fish Fish. 10, 361–395. doi: 10.1111/j.1467-2979.2008.00324.x
Riaz, J., Walters, A., Trebilco, R., Bestley, S., and Lea, M.-A. (2020). Stomach content analysis of mesopelagic fish from the southern Kerguelen Axis. Deep Sea Res. Part II Top. Stud. Oceanogr. 174:104659. doi: 10.1016/j.dsr2.2019.104659
Riginella, E., Mazzoldi, C., Ashford, J., Jones, C. D., Morgan, C., and La Mesa, M. (2016). Life history strategies of the Scotia Sea icefish, Chaenocephalus aceratus, along the Southern Scotia Ridge. Polar Biol. 39, 497–509. doi: 10.1007/s00300-015-1802-0
Rintoul, S. R., and da Silva, C. E. (2019). “Antarctic circumpolar current,” in Encyclopedia of Ocean Sciences (Third Edition), eds J. K. Cochran, H. J. Bokuniewicz, and P. L. Yager (Oxford: Academic Press), 248–261.
Roberts, J., Xavier, J. C., and Agnew, D. (2011). The diet of toothfish species Dissostichus eleginoides and Dissostichus mawsoni with overlapping distributions. J. Fish Biol. 79, 138–154. doi: 10.1111/j.1095-8649.2011.03005.x
Robison, B. H. (2003). What drives the diel vertical migrations of Antarctic midwater fish? J. Mar. Biol. Assoc. United Kingdom 83, 639–642. doi: 10.1017/S0025315403007586h
Rodhouse, P., Griffiths, H., and Xavier, J. (2014a). “Southern ocean squid,” in Biogeographic Atlas of the Southern Ocean, eds C. De Broyer, P. Koubbi, H. J. Griffiths, B. Raymond, A. Van De Putte, B. Danis, et al. (Cambridge: Scientific Committee on Antarctic Research), 284–289.
Rodhouse, P. G. (2013). Role of squid in the Southern Ocean pelagic ecosystem and the possible consequences of climate change. Deep Sea Res. Part II Top. Stud. Oceanogr. 95, 129–138. doi: 10.1016/j.dsr2.2012.07.001
Rodhouse, P. G., Pierce, G. J., Nichols, O. C., Sauer, W. H., Arkhipkin, A. I., Laptikhovsky, V. V., et al. (2014b). Environmental effects on cephalopod population dynamics: implications for management of fisheries. Adv. Mar. Biol. 67, 99–233. doi: 10.1016/b978-0-12-800287-2.00002-0
Rodhouse, P. G., White, M. G., and Jones, M. (1992). Trophic relations of the cephalopod Martialia hyadesi (Teuthoidea: Ommastrephidae) at the Antarctic Polar Front, Scotia Sea. Mar. Biol. 114, 415–421. doi: 10.1007/bf00350032
Rogers, A., Morley, S., Fitzcharles, E., Jarvis, K., and Belchier, M. (2006). Genetic structure of Patagonian toothfish (Dissostichus eleginoides) populations on the Patagonian Shelf and Atlantic and western Indian Ocean Sectors of the Southern Ocean. Mar. Biol. 149, 915–924. doi: 10.1007/s00227-006-0256-x
Rosa, R., Lopes, V. M., Guerreiro, M., Bolstad, K., and Xavier, J. C. (2017). Biology and ecology of the world’s largest invertebrate, the colossal squid (Mesonychoteuthis hamiltoni): a short review. Polar Biol. 40, 1871–1883. doi: 10.1007/s00300-017-2104-5
Santoro, M., Mattiucci, S., Work, T., Cimmaruta, R., Nardi, V., Cipriani, P., et al. (2013). Parasitic infection by larval helminths in Antarctic fishes: pathological changes and impact on the host body condition index. Dis. Aquat. Organ. 105, 139–148. doi: 10.3354/dao02626
Saunders, R. A., Fielding, S., Thorpe, S. E., and Tarling, G. A. (2013). School characteristics of mesopelagic fish at South Georgia. Deep Sea Res. Part I Oceanogr. Res. Pap. 81, 62–77. doi: 10.1016/j.dsr.2013.07.007
Saunders, R. A., Hill, S. L., Tarling, G. A., and Murphy, E. J. (2019). Myctophid fish (Family Myctophidae) are central consumers in the food web of the Scotia Sea (Southern Ocean). Front. Mar. Sci. 6:530. doi: 10.3389/fmars.2019.00530
Saunders, R. A., and Tarling, G. A. (2018). Southern Ocean mesopelagic fish comply with Bergmann’s rule. Am. Natural. 191, 343–351. doi: 10.1086/695767
