- 1Institute of Medical Microbiology, University Hospital Essen, University Duisburg-Essen, Essen, Germany
- 2Institute of Biology, Humboldt University, Berlin, Germany
- 3Parasitology Unit, Max Planck Institute for Infection Biology, Berlin, Germany
The cytokine IL-10 plays a crucial role during malaria infection by counteracting the pro-inflammatory immune response. We and others demonstrated that Plasmodium yoelii infection results in enhanced IL-10 production in CD4+ T cells accompanied by the induction of an immunosuppressive phenotype. However, it is unclear whether this is a direct effect caused by the parasite or an indirect consequence due to T cell activation by IL-10-producing antigen-presenting cells. Here, we demonstrate that CD11c+CD11b+CD8− dendritic cells (DCs) produce elevated levels of IL-10 after P. yoelii infection of BALB/c mice. DC-specific ablation of IL-10 in P. yoelii-infected IL-10flox/flox/CD11c-cre mice resulted in increased IFN-γ and TNF-α production with no effect on MHC-II, CD80, or CD86 expression in CD11c+ DCs. Accordingly, DC-specific ablation of IL-10 exacerbated systemic IFN-γ and IL-12 production without altering P. yoelii blood stage progression. Strikingly, DC-specific inactivation of IL-10 in P. yoelii-infected mice interfered with the induction of IL-10-producing CD4+ T cells while raising the frequency of IFN-γ-secreting CD4+ T cells. These results suggest that P. yoelii infection promotes IL-10 production in DCs, which in turn dampens secretion of pro-inflammatory cytokines and supports the induction of CD4+IL-10+ T cells.
Introduction
Malaria is caused by the parasite Plasmodium and is one of the most important infectious diseases in humans worldwide. During blood stage propagation of Plasmodium infection, CD4+ T cells and humoral immune responses are essential components to control infection. However, immune responses against the parasite have to be tightly controlled to guarantee parasite clearance without excessive immune activation that might result in exacerbated tissue damage and increased mortality, emphasizing the need for immunoregulatory mechanisms (1, 2).
Recently, we demonstrated that infection of BALB/c mice with Plasmodium yoelii resulted in an expansion of naturally occurring CD4+Foxp3+ regulatory T cells (Tregs) (3, 4). Depletion of these cells by using the well-established DEREG mouse model (5) strongly increased T cell activation accompanied by a more efficient pathogen clearance (3). Besides Tregs, the cytokine IL-10 was identified as key immunoregulator during infection with different pathogens including Plasmodium ssp. (6). Injection of recombinant IL-10 protected susceptible mice from experimental cerebral malaria induced by Plasmodium berghei infection (7), and ablation of IL-10 resulted in higher plasma levels of pro-inflammatory cytokines and increased mortality of Plasmodium chabaudi-infected mice (8). IL-10-deficient mice infected with a non-lethal P. yoelii strain exhibited lower parasitemia (9), and ablation of IL-10 receptor (IL-10R) signaling resulted in decreased parasite burden in C57BL/6 mice infected with a virulent P. yoelii strain (10), underlining the importance of IL-10 in the regulation of immune responses during malaria infection.
It is well established that different immune cell types including B cells (11), macrophages, dendritic cells (DCs) (12), and several T cell subsets can produce IL-10 (13). During P. yoelii infection, we and other demonstrated elevated IL-10 expression by CD4+Foxp3+ Tregs and CD4+Foxp3− T cells (3, 9). Induction of IL-10 production in CD4+Foxp3− T cells during P. yoelii conferred immunosuppressive function to these cells, in terms of reduced proliferation and production of pro-inflammatory cytokines of cocultured responder cells (3). However, it remains unclear whether induction of these type 1 regulatory T cells (Tr1) is a parasite-driven effect or results from cytokine release of other immune cells and/or interaction with antigen-presenting cells (APCs), such as DCs, during the course of infection.
Dendritic cells are a heterogenous population of APCs playing a crucial role in the induction and regulation of cell-mediated immune responses (14). They can be subdivided into several subpopulations based on their expression of a variety of cell surface markers and their responses to pathogen molecules. Conventional CD11c+ dendritic cells mainly compromise of CD11b−CD8+ and CD11b+CD8− subsets, which have been suggested to exhibit different functions. Whereas CD8+ DCs may have a superior ability to prime CD8+ T cells, CD11b+ DCs are thought to be more efficient in MHC-II antigen presentation to CD4+ T cells (15), the T cell type that plays an important role in the blood stage of malaria infection (16).
One immunoregulatory property of DCs is the production of IL-10 in the course of inflammatory responses (17–19) and infectious diseases (20–22). During parasitic infection ablation of IL-10R expression specifically in DCs resulted in enhanced immune responses associated with elevated Th1 responses and reduced parasitemia (23), comparable to ubiquitous IL-10-deficient mice (24), demonstrating that IL-10 might act in a paracrine and autocrine manner. IL-10 plays also an important role during interaction of DCs with T cells, since IL-10 signaling in DCs was suggested to be dispensable during naïve T cell priming but critical to prevent exaggerated effector T cell responses during skin inflammation (25). On the other hand, interaction of naïve T cells with IL-10-producing DCs was shown to induce immunosuppressive function (26–28). Since we and others detected the induction of IL-10-producing CD4+ Tr1 cells in the course of P. yoelii infection (3, 9), we asked whether DC-derived IL-10 contributes to this process. Therefore, we analyzed IL-10 secretion by DC subpopulations and its impact on the adaptive immune response in P. yoelii-infected mice.
Materials and Methods
Mice and Parasites
IL-10eGFP mice (29) (Jackson Laboratories, Bar Harbour, ME, USA), IL-10flox/flox mice (30) (provided by Axel Roers, Dresden, Germany and Werner Müller, Manchester, UK), CD11c-cre mice (31), all on BALB/c background and BALB/c mice (Harlan Laboratories, Borchen, Germany) were crossed and maintained under specific pathogen-free conditions at the Animal Facility of the University Hospital Essen, Germany. Cryopreserved P. yoelii 17XNL (non-lethal)-infected red blood cells (iRBCs) were passaged once through BALB/c mice before being used in experimental animals. For infection 1 × 105 iRBCs were injected i.v. The frequency of iRBCs (parasitemia) was determined by microscopic examination of Giemsa-stained blood films. The study was carried out in accordance with the guidelines of the German Animal Protection Law and the State Authority for Nature, Environment and Customer Protection, North Rhine-Westphalia, Germany. The protocol was approved by the State Authority for Nature, Environment and Customer Protection, North Rhine-Westphalia, Germany.
