- 1Parasitology Unit, Max Planck Institute for Infection Biology, Berlin, Germany
- 2Wellcome Trust Research Programme, Centre for Geographic Medicine Research-Coast, Kenya Medical Research Institute (KEMRI), Kilifi, Kenya
- 3Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
- 4Molecular Parasitology, Institute of Biology, Humboldt University, Berlin, Germany
Immunoepidemiological studies typically reveal slow, age-dependent acquisition of immune responses against Plasmodium falciparum sporozoites. Naturally acquired immunity against preerythrocytic stages is considered inadequate to confer protection against clinical malaria. To explore previously unrecognized antisporozoite responses, we measured serum levels of naturally acquired antibodies to whole Plasmodium falciparum sporozoites (Pfspz) and the immunodominant (NANP)5 repeats of the major sporozoite surface protein, circumsporozoite protein, in a well-characterized Kenyan cohort. Sera were sampled at the start of the malaria transmission season, and all subjects were prospectively monitored for uncomplicated clinical malaria in the ensuing 6 months. We used Kaplan–Meier analysis and multivariable regression to investigate the association of antisporozoite immunity with incidence of clinical malaria. Although naturally acquired humoral responses against Pfspz and (NANP)5 were strongly correlated (p < 0.0001), 37% of Pfspz responders did not recognize (NANP)5. The prevalence and magnitude of antisporozoite responses increased with age, although some high Pfspz responders were identified among children. Survival analysis revealed a reduced risk of and increased time to first or only episode of clinical malaria among Pfspz or (NANP)5 responders carrying microscopically detectable Plasmodium falciparum (Pf) parasitemia at the start of the transmission season (p < 0.03). Our Cox regression interaction models indicated a potentially protective interaction between high anti-Pfspz (p = 0.002) or anti-(NANP)5 (p = 0.001) antibody levels and microscopically detectable Pf parasitemia on the risk of subsequent clinical malaria. Our findings indicate that robust antisporozoite immune responses can be naturally acquired already at an early age. A potentially protective role of high levels of anti-Pfspz antibodies against clinical episodes of uncomplicated malaria was detected, suggesting that antibody-mediated preerythrocytic immunity might indeed contribute to protection in nature.
Introduction
Malaria transmission by Plasmodium-infected Anopheles mosquitoes results in at least 200 million malaria cases every year, and the majority of disease burden is caused by Plasmodium falciparum (Pf) infections in children (1).
During a blood meal, an infected mosquito typically injects fewer than 40 motile sporozoites into the human skin (2). After injection, a proportion of sporozoites reaches a blood capillary by active intradermal migration, breaches the endothelial cells of the blood vessel, and is passively transported to the liver through the blood circulation. Following a massive replication phase in hepatocytes, tens of thousands of liver merozoites are released to initiate asexual parasite propagation in erythrocytes, the only phase in the parasite life cycle causing malaria symptoms.
When experimentally inoculated in repeated high doses, arrested, but metabolically active, Plasmodium sporozoites are able to elicit protective and often lasting and sterile immune responses against homologous challenge in humans (3–5). Because the preerythrocytic phase of the life cycle is clinically silent, humans can be inoculated with high doses of sporozoites, providing a tantalizing rationale for preerythrocytic malaria vaccine approaches (6). Plasmodium sporozoites are considered immunologically silent in natural infections, because they are mostly inoculated in small numbers and are exposed to the immune system only for a brief period (7, 8).
Naturally acquired immune responses against whole Pf sporozoites (Pfspz) typically reveal age- and transmission-dependent increases in prevalence (9–11). Strong humoral responses are directed against the central repeat region (CRR) of the major sporozoite surface protein, circumsporozoite protein (CSP) (12–17). The CRR is composed of a varying number of NANP repeats, and a single Pf sporozoite inoculation is sufficient to trigger anti-NANP antibody responses (15, 18). However, in virtually all populations studied, a varying proportion of naturally exposed individuals remains unresponsive to NANP repeats, even after repeated exposures (19).
In addition to NANP repeats, immune responses can also target regions in the amino- or carboxy-termini of CSP (20–22) or additional sporozoite antigens, such as thrombospondin-related anonymous protein (23–25) or sporozoite threonine–asparagine-rich protein (26, 27). However, no single preerythrocytic antigen has yet been identified that serves as a correlate of naturally acquired protection against infection or clinical disease. Rather, immunoepidemiological studies show that the breadth of preerythrocytic antigens recognized by an individual is associated with a lower risk of and an extended time to reinfection (28, 29).
In this study, we analyzed humoral immune responses against Pfspz in a cohort of naturally exposed individuals in Kenya. We compared prevalence and titers of antibodies against whole Pfspz and NANP repeats of CSP and assessed potentially protective contributions of antisporozoite immunity against clinical malaria. We show previously unrecognized high anti-Pfspz responses in children, which are not captured by (NANP)5-enzyme-linked immunosorbent assay (ELISA), and demonstrate potentially protective interactions between high levels of anti-Pfspz responses and asymptomatic Pf slide positivity at the time of serum collection on the risk of clinical malaria.
Materials and Methods
Study Design and Cohort
This was a follow-up study from seroepidemiological cohort studies previously conducted in Chonyi village (Kilifi County, Kenya) (30–34). At the time the study was conducted, this region experienced year-round moderate malaria transmission (~20–100 infective bites/person/year), with a short rainy season from November to December and an extended one from June to August causing two seasonal peaks in transmission (35). Details of the cohort and study area have been published previously (36). For this study, serum samples of 514 individuals aged 1.6 months–82 years were analyzed from the cross-sectional bleed conducted in October 2000, immediately prior to the start of the malaria transmission season. At the time of this cross-sectional bleed, no individual experienced clinical malaria episodes, but 37% carried a microscopically detectable asymptomatic Pf blood stage infection, as assessed by thick and thin blood films. Asymptomatic infection was defined as any microscopically detectable parasitemia in an afebrile individual. We measured serum levels of antibodies to (i) whole Pfspz and (ii) the (NANP)5 repeats of CSP. Active weekly surveillance and passive case detection for development of clinical malaria during a 26-week follow-up permitted a retrospective analysis of potential associations of humoral antisporozoite immune responses with incidence of clinical malaria, which was defined according to the following age-specific criteria: for infants <1-year old and for older children and adults (≥15 years old), an axillary temperature of ≥37.5°C plus any parasitemia; for children aged 1–14 years, an axillary temperature of ≥37.5°C, combined with a parasitemia of ≥2,500 parasites/μl (36).