Schaafsma, F. L., Cherel, Y., Flores, H., Van Franeker, J. A., Lea, M.-A., Raymond, B., et al. (2018). The energetic value of zooplankton and nekton species of the Southern Ocean. Mar. Biol. 165, 1–35. doi: 10.1016/b978-012374473-9.00663-9
Schiaparelli, S., Danis, B., Wadley, V., and Michael Stoddart, D. (2013). “The census of antarctic marine life: the first available baseline for antarctic marine biodiversity,” in Adaptation and Evolution in Marine Environments, Volume 2: The Impacts of Global Change on Biodiversity, eds C. Verde and G. di Prisco (Berlin: Springer), 3–19. doi: 10.1007/978-3-642-27349-0_1
Schiavon, L., Dulière, V., La Mesa, M., Marino, I. A. M., Codogno, G., Boscari, E., et al. (2021). Species distribution, hybridization and connectivity in the genus Chionodraco: unveiling unknown icefish diversity in antarctica. Divers. Distribut. 27, 766–783. doi: 10.1111/ddi.13249
Seco, J., Aparício, S., Brierley, A. S., Bustamante, P., Ceia, F. R., Coelho, J. P., et al. (2021). Mercury biomagnification in a Southern Ocean food web. Environ. Pollut. 275:116620. doi: 10.1016/j.envpol.2021.116620
Seco, J., Xavier, J. C., Brierley, A. S., Bustamante, P., Coelho, J. P., Gregory, S., et al. (2020). Mercury levels in Southern Ocean squid: variability over the last decade. Chemosphere 239, 124785. doi: 10.1016/j.chemosphere.2019.124785
Simmonds, J., and MacLennan, D. (2005). Fisheries Acoustics: Theory and Practice. Hoboken, NJ: Wiley-Blackwell.
Smeele, Z. E., Ainley, D. G., and Varsani, A. (2018). Viruses associated with Antarctic wildlife: from serology based detection to identification of genomes using high throughput sequencing. Virus Res. 243, 91–105. doi: 10.1016/j.virusres.2017.10.017
Smith, P., and McVeagh, M. (2000). Allozyme and microsatellite DNA markers of toothfish population structure in the Southern Ocean. J. Fish Biol. 57, 72–83. doi: 10.1111/j.1095-8649.2000.tb02245.x
Son, K.-T., Kwon, J.-Y., Jo, M.-R., Yoon, M., Song, K.-C., Choi, W.-S., et al. (2014). Total mercury contents of Antarctic toothfish Dissostichus Mawsoni caught in the Antarctic Sea. Fish. Aquat. Sci. 17, 427–431. doi: 10.5657/fas.2014.0427
Stevens, D. W., Dunn, M. R., Pinkerton, M. H., and Forman, J. S. (2014). Diet of Antarctic toothfish (Dissostichus mawsoni) from the continental slope and oceanic features of the Ross Sea region, Antarctica. Antarctic Sci. 26, 502–512. doi: 10.1017/S095410201300093X
Stowasser, G., Atkinson, A., McGill, R., Phillips, R., Collins, M. A., and Pond, D. (2012). Food web dynamics in the Scotia Sea in summer: a stable isotope study. Deep Sea Res. Part II Top. Stud. Oceanogr. 59, 208–221. doi: 10.1016/j.dsr2.2011.08.004
Strobel, A., Bennecke, S., Leo, E., Mintenbeck, K., Pörtner, H. O., and Mark, F. C. (2012). Metabolic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and P CO2. Front. Zool. 9:28. doi: 10.1186/1742-9994-9-28
Strobel, A., Leo, E., Pörtner, H. O., and Mark, F. C. (2013). Elevated temperature and P CO2 shift metabolic pathways in differentially oxidative tissues of Notothenia rossii. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 166, 48–57. doi: 10.1016/j.cbpb.2013.06.006
Subramaniam, R. C., Melbourne-Thomas, J., Corney, S. P., Alexander, K., Péron, C., Ziegler, P., et al. (2020). Time-dynamic food web modeling to explore environmental drivers of ecosystem change on the Kerguelen Plateau. Front. Mar. Sci. 7:641. doi: 10.3389/fmars.2020.00641
Taillebois, L., Barton, D. P., Crook, D. A., Saunders, T., Taylor, J., Hearnden, M., et al. (2017). Strong population structure deduced from genetics, otolith chemistry and parasite abundances explains vulnerability to localized fishery collapse in a large Sciaenid fish. Protonibea diacanthus. Evol. Appl. 10, 978–993. doi: 10.1111/eva.12499