Cell Isolation
Single cell suspensions of splenocytes were generated by rinsing spleens with erythrocyte lysis buffer and washing with PBS supplemented with 2% FCS and 2 mM EDTA. For the isolation of CD11c+ DCs, splenocytes were separated from contaminating superparamagnetic splenic red pulp cells (32) by using the AutoMACS Pro (Miltenyi Biotec, Bergisch Gladbach, Germany) before using MACS CD11c Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s recommendations. CD4+ T cells were isolated from splenocytes using the CD4+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s protocol, followed by anti-CD4 staining and cell sorting using an Aria II Cell Sorter (BD Biosciences, Heidelberg, Germany).
Antibodies and Flow Cytometry
Anti-CD4, anti-CD8, anti-CD11c, anti-CD11b, anti-MHC-II, anti-CD80, anti-CD86, anti-CD335, anti-CD3, anti-IFN-γ (all BD Biosciences, Heidelberg, Germany), anti-CD62L, anti-TNF-α, anti-Foxp3 (all eBioscience, Frankfurt, Germany), anti-CD160, anti-CD138, and anti-IL-10 (Biolegend, London, UK) were used as fluorescein isothiocyanate, pacific blue, phycoerythrin (PE), BD Horizon V450, allophycocyanin, AlexaFlour647, PE-cyanin 7, or peridinin-chlorophyll protein conjugates. Dead cells were identified by staining with the fixable viability dye eFlour 780 (eBioscience, Frankfurt, Germany). Intracelluar staining for Foxp3 was performed with the Foxp3 staining kit (eBioscience, Frankfurt, Germany) according to the manufacturer’s recommendations. Cytokine production from freshly isolated splenocytes was measured by stimulating cells with 10 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, München, Germany) and 100 µg/ml ionomycin (Sigma-Aldrich, München, Germany) for 4 or 6 h (IL-10), respectively, in the presence of 5 µg/ml Brefeldin A (for IFN-γ, TNF-α staining) and 5 µg/ml Monensin (for IL-10 staining), treating with 2% paraformaldehyde and 0.1% NP40, and staining with the respective antibody cocktail. Flow cytometric expression analyses were performed with an LSR II instrument using DIVA software (BD Biosciences, Heidelberg, Germany).
Serum Cytokine and IL-10 Production by DCs and CD4+ T Cells
Blood samples were collected, incubated at room temperature and centrifuged at 1,000 × g. Splenic CD11c+ DCs were stimulated overnight with 100 ng/ml liposaccharide (Sigma-Aldrich, München, Germany). Cytokines were quantified in the supernatants from stimulated DCs or CD4+ T cells, and in sera by using a Luminex Screening assay (R&D Systems, Wiesbaden, Germany) and a Luminex 200 system with Luminex IS software (Luminex Corporation, MV’s-Hertogenbosch, Netherlands) according to the manufacturer’s instructions. The detection limits were 1.32 pg/ml for IL-10, 0.73 pg/ml for IFN-γ, 0.13 pg/ml for TNF-α, and 1.31 pg/ml for IL-12, respectively.
Statistical Analysis
Statistical analyses were performed with one-way ANOVA, Student’s t-test for parametric and Mann–Whitney test for non-parametric distributed data as indicated with significance set at the levels of *p < 0.05, **p < 0.01, and ***p < 0.001. Normality was tested using the D’Agostino–Pearson omnibus and Kolmogorov–Smirnov test. All analyses were calculated with Graph Pad Prism 5.0 Software (Graph Pad Software, La Jolla, CA, USA).
Results
Enhanced Frequencies of IL-10-Producing CD11c+CD11b+CD8− DCs in P. yoelii-Infected Mice
IL-10 plays an important role in the regulation of P. yoelii infection, and CD4+ T cells have been shown to secrete high amounts of IL-10 upon infection (3, 9). To gain further insights into the question whether IL-10 induction in CD4+ T cells is a direct effect of the parasite or results from IL-10 secretion by APCs, we analyzed the frequency of IL-10-producing (eGFP+) CD11c+CD11b+CD8− DCs and CD11c+CD11b−CD8+ DCs in spleen of IL-10eGFP reporter mice at different time points post-P. yoelii infection by flow cytometry (Figure 1A). Whereas we observed significantly elevated percentages of IL-10-expressing CD11c+CD11b+CD8− DCs in the course of infection (Figure 1B), the frequency of IL-10+ CD11c+CD11b−CD8+ DCs was reduced at day 5 postinfection but recovered to levels of non-infected mice at day 7, 10, and 14 postinfection (Figure 1C).