Generation of Pf Sporozoites
Plasmodium falciparum gametocyte cultures were derived from high parasitemic (5–8%) asexual PfNF54 parasites, cultured in pooled human O+ red blood cells and RPMI-HEPES medium, supplemented with hypoxanthine, sodium carbonate, l-glutamine, and pooled, non-immune, heat-inactivated human A+ serum. The asexual Pf culture was diluted to 1% parasitemia into culture flasks containing 6 ml prewarmed medium, resulting in a final hematocrit of 5%, and gas flushed with 5% O2 and 5% CO2. Medium was replaced daily. From day 14 onward, cultures were examined daily for stage V gametocytemia, male:female ratio, and exflagellation rates. Infection of Anopheles stephensi mosquitoes was done by membrane feeding for 15 min at 26°C in the dark. Mosquitoes were maintained at 26°C and 80% humidity. Salivary glands were liberated between days 18 and 20 after infection by hand dissection in RPMI/3% BSA. Salivary glands were gently ground in a test tube, and the sporozoite suspension was centrifuged at 20 × g for 3 min to remove mosquito tissue debris. The mean sporozoite number per mosquito was 7,400. Two thousand to four thousand sporozoites per well were added to eight-well chamber slides (Medco), air dried, and stored at room temperature.
Detection of Anti-Pfspz Antibodies by Immunofluorescence Assay (IFA)
For fixation, sporozoite slides were dipped in cold acetone for 1 min and rehydrated in PBS for 30 min. All incubation steps were done in a dark humid chamber for 1 h at 37°C. Blocking was done with PBS/3% BSA. After washing with PBS/0.5% BSA, human sera were added in serial dilutions ranging from 1:50 to 1:24,300 in PBS/0.5% BSA, followed by extensive washing with PBS/0.5% BSA. AlexaFluor488 goat anti-human IgG (1:500; Invitrogen) and Hoechst (1:1,000; Invitrogen) were used as secondary fluorescent antibody and nuclear stain, respectively. Slides were sealed after addition of Fluoromount (Southern Biotech) and analyzed by fluorescence microscopy. For the negative control, one well per slide was stained with pooled sera from 20 unexposed individuals from the United Kingdom. The pooled negative control sera did not deliver any IFA signal detectable by visual examination even at the lowest dilution. Cohort sera displaying a comparable response were considered to be IFA negative or unresponsive to Pfspz. For the positive control, one well per slide was stained with an anti-PfCSP antibody (2A10) (37) or a Kenyan adult hyper immune serum. All IFA slides were randomized prior to microscopic screening to avoid biased evaluation, and arbitrary sample batches were repeated to exclude day-to-day variation of the assay. The reciprocal antisporozoite end titer was defined as the last dilution at which immunofluorescence was detectable by fluorescence microscopy.
Detection of Serum Antibodies by ELISA
To quantify serum antibody titers against the CRR of CSP, a standard ELISA was performed using the (NANP)5-icosapeptide (Peptides and Elephants, Potsdam, Germany). A peptide stock solution (1 µg/µl) was prepared in sodium carbonate buffer (pH 9.3) and stored at −80°C. For experiments, the stock solution was diluted to a concentration of 400 ng/100 μl. F96 MaxiSorp plates (Nunc) were coated with peptide overnight at 4°C (400 ng/well). After extensive washing with PBS/0.05% Tween, unspecific binding was blocked with 100 µl casein (Thermo Scientific Blocker). After an additional wash, human sera (1:200 in 100 µl casein) were incubated for 1 h at 37°C. After extensive washing, plates were incubated for 1 h at 37°C with horseradish peroxidase-conjugated rabbit anti-human IgG (Dako Ltd.) at a 1:3,000 dilution in 100 µl casein. Following washing, bound human IgG was measured with OPD substrate (Sigmafast). The reactions were stopped with 50 µl 2 M H2SO4, and absorbance was read in an ELISA plate reader at 492 nm. To assess the variability of the ELISA assay, all sera were tested in duplicates, and the coefficient of variation was calculated for each paired serum sample. Samples that varied by ≥20% were tested again. A hyper immune Kenyan adult serum was used in duplicates on each ELISA plate as a positive control. The ratio of mean optical densities (ODs) of the positive serum samples was computed to assess plate to plate and day-to-day variability. For ratios ranging between 1.1 and 1.3 or 0.7 and 0.89, OD values were multiplied to adjust the data to a reference plate. For ratios <0.7 or >1.3, the experiment was repeated. Twenty sera from unexposed individuals from the United Kingdom were used as negative controls. The mean OD plus three SDs from all negative controls was determined as a cutoff for (NANP)5 seropositivity. ELISAs to measure serum levels of antibodies against Pf schizont extract and blood stage merozoite antigens were described previously (31).
Statistical Analysis
A Spearman’s correlation was run to assess the relationship between ELISA ODs and IFA end titers. The Kruskal–Wallis test was applied for non-parametric analyses of variance in levels of antisporozoite antibodies across age groups. The Pearson’s χ2 test was used to determine the statistical significance of differences between proportions. The Mann–Whitney test determined statistically significant differences in antibody titer between two subsets of individuals. A binomial general linear model was used to calculate risk ratios in univariate analysis. Kaplan–Meier survival analysis and the log-rank Mantel–Cox test were used to compare the incidence of clinical malaria in distinct subsets of the cohort.
For multivariable analyses, we used a fractional polynomial Cox regression model that best predicts the time to first or only episode of clinical malaria for individuals with different levels of antisporozoite antibodies and predicted the log relative hazard against increasing levels of antibodies. Stepwise regression was conducted to select the most parsimonious set of covariates that are best predictors of clinical malaria. The variables with least significant coefficients (p > 0.2) were dropped one at a time until the final model was obtained. Likelihood ratio tests were run to compare the fit of the models with and without each variable. The final model was adjusted for the following confounders: age, plasma levels of antibodies against blood stage antigens [merozoite surface proteins 1-3 (MSP1-3), apical membrane antigen-1 (AMA1), erythrocyte binding antigen 175 (EBA-175), and Pf schizont extract], and malaria exposure, or unweighted local malaria prevalence index, as measured by the proportion of infected individuals within a 1 km radius of the index case. The malaria exposure index was calculated over the 6-month follow-up period using weekly slide data for acute episodes only (active case detection) and using the cross-sectional slide data (38).