Tavares, S., Xavier, J., Phillips, R., Pereira, M., and Pardal, M. (2013). Influence of age, sex and breeding status on mercury accumulation patterns in the wandering albatross Diomedea exulans. Environ. Pollut. 181, 315–320. doi: 10.1016/j.envpol.2013.06.032
Tavernier, E., and Giraldo, C. (2017). “Trophic ecology of early developmental stages of Antarctic Silverfish,” in The Antarctic Silverfish: A Keystone Species in a Changing Ecosystem, eds M. Vacchi, E. Pisano, and L. Ghigliotti (Cham: Springer International Publishing), 113–130. doi: 10.1007/978-3-319-55893-6_6
Testa, J., Siniff, D., Ross, M., and Winter, J. (1985). “Weddell seal—Antarctic cod interactions in McMurdo Sound, Antarctica,” in Antarctic Nutrient Cycles and Food Webs, eds M. R. Siegfried, P. R. Condy, and R. M. Laws (Berlin: Springer), 561–565. doi: 10.1007/978-3-642-82275-9_76
Thompson, A. F., Stewart, A. L., Spence, P., and Heywood, K. J. (2018). The antarctic slope current in a changing climate. Rev. Geophys. 56, 741–770. doi: 10.1029/2018RG000624
Todgham, A. E., Hoaglund, E. A., and Hofmann, G. E. (2007). Is cold the new hot? Elevated ubiquitin-conjugated protein levels in tissues of Antarctic fish as evidence for cold-denaturation of proteins in vivo. J. Comp. Physiol. B 177, 857–866. doi: 10.1007/s00360-007-0183-2
Toomey, L., Welsford, D., Appleyard, S. A., Polanowski, A., Faux, C., Deagle, B. E., et al. (2016). Genetic structure of Patagonian toothfish populations from otolith DNA. Antarctic Sci. 28, 1–14. doi: 10.1017/S0954102016000183
Trebilco, R., Melbourne-Thomas, J., and Constable, A. J. (2020). The policy relevance of Southern Ocean food web structure: implications of food web change for fisheries, conservation and carbon sequestration. Mar. Policy 115:103832. doi: 10.1016/j.marpol.2020.103832
Troccoli, G. H., Aguilar, E., Martínez, P. A., and Belleggia, M. (2020). The diet of the Patagonian toothfish Dissostichus eleginoides, a deep-sea top predator off Southwest Atlantic Ocean. Polar Biol. 43, 1595–1604. doi: 10.1007/s00300-020-02730-2
UNEP (2011). “Climate change and POPs: predicting the impacts,” in Proceedings of the Report of the UNEP/AMAP Expert Group (Geneva: Secretariat of the Stockholm Convention).
Vacchi, M., DeVries, A. L., Evans, C. W., Bottaro, M., Ghigliotti, L., Cutroneo, L., et al. (2012). A nursery area for the Antarctic silverfish Pleuragramma antarcticum at Terra Nova Bay (Ross Sea): first estimate of distribution and abundance of eggs and larvae under the seasonal sea-ice. Polar Biol. 35, 1573–1585. doi: 10.1007/s00300-012-1199-y
Vacchi, M., Pisano, E., and Ghigliotti, L. (2017). The Antarctic Silverfish: A Keystone Species in a Changing Ecosystem. Berlin: Springer.
Van de Putte, A. P., Jackson, G. D., Pakhomov, E., Flores, H., and Volckaert, F. A. (2010). Distribution of squid and fish in the pelagic zone of the Cosmonaut Sea and Prydz Bay region during the BROKE-West campaign. Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 956–967. doi: 10.1016/j.dsr2.2008.02.015
Van Doorslaer, K., Kraberger, S., Austin, C., Farkas, K., Bergeman, M., Paunil, E., et al. (2018). Fish polyomaviruses belong to two distinct evolutionary lineages. J. Gen. Virol. 99, 567. doi: 10.1099/jgv.0.001041
Vernet, M., Geibert, W., Hoppema, M., Brown, P. J., Haas, C., Hellmer, H. H., et al. (2019). The Weddell Gyre, Southern Ocean: present knowledge and future challenges. Rev. Geophys. 57, 623–708. doi: 10.1029/2018RG000604
Voskoboinikova, O. (1997). Osteological development of the Channichthyidae (Teleostei: Notothenioidei). Cybium 21, 369–379.