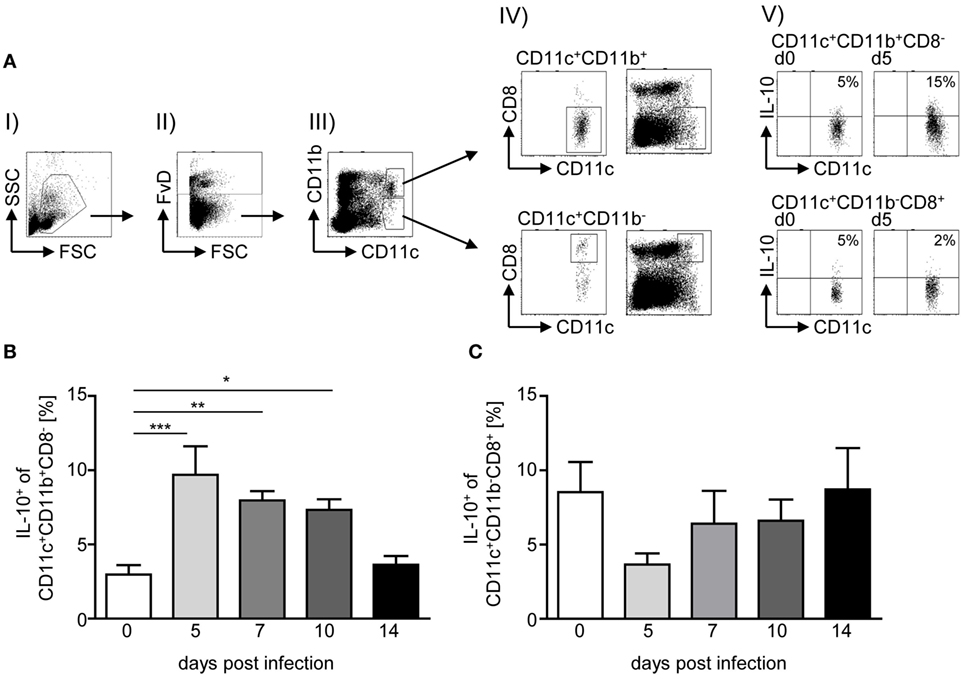
Figure 1. Elevated IL-10 production of CD11c+CD11b+CD8− dendritic cells (DCs) upon Plasmodium yoelii infection. IL-10eGFP reporter mice were infected with infected red blood cells at day 0. At day 5, 7, 10, and 14 postinfection (p.i.), the percentages of IL-10-expressing (eGFP+) (B) CD11c+CD11b+CD8− DCs and (C) CD11c+CD11b−CD8+ DCs were determined by flow cytometry. (A) Representative dot plots illustrate the gating strategy: (I) selection of mononuclear cells, (II) gating on viable cells, (III) gating on CD11c+CD11b+ and CD11c+CD11b− cells, respectively, and (IV) selection of CD8− from gated CD11c+CD11b+ (upper panel) and CD8+ from gated CD11c+CD11b− cells (lower panel). Additionally, we depicted all viable cells together with gated CD8− from CD11c+CD11b+ and CD8+ from gated CD11c+CD11b− cells, respectively (right panel). (V) Representative dot plots showing IL-10-expressing CD11c+ subsets as indicated. Data from n = 6–12 mice per time point out of at least two individual experiments are summarized as mean ± SEM. One-way ANOVA with Dunnett’s posttest was used for statistical analysis (*p < 0.05, **p < 0.01, ***p < 0.001).
DC-Specific IL-10 Inactivation Results in Elevated Production of Pro-inflammatory Cytokines by CD11c+ DCs during P. yoelii Infection
To investigate the impact of DC-derived IL-10 on the immune response during P. yoelii infection of BALB/c mice, we made use of IL-10flox/flox/CD11c-cre mice to specifically ablate IL-10 expression in CD11c+ DCs. To confirm functional inactivation of IL-10, we isolated CD11c+ DCs from IL-10flox/flox/CD11c-cre mice and IL-10flox/flox littermates, stimulated them in vitro with LPS and analyzed the amount of IL-10 in the supernatant by Luminex technology. As depicted in Figure 2A, CD11c+ DCs from IL-10flox/flox control mice secreted approximately 22 pg/ml IL-10 to the culture medium, whereas cells from DC-specific IL-10-deficient (IL-10flox/flox/CD11c-cre) mice produced significantly less IL-10 upon LPS stimulation (approximately 5 pg/ml). Hence, the IL-10flox/flox/CD11c-cre mouse line is a suitable model to investigate the impact of DC-derived IL-10 on the course of P. yoelii infection.
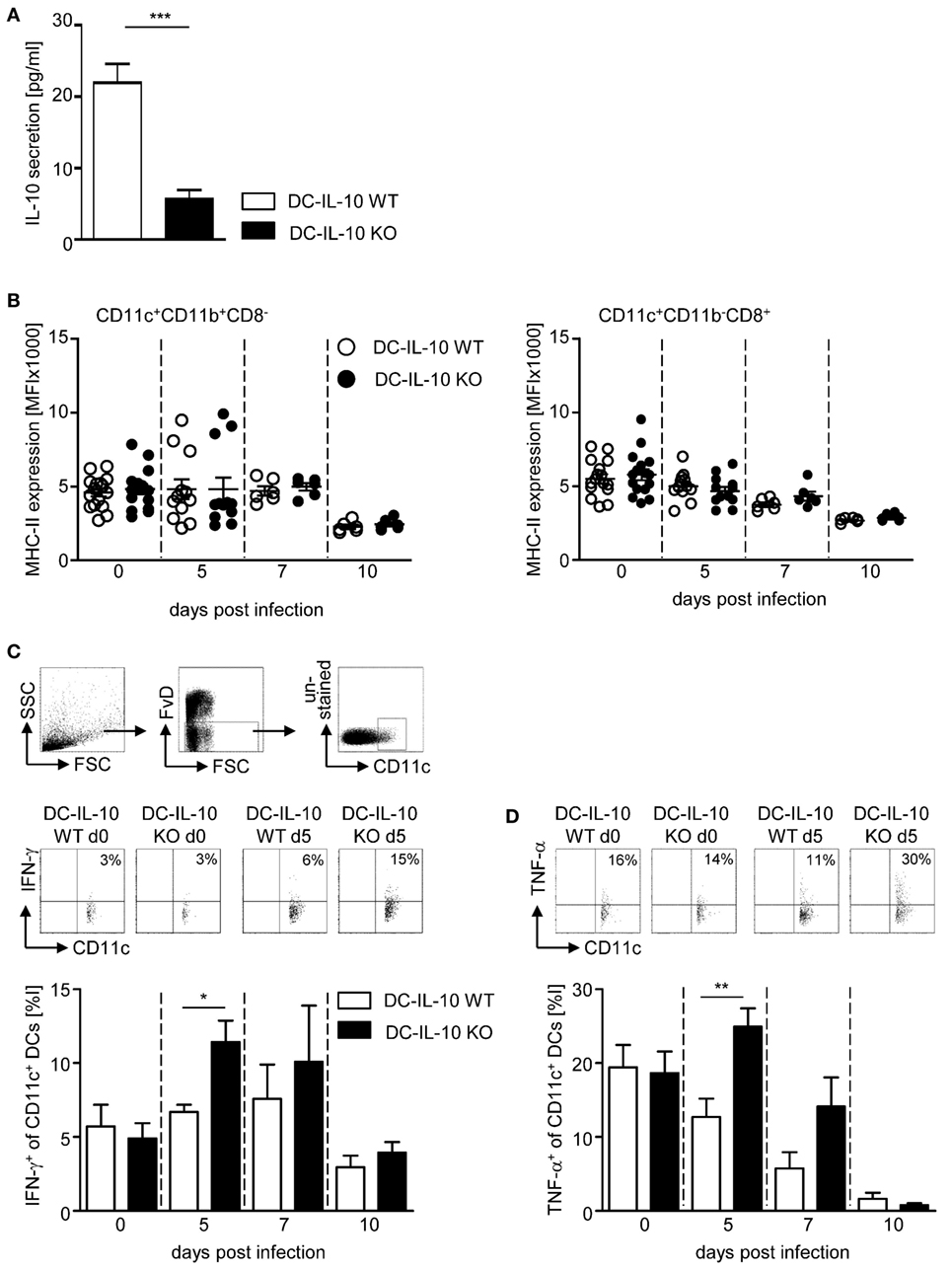
Figure 2. Inactivation of IL-10 in IL-10flox/flox/CD11c-cre mice results in enhanced IFN-γ and TNF-α production of CD11c+ dendritic cells (DCs) during Plasmodium yoelii infection. (A) IL-10 secretion of LPS-stimulated CD11c+ DCs isolated from spleen of IL-10flox/flox (DC-IL-10 WT) and IL-10flox/flox/CD11c-cre (DC-IL-10 KO) was analyzed by Luminex technology. IL-10flox/flox and IL-10flox/flox/CD11c-cre mice were infected with P. yoelii. At indicated time points postinfection, (B) the expression level (MFI) of MHC-II was analyzed on gated CD11c+CD11b+CD8− DCs and CD11c+CD11b−CD8+ DCs, and the (C) frequencies of IFN-γ and (D) TNF-α-expressing CD11c+ DCs were determined by flow cytometry. The gating strategy of CD11c+ DCs and representative dot plots are shown in the upper panel. Results from at least two independent experiments with n = 6–16 mice per time point were summarized as mean ± SEM. Student’s t-test was used for statistical analysis (*p < 0.05, **p < 0.01, ***p < 0.01).