To examine the effect of interactions between baseline antisporozoite antibody levels and microscopically detectable Pf parasitemia on malaria risk, hazard ratios were computed through a Cox regression model with and without one-, two-, or three-way interactions between antisporozoite antibody levels, microscopically detectable Pf infection, and malaria exposure index. Models with and without interactions were compared using the log likelihood ratio test.
The fractional polynomial Cox regression model and the interaction model are presented using anti-(NANP)5 ODs as a continuous variable. To reduce the skewness while maintaining the direction of association, anti-(NANP)5 OD values were transformed to negative inverse values before running the models.
Data were compared for statistically significant differences at a significance level of α = 0.05. The Bonferroni correction was applied to control for the increasing familywise error rate through multiple comparisons. Four families of statistical tests were defined based on the common dependent variable and null hypothesis tested. For each family composed of k significance tests, a new critical value was defined as α/k. Individual tests within these families were considered significant at α = 0.05 level if p < (α/k). The following families were defined:
1. Association of anti-Pfspz or anti-(NANP)5 antibody prevalence or titers with age; k = 8; αnew = 0.05/8 = 0.006.
2. Association of anti-Pfspz or anti-(NANP)5 antibody prevalence or titers with blood film status (for each separate age category), k = (4 tests × 9 age categories) = 36; αnew = 0.05/36 = 0.001.
3. Association of anti-Pfspz or anti-(NANP)5 antibody levels with incidence of clinical malaria; k = 4; αnew = 0.05/4 = 0.013.
4. Interaction of anti-Pfspz or anti-(NANP)5 antibody levels with blood film positivity status and association with incidence of clinical malaria; k = 14; αnew = 0.05/14 = 0.004.
All statistical analyses were performed using STATA/SE12 (StataCorp).
Results
Incidence of Clinical Malaria
Overall, the risk of developing at least one episode of clinical malaria during 6 months of follow-up decreased with age (p < 0.0001), with an overall rate of 0.4 episodes/person/year (Table S1 in Supplementary Material).
Asymptomatic Pf blood stage infection at the beginning of the malaria transmission season was detected in 37% of individuals, although this proportion was higher in younger age groups (3–15 years) (Table S1 in Supplementary Material). One or more malaria episodes were reported in 22% of blood film-positive individuals (Table S1 in Supplementary Material). This proportion was significantly lower (11%) among subjects who were blood film negative at the beginning of the observation period (p = 0.001) (Table S1 in Supplementary Material). This group may include parasite-free individuals and subjects carrying Pf parasites below the detection threshold by microscopy.
Naturally Acquired Antibody Responses against Pf Sporozoites
A substantial fraction (63%) of the Chonyi sera recognized air-dried, whole Pfspz by IFA (Figure 1). The proportion of anti-(NANP)5 responders was substantially lower (27%), and all (NANP)5-reactive individuals except three were also classified as Pfspz responders (Figure 1). A large subset (36%) of sera recognized Pfspz but not (NANP)5. We observed an overall correlation of increased intensity of (NANP)5 responses in individuals with high anti-Pfspz titers (p < 0.0001) (Figure 1). However, even among the sera that produced the highest Pfspz reactivities by IFA, some remained (NANP)5 negative (Figure 1).
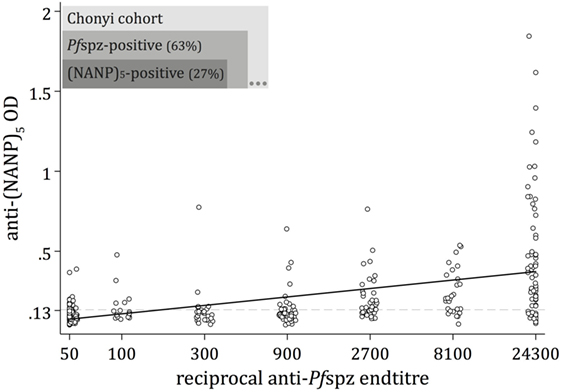
Figure 1. Naturally acquired antibody responses against Plasmodium falciparum (Pf) sporozoites. Antibody responses to whole Pf sporozoites (Pfspz) and to the central repeat region of the circumsporozoite protein were measured by immunofluorescence assay (IFA) on air-dried Pfspz and enzyme-linked immunosorbent assay (ELISA) using (NANP)5 peptide, respectively. Anti-Pfspz end titers were defined as the last dilution at which sera recognized Pfspz by IFA. Anti-Pfspz antibody titers are log transformed. The (NANP)5-ELISA seropositivity cutoff (dashed line) was defined as the mean optical density (OD) plus 3 SDs of 20 sera from unexposed individuals (OD = 0.135). Serum from a hyperimmune Kenyan adult (20H) served as a positive control; black line: fitted regression line; Spearman’s ρ = 0.668; p < 0.0001.
Stratification of the Chonyi cohort revealed age-dependent acquisition of anti-Pfspz antibodies (p < 0.0001). The age-related increase in prevalence was particularly accentuated among blood film-negative individuals (p < 0.001) (Figure 2A). This was in marked contrast with blood film-positive individuals (p = 0.72), where prevalence of anti-Pfspz responses reached 68% in children younger than 6 years (Figure 2A). In individual age groups, the proportion of Pfspz responders was consistently higher among blood film-positive individuals, although this difference was only significant among individuals aged 7–8 years (p = 0.001) (Figure 2A).