Waller, C. L., Griffiths, H. J., Waluda, C. M., Thorpe, S. E., Loaiza, I., Moreno, B., et al. (2017). Microplastics in the Antarctic marine system: an emerging area of research. Sci. Total Environ. 598, 220–227. doi: 10.1016/j.scitotenv.2017.03.283
Ward, P., Atkinson, A., Venables, H. J., Tarling, G. A., Whitehouse, M. J., Fielding, S., et al. (2012). Food web structure and bioregions in the Scotia Sea: a seasonal synthesis. Deep Sea Res. Part II Top. Stud. Oceanogr. 59, 253–266. doi: 10.1016/j.dsr2.2011.08.005
Warren, J. D. (2012). Counting critters in the sea using active acoustics. Acoust. Today 8, 25–34. doi: 10.1121/1.4753914
Wiener, J., Bodaly, R., Brown, S., Lucotte, M., Newman, M., Porcella, D., et al. (2007). “Monitoring and evaluating trends in methylmercury accumulation in aquatic biota,” in Ecosystem Responses to Mercury Contamination: Indicators of Change, eds R. Harris, D. P. Krabbenhoft, R. P. Mason, M. W. Murray, R. J. Reash, and T. Saltman (Pensacola: Taylor and Francis), 87–122. doi: 10.1201/9780849388897.ch4
William, K. (1997). Abrupt mid-twentieth-century decline in Antarctic sea-ice extent from whaling records. Nature 389, 57–60. doi: 10.1038/37956
Williams, R., and Tuck, G. (2002). Movement, growth and available abundance to the fishery of Dissostichus eleginoides Smitt, 1898 at Heard Island, derived from tagging experiments. CCAMLR Science 9, 33–48.
Woods, B., Walters, A., Hindell, M., and Trebilco, R. (2020). Isotopic insights into mesopelagic niche space and energy pathways on the southern Kerguelen Plateau. Deep Sea Res. Part II Top. Stud. Oceanogr. 174:104657. doi: 10.1016/j.dsr2.2019.104657
Xavier, J., and Cherel, Y. (2021). Cephalopod Beak Guide for the Southern Ocean: An Update on Taxonomy. Cambridge: British Antarctic Survey.
Xavier, J., Croxall, J., and Reid, K. (2003). Interannual variation in the diets of two albatross species breeding at South Georgia: implications for breeding performance. Ibis 145, 593–610. doi: 10.1046/j.1474-919x.2003.00196.x
Xavier, J. C., Cherel, Y., Allcock, L., Rosa, R., Sabirov, R. M., Blicher, M. E., et al. (2018). A review on the biodiversity, distribution and trophic role of cephalopods in the Arctic and Antarctic marine ecosystems under a changing ocean. Mar. Biol. 165:93. doi: 10.1007/s00227-018-3352-9
Xavier, J. C., Raymond, B., Jones, D. C., and Griffiths, H. (2016). Biogeography of cephalopods in the Southern Ocean using habitat suitability prediction models. Ecosystems 19, 220–247. doi: 10.1007/s10021-015-9926-1
Xavier, J. C., Trathan, P. N., Ceia, F. R., Tarling, G. A., Adlard, S., Fox, D., et al. (2017). Sexual and individual foraging segregation in Gentoo penguins Pygoscelis papua from the Southern Ocean during an abnormal winter. PLos One 12:e0174850. doi: 10.1371/journal.pone.0174850
Yates, P., Ziegler, P., Welsford, D., Wotherspoon, S., Burch, P., and Maschette, D. (2019). Distribution of Antarctic toothfish Dissostichus mawsoni along East Antarctica: environmental drivers and management implications. Fish. Res. 219:105338. doi: 10.1016/j.fishres.2019.105338
Yoon, M., Jo, M. R., Kim, P. H., Choi, W. S., Kang, S. I., Choi, S. G., et al. (2018). Total and methyl mercury concentrations in Antarctic toothfish (Dissostichus mawsoni): health risk assessment. Bull. Environ. Contamin. Toxicol. 100, 748–753. doi: 10.1007/s00128-018-2326-4
Young, E. F., Belchier, M., Hauser, L., Horsburgh, G. J., Meredith, M. P., Murphy, E. J., et al. (2015). Oceanography and life history predict contrasting genetic population structure in two Antarctic fish species. Evol. Appl. 8, 486–509. doi: 10.1111/eva.12259
Young, E. F., Rock, J., Meredith, M. P., Belchier, M., Murphy, E. J., and Carvalho, G. R. (2012). Physical and behavioural influences on larval fish retention: contrasting patterns in two Antarctic fishes. Mar. Ecol. Prog. Ser. 465, 201–215. doi: 10.3354/meps09908
Young, E. F., Tysklind, N., Meredith, M. P., Bruyn, M., Belchier, M., Murphy, E. J., et al. (2018). Stepping stones to isolation: impacts of a changing climate on the connectivity of fragmented fish populations. Evol. Appl. 11, 978–994. doi: 10.1111/eva.12613
Yukhov, V. L. (1971). The range of Dissostichus mawsoni Norman and some features of its biology. J. Ichthyol. 11, 8–18.