We infected DC-specific IL-10-deficient mice and WT littermates with P. yoelii and analyzed the expression of MHC-II on CD11c+CD11b+CD8− DCs and CD11c+CD11b−CD8+ DCs at different time points postinfection by flow cytometry. We observed only a slight, albeit non-significant, reduction in MHC-II expression levels in the course of infection on both DC subtypes, and this was irrespective of their IL-10 expression (Figure 2B). Similarly, CD80 and CD86 expression on DCs slightly decreased at later time points postinfection, but again with no significant differences between IL-10-deficient and WT DCs (Figures S1A,B in Supplementary Material). However, significant higher percentages of CD11c+ DCs from IL-10flox/flox/CD11c-cre mice produced IFN-γ and TNF-α at early time points after P. yoelii infection in comparison to WT controls (Figures 2C,D). To exclude contaminating NK, NKT, and T cells within the CD11c+ DC population, we extended our analysis for IFN-γ production to CD335−CD3−CD160−CD11c+ DCs from non-infected and P. yoelii-infected mice at day 5 postinfection and obtained same results, showing higher percentages of IFN-γ-expressing CD335−CD3−CD160−CD11c+ DCs from IL-10flox/flox/CD11c-cre mice than from control mice (Figure S2 in Supplementary Material). These results suggest that DC-derived IL-10 has no impact on the capability of DCs to present antigen and on their costimulatory activity, but transiently alters the cytokine profile of CD11c+ DCs during P. yoelii infection.
Increased IFN-γ Expression in CD4+ T Cells from DC-Specific IL-10-Deficient P. yoelii-Infected Mice
Next, we asked whether the altered cytokine profile of IL-10-deficient CD11c+ DCs during P. yoelii infection has an impact on the T cell response. For this purpose, we infected IL-10flox/flox/CD11c-cre and IL-10flox/flox control mice with P. yoelii and determined the frequency of CD62L-expressing CD4+ and CD8+ T cells. As expected, the percentage of CD62L-expressing T cells was reduced in P. yoelii-infected mice, but we detected no differences between mice with IL-10-deficient CD11c+ DCs and WT littermates (Figures S3A,B in Supplementary Material). However, functional inactivation of IL-10 in CD11c+ DCs resulted in significantly elevated IFN-γ production in CD4+ T cells (Figures 3A,B) and slightly, but not significantly, increased frequencies of IFN-γ-expressing CD8+ T cells (Figures 3A,C) at day 5 post-P. yoelii infection. Hence, inactivation of IL-10 in CD11c+ DCs does not impede T cell activation, but rather induces IFN-γ production in CD4+ T cells during P. yoelii infection.
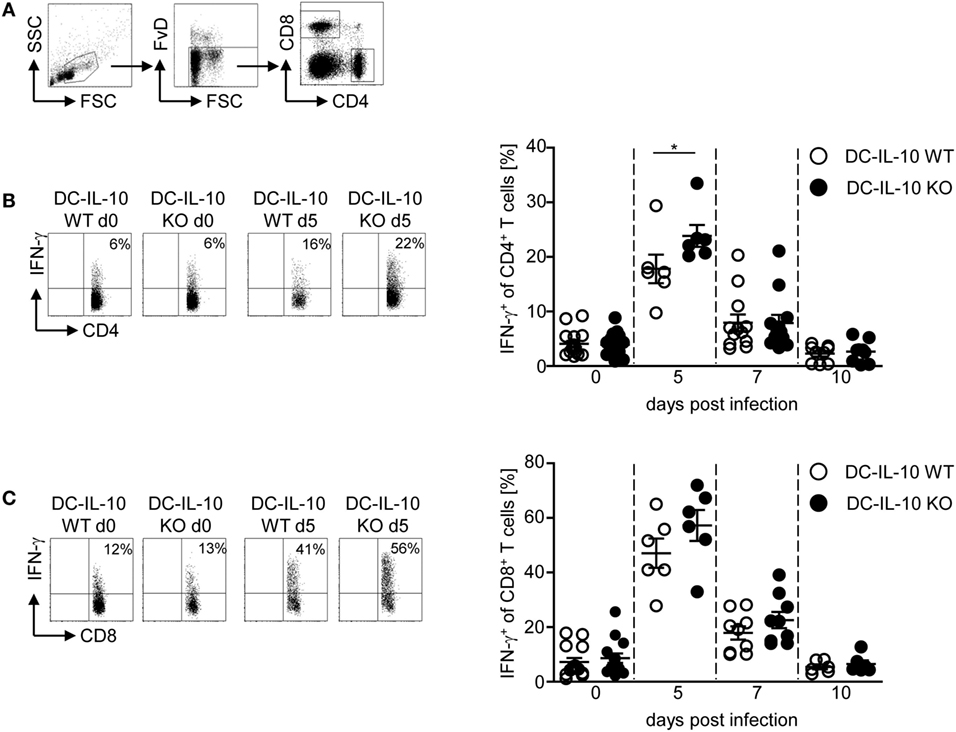
Figure 3. Increase of IFN-γ-producing CD4+ T cells in Plasmodium yoelii-infected IL-10flox/flox/CD11c-cre mice. (A) Representative gating strategy for CD4+ and CD8+ T cells. The frequency of IFN-γ-producing (B) CD4+ and (C) CD8+ T cells from non-infected and P. yoelii-infected IL-10flox/flox [dendritic cells (DC)-IL-10 WT] and IL-10flox/flox/CD11c-cre (DC-IL-10 KO) mice at day 5, 7, and 10 p.i. was determined by intracellular staining and flow cytometric analysis. Representative dot plots are shown in the left panel. Data from two to three independent experiments with n = 6–16 mice per time point are depicted as mean ± SEM. Mann–Whitney test was used for statistical analysis (*p < 0.05).