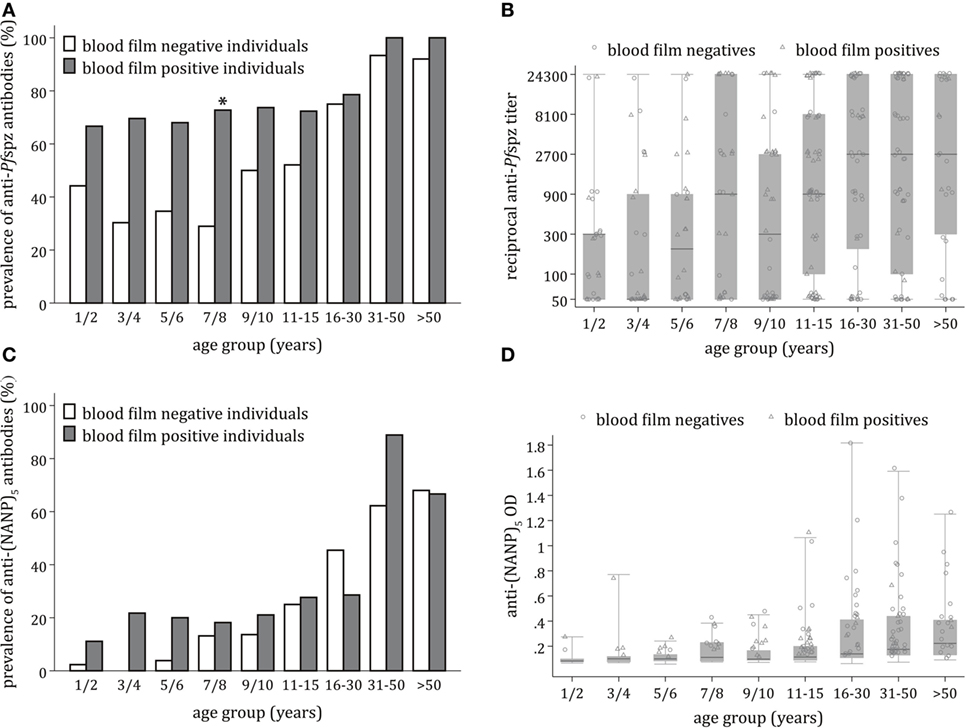
Figure 2. Age-dependent acquisition of antisporozoite antibodies and correlation with microscopically detectable Plasmodium falciparum (Pf) infection. (A) Proportion of sera responding to Pf spzorozoites (Pfspz) by immunofluorescence assay (IFA) in each age group, stratified by blood film positivity. Anti-Pfspz antibody prevalence was significantly correlated with age among blood film-negative individuals (Pearson’s χ2 = 73.2; p < 0.001), but not blood film-positive individuals (Pearson’s χ2 = 5.4; p = 0.719). In all age groups, the proportion of responders was higher among blood film-positive individuals, but this was only significant among young individuals aged 7–8 years (Pearson’s χ2 = 10.8; p = 0.001). (B) Distribution of anti-Pfspz antibody titers by age category, excluding IFA-non-responders. Anti-Pfspz antibody titers are plotted on a log scale. Median anti-Pfspz antibody titers increased significantly with increasing age (Kruskal–Wallis test: χ2 = 32.7; p < 0.001). Each serum was tested in threefold serial dilutions (1:50–1:24,300). The number of high responders was detected in all age groups. Within individual age groups, differences in antibody levels between blood film-positive individuals and blood film-negative individuals were non-significant (Mann–Whitney test: p > 0.05). Boxes and horizontal bars indicate the 75th and 25th percentiles and median, respectively. Circles and triangles represent individual sera of blood film-negative or blood film-positive individuals, respectively. Whiskers represent minimum and maximum values. (C) Proportion of sera responding to (NANP)5 by enzyme-linked immunosorbent assay (ELISA) in each age group, stratified by blood film positivity. The proportion of (NANP)5-seropositive sera increased with age in both blood film-negative subjects (Pearson’s χ2 = 95.9; p < 0.001) and blood film-positive individuals (Pearson’s χ2 = 23.9; p = 0.002). Within each age group, the proportion of responders was similar in blood film-positive individuals and blood film-negative individuals. (D) Distribution of anti-(NANP)5 antibody titers by age category. There was an age-dependent increase in median anti-(NANP)5 titers, but this was not statistically significant (Kruskal–Wallis test: χ2 = 11.2; p = 0.193). Differences in anti-(NANP)5 antibody levels among blood film-positive individuals or blood film-negative individuals were non-significant in all age groups (Mann–Whitney test: p < 0.05); dashed line: ELISA seropositivity cutoff = 0.135. Boxes and horizontal bars indicate the 75th and 25th percentiles and median, respectively. Circles and triangles represent individual sera of blood film-negative or blood film-positive individuals, respectively. Whiskers represent minimum and maximum values.
Despite the presence of several high responders among children, anti-Pfspz antibody titers significantly correlated with age (p < 0.001) (Figure 2B). This was significant among blood film-negative individuals (p = 0.001), but not blood film-positive individuals (p = 0.08). Differences in median end titer between the two cohort subsets were non-significant in all age groups.
Acquisition of antibodies against the PfCSP repetitive region (NANP)5 occurred in an age-dependent manner (p < 0.0001). Anti-(NANP)5 antibody acquisition correlated with age among blood film-negative individuals (p < 0.001) and blood film-positive individuals (p = 0.002) (Figure 2C). The proportion of (NANP)5-reactive sera was comparable between blood film-positive individuals and blood film-negative individuals.
There was an age-dependent increase in levels of anti-(NANP)5 antibodies, but this increase was not statistically significant (p = 0.19) (Figure 2D). Levels of anti-(NANP)5 antibodies were comparable between blood film-negative and blood film-positive individuals.
Correlation of Antibodies against Pf Sporozoites with Malaria Incidence
Univariate Analysis
In univariate analysis, there was no marked difference in the cumulative risk of clinical malaria depending on Pfspz responder status (p = 0.19) (Table S2 in Supplementary Material). However, the risk of clinical malaria was significantly reduced among Pfspz responders who were blood film positive at the beginning of follow-up (p = 0.015). This association was not observed among blood film-negative individuals.
Overall, individuals with a positive antibody response to (NANP)5 displayed a significantly lower risk of clinical malaria during follow-up (p = 0.006), although this association was only significant among blood film-positive individuals (p = 0.03) (Table S2 in Supplementary Material).
Survival Analysis
According to Kaplan–Meier analysis, antibodies against whole Pfspz did not have any impact on clinical malaria incidence among individuals who were blood film negative at the time of serum collection (p = 0.85) (Figure 3A). In marked contrast, low (1:50–1:100), moderate (1:300–1:2,700), and high (≥1:8,100) anti-Pfspz titers correlated with a reduced risk of and increased time to first (or only) episode of clinical malaria among blood film-positive individuals (p = 0.014) (Figure 3B).