Zane, L., Marcato, S., Bargelloni, L., Bortolotto, E., Papetti, C., Simonato, M., et al. (2006). Demographic history and population structure of the Antarctic silverfish Pleuragramma antarcticum. Mol. Ecol. 15, 4499–4511. doi: 10.1111/j.1365-294X.2006.03105.x
Zhu, G. P. (2018). “Progress report on collaborative research for otolith chemistry of Antarctic toothfish Dissostichus mawsoni in the Southern Ocean,” in CCAMLR Scientific Papers Working Group on Fish Stock Assessment WG-FSA-19/61.
Ziegler, P., and Welsford, D. (2019). “The Patagonian toothfish (Dissostichus eleginoides) fishery at Heard Island and McDonald Islands (HIMI) – population structure and history of the fishery stock assessment,” in The Kerguelen Plateau: Marine Ecosystem and Fisheries, eds G. Duhamel and D. Welsford (Kingston, TAS: Société française d’ichtyologie).
Keywords: marine ecosystem assessment, climate change, conservation management, Antarctic, fisheries, notothenioids, myctophids, squid
Citation: Caccavo JA, Christiansen H, Constable AJ, Ghigliotti L, Trebilco R, Brooks CM, Cotte C, Desvignes T, Dornan T, Jones CD, Koubbi P, Saunders RA, Strobel A, Vacchi M, van de Putte AP, Walters A, Waluda CM, Woods BL and Xavier JC (2021) Productivity and Change in Fish and Squid in the Southern Ocean. Front. Ecol. Evol. 9:624918. doi: 10.3389/fevo.2021.624918
Received: 01 November 2020; Accepted: 10 May 2021;
Published: 25 June 2021.
Edited by:
Orsolya Valkó, Centre for Ecological Research, Hungarian Academy of Sciences, HungaryReviewed by:
Xabier Irigoien, King Abdullah University of Science and Technology, Saudi ArabiaMasato Moteki, Tokyo University of Marine Science and Technology, Japan
Copyright © 2021 Caccavo, Christiansen, Constable, Ghigliotti, Trebilco, Brooks, Cotte, Desvignes, Dornan, Jones, Koubbi, Saunders, Strobel, Vacchi, van de Putte, Walters, Waluda, Woods and Xavier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jilda Alicia Caccavo, ergo@jildacaccavo.com
 Jilda Alicia Caccavo
Jilda Alicia Caccavo Henrik Christiansen
Henrik Christiansen Andrew J. Constable
Andrew J. Constable Laura Ghigliotti
Laura Ghigliotti Rowan Trebilco
Rowan Trebilco Cassandra M. Brooks
Cassandra M. Brooks Cédric Cotte
Cédric Cotte Thomas Desvignes
Thomas Desvignes Tracey Dornan
Tracey Dornan Christopher D. Jones
Christopher D. Jones Philippe Koubbi15,16
Philippe Koubbi15,16  Ryan A. Saunders
Ryan A. Saunders Anneli Strobel
Anneli Strobel Anton P. van de Putte
Anton P. van de Putte Andrea Walters
Andrea Walters Claire M. Waluda
Claire M. Waluda Briannyn L. Woods
Briannyn L. Woods José C. Xavier
José C. Xavier