DC-Derived IL-10 Promotes the Induction of IL-10-Producing CD4+ Tr1 Cells in P. yoelii-Infected Mice with Normal Parasite Propagation
To determine whether DC-derived IL-10 has an impact on CD4+ Treg subsets, we next analyzed the effect of DC-specific IL-10 ablation during P. yoelii infection on the amount of Foxp3+ Tregs and IL-10+ Tr1 cells. As depicted in Figure 4A, flow cytometric analyses revealed no differences in the frequency of Foxp3+ Tregs from P. yoelii-infected IL-10flox/flox/CD11c-cre mice in comparison to IL-10flox/flox littermates (Figure 4A). Strikingly, we observed a significant lower induction of IL-10-producing CD4+ Tr1 cells in IL-10flox/flox/CD11c-cre mice than in IL-10flox/flox control mice at day 5 and 7 post-P. yoelii infection (Figure 4B). To exclude, that IL-10 expression in CD4+ T cells is affected by CD11c-driven cre expression, we analyzed IL-10 secretion of sorted CD4+ T cells from naive IL-10flox/flox/CD11c-cre mice and IL-10flox/flox control mice after stimulation in vitro by Luminex and detected no significant differences (Figure S4A in Supplementary Material). Moreover, CD4+ T cells from both DC-specific IL-10-deficient and control mice produced similar amounts of IFN-γ and TNF-α after stimulation in vitro (Figures S4B,C in Supplementary Material), indicating that CD11c-driven cre expression has no influence on IL-10, IFN-γ, and TNF-α production by CD4+ T cells from naïve mice. Hence, these results provide evidence for DC-derived IL-10 to be involved in the induction of IL-10-producing CD4+ Tr1 cells during P. yoelii infection of BALB/c mice.
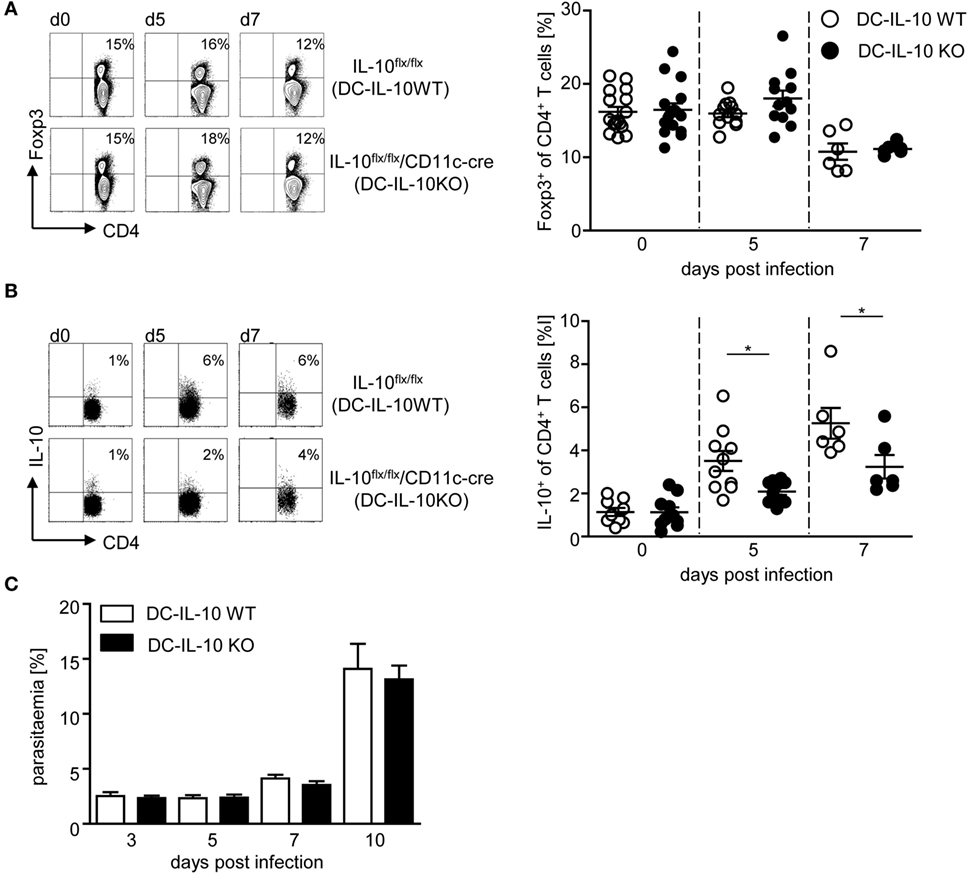
Figure 4. Impaired induction of IL-10-expressing CD4+ type 1 regulatory T cells and normal parasite propagation in Plasmodium yoelii-infected mice with IL-10-deficient CD11c+ dendritic cells (DCs). Percentages of (A) Foxp3 and (B) IL-10-expressing CD4+ T cells were measured by flow cytometry in non-infected and P. yoelii-infected IL-10flox/flox (DC-IL-10 WT) and IL-10flox/flox/CD11c-cre (DC-IL-10 KO) mice at indicated time points p.i. Representative dot plots are depicted in the left panels. (C) Parasitemia of P. yoelii-infected IL-10flox/flox (DC-IL-10 WT) and IL-10flox/flox/CD11c-cre (DC-IL-10 KO) mice was determined by Giemsa staining. Results from at least two independent experiments with n = 6–16 mice (A,B) and n = 18–32 mice (C) per time point are summarized as mean ± SEM. Mann–Whitney test was used for statistical analysis (*p < 0.05).