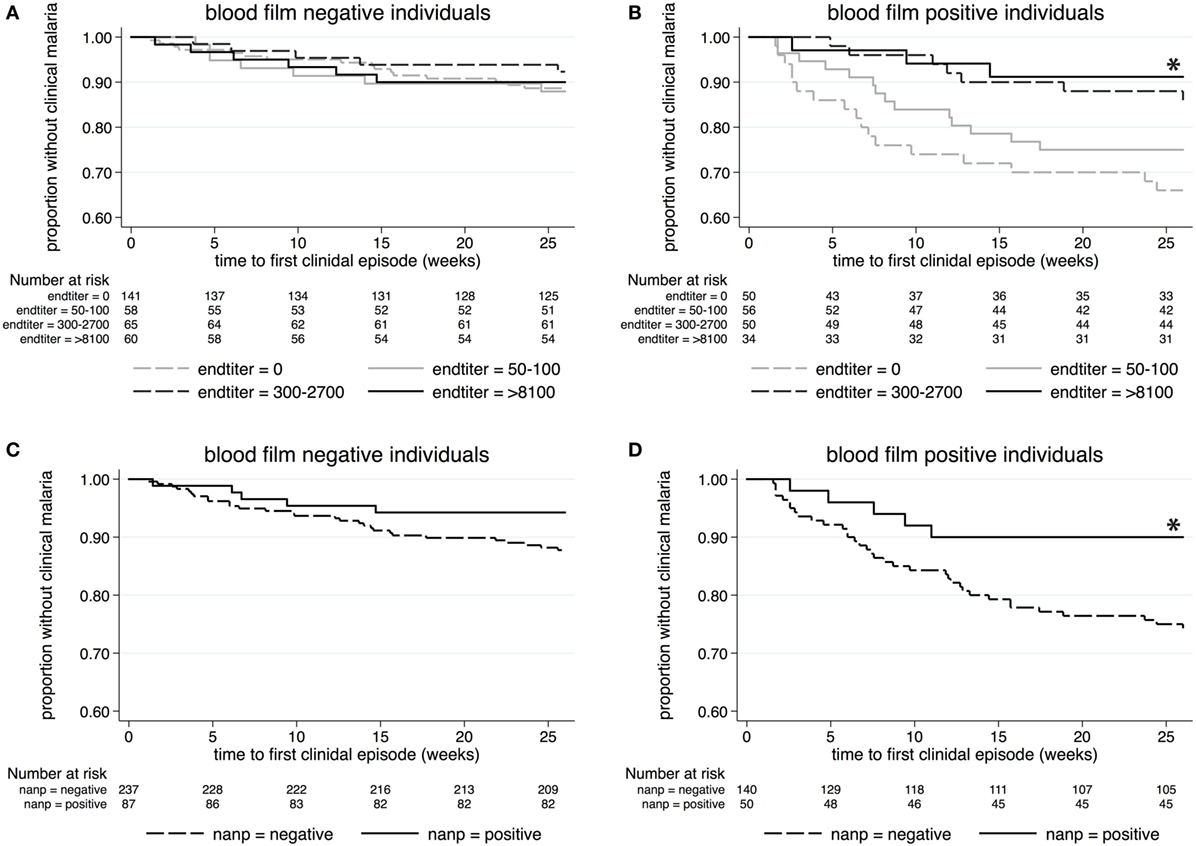
Figure 3. Kaplan–Meier analysis of time to clinical malaria during the 26-week follow-up. Survival curves display the proportion of malaria-free individuals over time, stratified by anti-Pfspz antibody titer (A,B) or (NANP)5-seropositivity status (C,D). (A) Among blood film-negative individuals, serum levels of anti-Pfspz antibodies did not have an impact on the incidence of clinical malaria (log-rank Mantel–Cox test: χ2 = 0.81; p = 0.847). (B) High anti-Pfspz antibody titers were significantly associated with a reduced risk of and increased time to first clinical episode among blood film-positive individuals (log-rank Mantel–Cox test: χ2 = 10.65; p = 0.014). (C) Anti-(NANP)5 seropositivity did not correlate with malaria incidence among blood film-negative individuals (log-rank Mantel–Cox test: χ2 = 2.74; p = 0.098). (D) Anti-(NANP)5 seropositivity was significantly associated with reduced malaria incidence among blood film-positive individuals (log-rank Mantel–Cox test: χ2 = 5.02; p = 0.025).
(NANP)5 responses did not correlate with the incidence of febrile malaria among blood film-negative individuals (p = 0.1) (Figure 3C), but (NANP)5 seropositivity was significantly associated with the reduced risk of and increased time to clinical malaria among blood film-positive individuals (p = 0.025) (Figure 3D).
Multivariate Analysis
To minimize bias through potential confounders, we developed a multivariable polynomial Cox regression model including the three critical covariates (i) age, (ii) malaria exposure, and (iii) plasma levels of antimerozoite stage antibodies. Increasing levels of anti-Pfspz antibodies were independently associated with a significantly increased risk of experiencing clinical malaria during follow-up among blood film-negative individuals (p = 0.006) (Figure 4A). The model showed a similar trend among anti-(NANP)5 responders, with a significantly increased hazard of clinical malaria among blood film-negative individuals (p = 0.007) (Figure 4C). Among blood film-positive individuals, increasing levels of anti-Pfspz antibodies were associated with a decreased risk of clinical malaria during follow-up, although this association was not significant after the Bonferroni correction was applied (p = 0.035) (Figure 4B). Anti-(NANP)5 antibody levels were not associated with risk of clinical malaria during follow-up among blood film-positive individuals (p = 0.99) (Figure 4D).
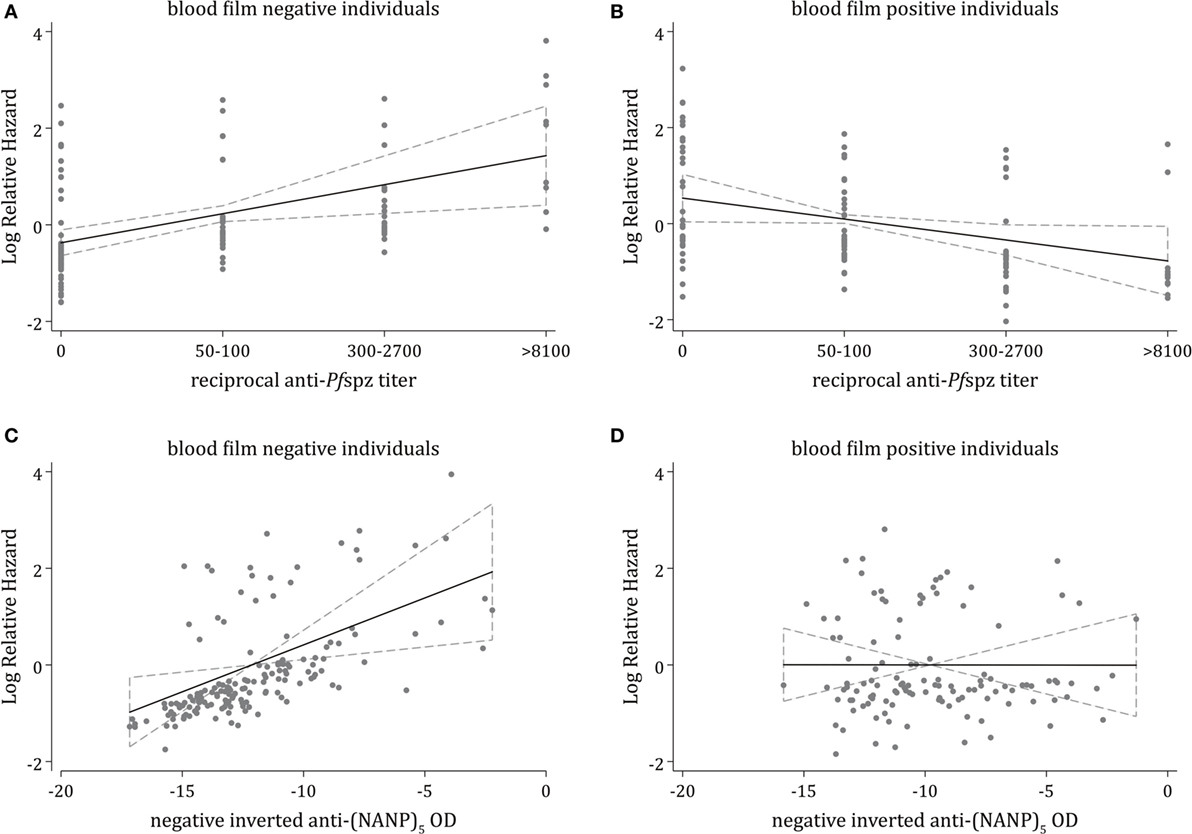
Figure 4. Multivariable analysis of associations of antisporozoite antibodies against clinical malaria. Multivariable fractional polynomial Cox regression models estimating the independent effect of different levels of anti-Pfspz (A,B) or anti-(NANP)5 (C,D) antibodies on the incidence of clinical malaria during follow-up, stratified by blood film positivity and adjusted for potential confounding variables, including age, malaria exposure, and plasma levels of antibodies against blood stage antigens (MSP1-3, AMA1, EBA175, and Pf schizont extract); malaria exposure was measured by the proportion of infected individuals within a 1-km radius of an index case, calculated using weekly slide data for acute episodes, and using the cross-sectional slide data (38). (A) Among blood film-negative individuals, increasing anti-Pfspz levels were associated with a significantly increased rate of clinical malaria during follow-up [hazard ratio (HR) = 1.82; 95% CI = 1.19–2.81; p = 0.006]. (B) Among blood film-positive individuals, increasing anti-Pfspz levels were associated with a reduced rate of clinical malaria during follow-up, although these results were not statistically significant after applying the Bonferroni correction (HR = 0.65; 95% CI = 0.43–0.97; p = 0.035). (C) Increasing anti-(NANP)5 levels were associated with a significantly increased rate of clinical malaria during follow-up among blood film negatives (HR = 1.21; 95% CI = 1.05–1.40; p = 0.007). To reduce the skewness while maintaining the direction of association, anti-(NANP)5 optical density (OD) values were transformed to negative inverse values. (D) No association between increasing anti-(NANP)5 levels and a reduced rate of clinical malaria during follow-up was observed among blood film-positive individuals (HR = 0.99; 95% CI = 0.88–1.13; p = 0.992).
Interaction Analysis
The relationship between antisporozoite antibodies, microscopically detectable Pf parasitemia, and the incidence of clinical malaria was further analyzed through a Cox regression interaction model assessing (i) the effect of antisporozoite antibodies among blood film-negative individuals, (ii) the effect of microscopically detectable Pf parasitemia among sporozoite non-responders, and (iii) the effect of interactions between antisporozoite antibody levels and microscopically detectable Pf parasitemia on the time to first or only clinical malaria episode during follow-up, independent of age and malaria exposure.
Among blood film-negative individuals, the model indicated an increased hazard of clinical malaria in high Pfspz responders, although this association was not significant after the Bonferroni correction (p = 0.014) (Table 1). Microscopically detectable Pf parasitemia significantly increased the hazard of clinical malaria among individuals who did not react to Pfspz (p < 0.001). Increasing titers of anti-Pfspz antibodies among blood film-positive individuals conferred a strong protection against clinical malaria, independent of age and malaria exposure (p = 0.002) (Table 1). Hence, the model indicated a strong interaction between the effect of anti-Pfspz antibody levels and Pf parasitemia on the risk of clinical malaria. The effect of anti-Pfspz antibody levels was protective in blood film-positive individuals and predicted increased risk of first or only clinical episode in blood film-negative individuals. There was a significant difference between the model with and without the interaction term (likelihood ratio test: χ2 = 26.05; p = 0.0005). A similar model identified an association between increasing anti-(NANP)5 antibody levels and microscopically detectable Pf parasitemia on the risk of clinical malaria (p = 0.001) (Table 2). Again, there was a significant difference between the model with and without interaction term (likelihood ratio test: χ2 = 24.38; p < 0.0001).
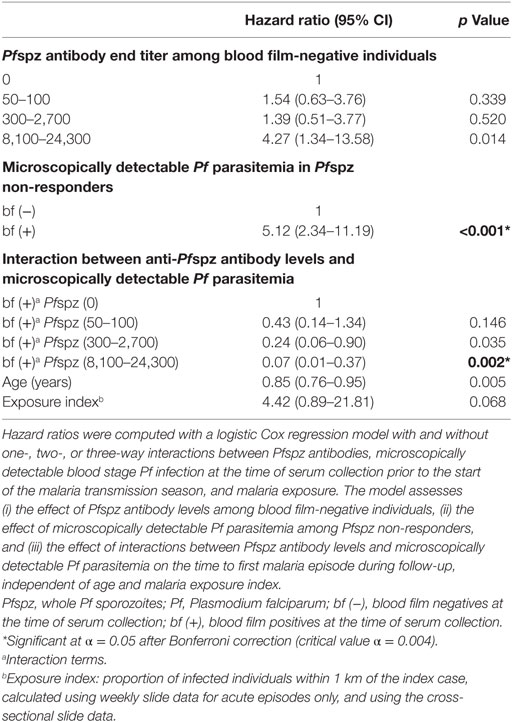
Table 1. Effect of interactions between anti-Pfspz antibody levels and microscopically detectable Pf parasitemia on the risk of clinical malaria.