Since DC-specific IL-10 ablation in P. yoelii-infected mice interfered with the induction of IL-10-producing CD4+ Tr1 cells and resulted in elevated production of pro-inflammatory cytokines, we monitored progression of blood infection by microscopic examination of Giemsa-stained blood films. We did not detect any significant effect on parasite clearance (Figure 4C), confirming that the observed differences can be attributed directly to immune signaling and are not an indirect effect of lower parasite burden.
P. yoelii Infection of DC-Specific IL-10-Deficient Mice Results in Elevated IL-12 Serum Levels
Our results indicate that DC-specific ablation of IL-10 triggers the expression of pro-inflammatory cytokines in DCs and T cells, while dampening the induction of Tr1 cells during P. yoelii infection. To investigate whether these effects have also systemic implications, we analyzed the concentration of different cytokines in sera of P. yoelii-infected IL-10flox/flox/CD11c-cre mice and IL-10flox/flox littermates. As depicted in Figure 5, DC-specific inactivation of IL-10 did not change TNF-α serum concentration (Figure 5A), but resulted in a slightly, albeit non-significantly increased systemic IFN-γ production (Figure 5B) and significantly elevated IL-12 levels (Figure 5C) in sera of P. yoelii-infected mice at day 5 postinfection.
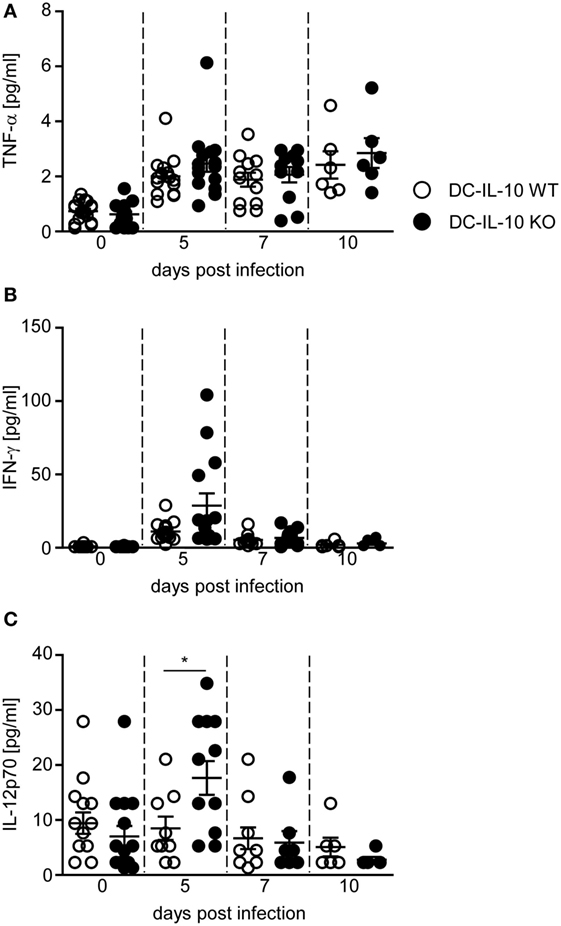
Figure 5. IL-10 deficiency in CD11c+ dendritic cells (DCs) results in elevated IFN-γ and IL-12 serum level. (A) TNF-α, (B) IFN-γ, and (C) IL-12 serum levels of Plasmodium yoelii-infected IL-10flox/flox (DC-IL-10 WT) and IL-10flox/flox/CD11c-cre (DC-IL-10 KO) mice were determined by Luminex technology at indicated time points p.i. Results from at least two independent experiments with n = 6–12 mice per time point are summarized as mean ± SEM. Mann–Whitney test was used for statistical analysis (*p < 0.05).
Discussion
The cytokine IL-10 has been identified as a key regulator to keep the balance between pro- and anti-inflammatory responses during the blood stage of Plasmodium infection. IL-10 deficiency resulted in a more efficient parasitic clearance upon P. yoelii infection and increased mortality of normally avirulent P. chabaudi infection (9, 33). Although IL-10 is a secreted cytokine that can have systemic effects, its cellular source appears to profoundly affect the resulting immune responses (34). We and others identified CD4+ T cells to produce high amounts of IL-10 and exhibiting suppressive activity during Plasmodium infection (3, 9, 21). However, it remains unclear whether induction of IL-10+ Tr1 cells results from direct interaction with the parasite or as a consequence of CD4+ T cell activation in the presence of IL-10; provided by professional APCs such as DCs, B cells, or macrophages (35).
Here, we demonstrate that the frequency of CD11c+CD11b+CD8− IL-10-producing DCs was significantly elevated upon P. yoelii infection of BALB/c mice. Well in line with our results, an increase in IL-10 expression was also observed in CD11chigh DCs from P. chabaudi-infected and P. berghei-infected C57BL/6 mice (21, 22). However, to our knowledge, the role of DC-derived IL-10 during Plasmodium infection has not been analyzed in detail. Cell type-specific inactivation of IL-10 in CD11c+ DCs by using IL-10flox/flox/CD11c-cre mice resulted in higher frequencies of IFN-γ- and TNF-α-producing DCs (Figures 2C,D) accompanied by elevated IFN-γ production in CD4+ T cells (Figure 3B) from P. yoelii-infected mice. Additionally, our study revealed that DC-specific inactivation of IL-10 dampens the percentage of IL-10-expressing immunosuppressive CD4+ Tr1 cells at day 5 and 7 postinfection (Figure 4B). Therefore, we postulate that DC-derived IL-10 rather than direct interaction with the parasite promotes IL-10 production in CD4+ T cells upon P. yoelii infection. During P. berghei infection of C57BL/6 mice B cell-derived IL-10 was proposed to be involved in the induction of IL-10-producing CD4+ Tr1 cells. Coculture of purified IL-10+ B cells, but not IL-10− B cells isolated from P. berghei-infected mice induced IL-10 production by CD4+ T cells (22) and B cell-specific inactivation of IL-10 lowered the number of IL-10 secreting CD4+ T cells during P. chabaudi infection (21). In our experiments, we have not detected a significant increase of IL-10 expression in CD19+ B cells or CD19−CD138+ plasma cells in the course of P. yoelii infection (Figures S5A,B in Supplementary Material). Hence, it seems to be unlikely that B cell-derived IL-10 contributes to elevated IL-10 expression in CD4+ T cells from P. yoelii-infected BALB/c mice. However, we cannot formally exclude that IL-10 produced by other cell types might also have an impact on the induction of CD4+ Tr1 cells during P. yoelii infection of BALB/c mice.