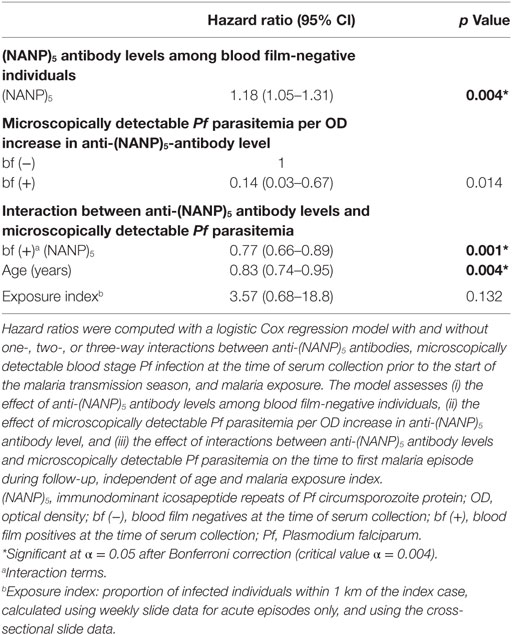
Table 2. Effect of interactions between anti-(NANP)5 antibody levels and microscopically detectable Pf parasitemia on the risk of clinical malaria.
Discussion
In this study, we analyzed the association of naturally acquired antibody responses against whole Pfspz and the PfCSP CRR (NANP)5 with microscopically detectable Pf parasitemia and clinical malaria in the longitudinally monitored Chonyi cohort. The data indicate that robust antisporozoite immune responses can be naturally acquired early in life and reveal a potentially protective interaction between the effect of anti-Pfspz antibodies and microscopically detectable Pf parasitemia on the risk of uncomplicated malaria.
Naturally Acquired Humoral Immunity against Pf Sporozoites
In good agreement with previous studies (15, 39), prevalence and intensity of antibody responses to Pfspz and (NANP)5 were correlated within the Chonyi cohort. However, even among high Pfspz responders, some sera remained (NANP)5 negative. Slow acquisition of anti-(NANP)5 antibodies has already been reported (17). Previous studies demonstrated that endemic sera retain IFA signals despite depletion of anti-(NANP)n antibodies by the cognate peptide (39). Thus, the sera used in this study may have reacted to CSP antigens other than (NANP)5 or non-CSP epitopes present internally or on the sporozoite surface (40), suggesting that exclusive quantification of anti-(NANP)n titers may largely underestimate the prevalence and breadth of naturally acquired preerythrocytic humoral immune responses. This discrepancy may be, at least partly, explained by different sensitivities of the assays used. In particular, the use of air-dried, acetone fixed sporozoites may inactivate some epitopes on the sporozoite surface and instead expose others that are usually inaccessible to protective antibodies. However, since our serology data show that fixed sporozoites are well recognized in IFA, we anticipate that live sporozoites tested in follow-up studies will likely display comparable activity.
In this study, we confirmed an age-dependent pattern of anti-Pfspz antibody acquisition (9–11), although we also identified several high responders among children, whose sera reacted to Pfspz at dilutions of up to 1:24,000. This is remarkable, considering that virtually all sera from adult volunteers immunized five times intravenously with ~135,000 metabolically active, irradiation-attenuated Pf sporozoites recognized Pfspz at ≤1:7,000 in a similar IFA assay (41). This would indicate that naturally acquired immune recognition of Pfspz fundamentally differs from syringe-injected, purified, and cryopreserved parasites.
Especially among younger age groups, the prevalence of antisporozoite responders was consistently higher among blood film-positive subjects. Whether the increased reactivity against sporozoites in this group could be attributable to recent or recurrent exposure to Pf could not be inferred based on the available data. Since the presence of asymptomatic Pf infection was determined by microscopy, we cannot exclude that a significant proportion of blood film-negative individuals may have carried blood stage parasites below the microscopy detection level. In future studies, more sensitive methodologies, such as quantitative polymerase chain reaction or loop-mediated isothermal amplification, may provide a more accurate estimate of the prevalence of asymptomatic Pf infection among study participants.
Despite the overall lower reactivity, an age distribution of the prevalence of anti-(NANP)5 antibodies was detectable in this cohort, confirming previous findings (14, 15, 42). Microscopically detectable Pf parasitemia did not seem to affect acquisition of the anti-(NANP)5 antibody repertoire, corroborating previous observations that anti-(NANP)5 antibodies do not necessarily correlate with exposure (43, 44). On the other hand, several studies showed that anti-(NANP)n antibody responses fluctuate with seasonal or geographical transmission variation (13, 16), and outbreak investigations showed that a single sporozoite inoculation is able to induce humoral immunity against (NANP)n (15, 18). These conflicting reports may reflect epidemiological differences between the geographically distinct study areas.
Together, these observations support the notion of naturally induced acquisition of antibodies against the whole sporozoite and, to a lesser extent, the dominant repeat region of its main surface antigen. Whether the prevalence and intensity of anti-Pfspz responses may represent a valuable marker for recent and/or repeated exposure remains to be elucidated in further longitudinal cohort studies, which should include entomological data to estimate the temporospatial dynamics of sporozoite transmission. Additional immune markers are needed to ultimately disentangle recent and past Plasmodium exposures.
Potentially Protective Associations of Antisporozoite Antibodies with Clinical Malaria
Previous investigations in the Chonyi cohort have shown that the correlation of naturally acquired antibodies to blood stage antigens with the risk of disease varies depending on the presence of microscopically detectable Pf infection (31, 34, 45, 46). Therefore, the relationship of antisporozoite antibodies with malaria incidence was analyzed separately in individuals who were blood film positive or blood film negative at sampling.
Our analysis revealed dual features of antisporozoite antibodies. Among individuals who were parasite free or carrying Pf parasites below the level of detection by microscopy at the start of the malaria transmission season, high anti-Pfspz and anti-(NANP)5 antibody titers were significantly correlated with increased hazard of clinical malaria during follow-up. Acute antibody responses against the sporozoite are most likely elicited upon Pf inoculation. Blood film-negative individuals displaying higher antisporozoite antibody titers may have been recently exposed to a strong inoculation dose and, as a consequence, may be at higher risk of clinical malaria. Since our study only assessed humoral immune responses against the NF54 Pf strain, we cannot formally exclude that these individuals may have been infected by novel Pf strains to which they were immunologically unresponsive.