Analysis of systemic cytokine levels revealed increased IFN-γ and IL-12 serum concentrations at day 5 post-P. yoelii infection in IL-10flox/flox/CD11c-cre mice compared to WT mice, suggesting that IL-10 produced by either DCs or CD4+ T cell or both immune cell subsets modulates the production of circulating pro-inflammatory cytokines. Well in line, significantly higher plasma levels of IFN-γ and IL-12 have also been observed in P. chabaudi-infected IL-10−/− mice (33) underlining the importance of IL-10 in regulating pro-inflammatory immune responses during Plasmodium infection.
Our results indicate that the parasite P. yoelii promotes IL-10 production in CD11c+CD11b+CD8− DCs, which in turn induces IL-10-expressing Tr1 cells. Evidence for parasite-induced IL-10 expression in DCs due to direct interaction was provided by coculture experiments of DCs with infected RBCs resulting in elevated IL-10 expression (36, 37). However, it remains unclear by which mechanism the parasite stimulates IL-10 production in DCs. Previous studies proposed that heme, which is released after lysis of iRBCs or hemozoin, the product of hemoglobin degradation by Plasmodium parasites is involved in the induction of IL-10 in murine and human macrophages (38, 39) and resulted in downregulation of IL-12 in human PBMCs and CD14+ monocytes (40). Hemozoin (41) or a complex of hemozoin, DNA, and unknown proteins (42) have been described to bind and trigger toll-like receptor 9 signaling. In addition, intact Plasmodium falciparum-infected erythrocytes can bind directly to CD36 and treatment of human DCs with anti-CD36 resulted in elevated IL-10 expression to comparable levels as after treatment with iRBCs (36). However, which receptors and signaling pathways are involved in the induction of IL-10 expression in CD11c+CD11b+CD8− DCs during P. yoelii infection has to be elucidated in further experiments.
Interestingly, our data show that DC-derived IL-10 has no impact on pathogen clearance, although we detected elevated cellular and systemic pro-inflammatory cytokine levels and lower percentages of IL-10-producing CD4+ Tr1 cells in P. yoelii-infected IL-10flox/flox/CD11c-cre mice than in respective control animals. T cell-specific inactivation of IL-10 had also no impact on parasitemia in P. yoelii-infected BALB/c mice in our hands (3) and on parasite burden in C57BL/6 mice infected with P. chabaudi (21). In contrast, Couper and colleagues reported a significant influence of T cell-derived IL-10 on parasite clearance. The apparent discrepancy can likely be attributed to the different experimental settings, parasite propagation in T cell-specific IL-10-deficient mice (3, 21) and adoptive transfer of CD4+ T cells from IL-10-deficient C57BL/6 to P. yoelii-infected Rag1KO mice, which also lack CD8+ T cells and B cells, in addition to endogenous CD4+ T cells (9).
In summary, our results provide evidence that P. yoelii infection of BALB/c mice results in elevated IL-10 expression in CD11c+CD11b+CD8− DCs, which in turn dampens the cellular and systemic production of pro-inflammatory cytokines and induces IL-10-secreting CD4+ Tr1 cells. These results contribute to a better understanding in the impact of DC-derived IL-10 on the dysregulated immune responses during Plasmodium infection.
Author Contributions
KL, KU, SA, and MH designed and performed the experiments and analyzed data. JB, AW, and KM were involved in the data discussion and in drafting the manuscript. WH initiated, organized, and designed the study and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Axel Roers (Dresden) and Werner Müller (Manchester) for providing them with IL-10flox/flox mice and to Ari Waisman (Mainz) for CD11c-cre mice. The authors thank Sina Luppus for excellent technical assistance and Witold Bartosik and Christian Fehring for cell sorting. This project was supported by German Research Foundation (DFG) to WH and JB (GRK 1949).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00152/full#supplementary-material.
Abbreviations
APC, antigen-presenting cell; DC, dendritic cell; iRBC, infected red blood cells; P. yoelii, Plasmodium yoelii; Tr1, type 1 regulatory T cell; Treg, regulatory T cell.
References
1. Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol (2008) 9:725–32. doi:10.1038/ni.f.205
2. Riley EM, Stewart VA. Immune mechanisms in malaria: new insights in vaccine development. Nat Med (2013) 19:168–78. doi:10.1038/nm.3083
3. Abel S, Lückheide N, Westendorf AM, Geffers R, Roers A, Müller W, et al. Strong impact of CD4+ Foxp3+ regulatory T cells and limited effect of T cell-derived IL-10 on pathogen clearance during Plasmodium yoelii infection. J Immunol (2012) 188:5467–77. doi:10.4049/jimmunol.1102223
4. Abel S, Ueffing K, Tatura R, Hutzler M, Hose M, Matuschewski K, et al. Plasmodium yoelii infection of BALB/c mice results in expansion rather than induction of CD4(+) Foxp3(+) regulatory T cells. Immunology (2016) 148:197–205. doi:10.1111/imm.12602
5. Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med (2007) 204:57–63. doi:10.1084/jem.20061852
6. Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol (2008) 180:5771–7. doi:10.4049/jimmunol.180.9.5771
7. Kossodo S, Monso C, Juillard P, Velu T, Goldman M, Grau GE. Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology (1997) 91:536–40. doi:10.1046/j.1365-2567.1997.00290.x
8. Li C, Sanni LA, Omer F, Riley E, Langhorne J. Pathology of Plasmodium chabaudi chabaudi infection and mortality in interleukin-10-deficient mice are ameliorated by anti-tumor necrosis factor alpha and exacerbated by anti-transforming growth factor beta antibodies. Infect Immun (2003) 71:4850–6. doi:10.1128/IAI.71.9.4850-4856.2003
9. Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog (2008) 4:e1000004. doi:10.1371/journal.ppat.1000004
10. Omer FM, de Souza JB, Riley EM. Differential induction of TGF-beta regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J Immunol (2003) 171:5430–6. doi:10.4049/jimmunol.171.10.5430
11. Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol (2009) 183:2312–20. doi:10.4049/jimmunol.0900185
12. Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol (2001) 19:683–765. doi:10.1146/annurev.immunol.19.1.683
13. Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature (2002) 420:502–7. doi:10.1038/nature01152
14. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol (2000) 18:767–811. doi:10.1146/annurev.immunol.18.1.767
15. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol (2013) 31:563–604. doi:10.1146/annurev-immunol-020711-074950
16. Wykes MN, Good MF. What really happens to dendritic cells during malaria? Nat Rev Microbiol (2008) 6:864–70. doi:10.1038/nrmicro1988
17. Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest (2010) 120:559–69. doi:10.1172/JCI40008
18. Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol (2001) 2:725–31. doi:10.1038/90667
19. Igyarto BZ, Jenison MC, Dudda JC, Roers A, Müller W, Koni PA, et al. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol (2009) 183:5085–93. doi:10.4049/jimmunol.0901884
20. Jang S, Uematsu S, Akira S, Salgame P. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J Immunol (2004) 173:3392–7. doi:10.4049/jimmunol.173.5.3392
21. Freitas do Rosário AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol (2012) 188:1178–90. doi:10.4049/jimmunol.1102755
22. Liu Y, Chen Y, Li Z, Han Y, Sun Y, Wang Q, et al. Role of IL-10-producing regulatory B cells in control of cerebral malaria in Plasmodium berghei infected mice. Eur J Immunol (2013) 43:2907–18. doi:10.1002/eji.201343512
23. Girard-Madoux MJ, Kautz-Neu K, Lorenz B, Ober-Blobaum JL, von SE, Clausen BE. IL-10 signaling in dendritic cells attenuates anti-Leishmania major immunity without affecting protective memory responses. J Invest Dermatol (2015) 135:2890–4. doi:10.1038/jid.2015.236
24. Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med (2001) 194:1497–506. doi:10.1084/jem.194.10.1497
25. Girard-Madoux MJ, Kel JM, Reizis B, Clausen BE. IL-10 controls dendritic cell-induced T-cell reactivation in the skin to limit contact hypersensitivity. J Allergy Clin Immunol (2012) 129:143–50. doi:10.1016/j.jaci.2011.08.032
26. Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol (2003) 3:984–93. doi:10.1038/nri1246
27. Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood (2005) 105:1162–9. doi:10.1182/blood-2004-03-1211
28. McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med (2002) 195:221–31. doi:10.1084/jem.20011288
29. Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galán JE, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity (2006) 25:941–52. doi:10.1016/j.immuni.2006.09.013
30. Roers A, Siewe L, Strittmatter E, Deckert M, Schlüter D, Stenzel W, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med (2004) 200:1289–97. doi:10.1084/jem.20041789
31. Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med (2007) 204:1653–64. doi:10.1084/jem.20062648
32. Franken L, Klein M, Spasova M, Elsukova A, Wiedwald U, Welz M, et al. Splenic red pulp macrophages are intrinsically superparamagnetic and contaminate magnetic cell isolates. Sci Rep (2015) 5:12940. doi:10.1038/srep12940
33. Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun (1999) 67:4435–42.
34. Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med (2007) 204:239–43. doi:10.1084/jem.20070104
35. Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature (1997) 389:737–42. doi:10.1038/39614
36. Urban BC, Willcox N, Roberts DJ. A role for CD36 in the regulation of dendritic cell function. Proc Natl Acad Sci U S A (2001) 98:8750–5. doi:10.1073/pnas.151028698
37. Ocana-Morgner C, Mota MM, Rodriguez A. Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med (2003) 197:143–51. doi:10.1084/jem.20021072
38. Cambos M, Bazinet S, Abed E, Sanchez-Dardon J, Bernard C, Moreau R, et al. The IL-12p70/IL-10 interplay is differentially regulated by free heme and hemozoin in murine bone-marrow-derived macrophages. Int J Parasitol (2010) 40:1003–12. doi:10.1016/j.ijpara.2010.02.007
39. Deshpande P, Shastry P. Modulation of cytokine profiles by malaria pigment – hemozoin: role of IL-10 in suppression of proliferative responses of mitogen stimulated human PBMC. Cytokine (2004) 28:205–13. doi:10.1016/j.cyto.2004.08.002
40. Keller CC, Yamo O, Ouma C, Ong’echa JM, Ounah D, Hittner JB, et al. Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia. Infect Immun (2006) 74:5249–60. doi:10.1128/IAI.00843-06
41. Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med (2005) 201:19–25. doi:10.1084/jem.20041836
Keywords: rodent, parasitic protozoan, malaria, regulatory T cells, interleukin 10
Citation: Loevenich K, Ueffing K, Abel S, Hose M, Matuschewski K, Westendorf AM, Buer J and Hansen W (2017) DC-Derived IL-10 Modulates Pro-inflammatory Cytokine Production and Promotes Induction of CD4+IL-10+ Regulatory T Cells during Plasmodium yoelii Infection. Front. Immunol. 8:152. doi: 10.3389/fimmu.2017.00152
Received: 25 November 2016; Accepted: 30 January 2017;
Published: 28 February 2017
Edited by:
Karsten Kretschmer, DFG-Center for Regenerative Therapies Dresden (CRTD), GermanyReviewed by:
Ari Waisman, University of Mainz, GermanyAlf Hamann, Deutsches Rheuma-Forschungszentrum Berlin, Germany
Roland Lang, University Hospital Erlangen, Germany
Copyright: © 2017 Loevenich, Ueffing, Abel, Hose, Matuschewski, Westendorf, Buer and Hansen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wiebke Hansen, wiebke.hansen@uk-essen.de
 Katharina Loevenich
Katharina Loevenich Kristina Ueffing1
Kristina Ueffing1 Simone Abel
Simone Abel Matthias Hose
Matthias Hose Kai Matuschewski
Kai Matuschewski Wiebke Hansen
Wiebke Hansen