In marked contrast, higher levels of anti-Pfspz antibodies predicted a lower risk of and delayed progression to clinical malaria among those individuals who were blood film positive at the start of the malaria transmission season. Notably, we detected a strong and potentially protective interaction between the effect of both anti-Pfspz and anti-(NANP)5 antibody levels and microscopically detectable Pf parasitemia at the start of the transmission season on the risk of clinical malaria, which was independent of age and Pf exposure. The interaction analysis showed that the potentially protective effect of antisporozoite antibodies was greater among individuals with detectable baseline Pf parasitemia than those who were blood film negative at the beginning of the malaria transmission season. Because of this interaction, blood film-negative and -positive individuals displaying high antibody titers against sporozoites may experience different risks of clinical malaria. In contrast, the difference in risk of disease may be comparatively similar among blood film-negative or -positive individuals who display low antisporozoite titers. At higher antisporozoite antibody levels, blood film-positive individuals had significantly lower risk of malaria compared with blood film-negative individuals. This correlation was previously unrecognized and provides a rationale for future studies on the role(s) of preerythrocytic immunity in partial protection against malaria. Although highly speculative, one possibility is that members of the Chonyi cohort may have been infected by a diverse range of Pf strains, which in turn could have different immunological and pathogenic properties, thus influencing both individual immune responses and the patterns of clinical malaria incidence during the follow-up period. In such a scenario, selection through protective effects of antisporozoite antibodies could favor alternative strains.
One major limitation of this analysis is that data on participants’ Pf carrier status were only available from a cross-sectional measurement at the start of the malaria transmission season. It is plausible that the status of single individuals may have changed during the follow-up. Such changes may have affected the patterns of malaria incidence in the cohort over time, but this could not be taken into account in this analysis.
Although naturally induced antisporozoite immunity does not translate into sterile protection, partial preerythrocytic immunity might play a significant role in protection from clinical symptoms (28, 29). However, frequent inoculations over a longer time period might be required to induce short-lived, potentially protective anti-Pfspz antibodies. While such requirements might not have been met in the blood film-negative subset, the identification of children with high Pfspz antibody titers among the blood film-positive individuals indicates that high and potentially protective threshold levels may be acquired early in life. These hypotheses are based on the findings from this data set, but do not take into account the variability of the data. It is possible that other serologic data sets based on different cohorts or transmission settings may deliver contrasting results.
We cannot formally exclude that additional factors may have confounded this analysis. For instance, multiclonality of asymptomatic Pf infections correlates with a reduced risk of subsequent clinical malaria (47–49), and this association is dependent on the intensity of transmission (50). In children, sickle cell trait is independently associated with a delayed time to first clinical episode (51). Since these factors were not accounted for in our analysis, residual confounding may have occurred.
In addition, it is possible that the anti-Pfspz antibodies detected by IFA may actually recognize antigens that are shared between sporozoites and erythrocytic stages (52). Potentially protective anti-Pfspz antibodies detected in this study may be (i) antibodies that have been generated by blood stage infection but are also effective against sporozoites or (ii) antibodies that are effective against blood stages, in which case the IFA assay may have served as a proxy of protective blood stage responses.
Although there is currently scarce evidence that reducing the sporozoite load may reduce clinical malaria, possible scenarios for the contribution of antisporozoite antibodies to protection from clinical malaria include (i) reduction of sporozoite numbers by opsonization and subsequent phagocytosis through innate immune cells and (ii) blocking of essential parasite ligands for liver invasion receptors, as suggested by reduced sporozoite invasion of human liver cell cultures in vitro following the addition of endemic sera (53). Both effects may ultimately limit the scale of liver infection and, consequently, delay or mitigate the emergence of liver merozoites that initiate blood stage infection. Although the NANP repeats of CSP represent an immunodominant B-cell epitope (37), it is tempting to speculate that only broad recognition of multiple sporozoite antigens may represent a good marker for recent exposure and elicit protection against subsequent malaria incidence.
Conclusion
Observations in Kenya and other endemic areas indicated that only a very small fraction of mosquito inoculations results in reappearance of malaria after drug treatment (54–56). While these observations might be partly attributed to potent blood stage immune responses or inefficient sporozoite inoculation by infected mosquitoes, they could also suggest that malaria-exposed individuals are able to develop partially protective immunity against sporozoites. In this study, whole Pf sporozoites were employed for the first time as target antigen in a longitudinal study to assess the role(s) of naturally acquired antibodies in protective immunity against clinical malaria. A potentially protective contribution of antisporozoite antibodies was detected, suggesting that preerythrocytic immunity might indeed contribute to naturally acquired protection and that antibodies against several sporozoite antigens might be superior than responses to single antigens. Several obstacles complicate the interpretation of naturally acquired antibodies against sporozoites and their potentially protective roles. First, assessment of the relationship between disease and immune responses that never reach sterile protection is complex. In addition, endpoints in Pf malaria include microscopic detection of blood stage infection, while preerythrocytic stages remain undetectable. Additional functional assays, such as sporozoite invasion inhibition and antibody-dependent, cell-mediated immunity, will add further confidence to whether high antisporozoite titers can effectively reduce the burden of this vector-borne parasitic disease and exert a hitherto neglected benefit in preventing clinical malaria episodes.
Ethics Statement
Ethical approval was obtained from the Kenya National Research Ethics Committee (study protocol number REF CTMDR/SCC/1340). All participants (or parents/guardians of children aged ≤14 years) gave written informed consent (31, 34, 36). The local dialect was used to explain the study protocol to the participants, and a copy of the consent form was left in each household, so that all family members could review it prior to consenting.
Author Contributions
All authors delivered substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work; contributed to drafting the work or revising it critically for important intellectual content; gave final approval of the version to be published; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors did not at any time receive payment or services from a third party for any aspect of the submitted work. There are no financial relationships with entities that could be perceived to influence or that give the appearance of potentially influencing, what is written in the submitted work. There are no patents and copyrights, whether pending, issued, licensed, and/or receiving royalties that are relevant to this work. All the authors declare no conflict of interest.
Acknowledgments
We thank Philip Bejon and Steffen Borrmann for discussions and critical reading of this manuscript.
Funding
This work was supported by the Max Planck Society, the Kenya Medical Research Institute Center for Geographic Medicine Research-Coast (KEMRI-CGMRC), and the Wellcome Trust (grant numbers 092741 and 077092 to KEMRI-CGMRC). VT was also supported by a Wellcome Trust Intermediate Fellowship in Public Health and Tropical Medicine (grant number 084378/Z/07/A). This article is published with the permission of the director of KEMRI.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00488/full#supplementary-material.
Abbreviations
Pf, Plasmodium falciparum; Pfspz, whole Plasmodium falciparum sporozoites; CSP, circumsporozoite protein (major sporozoite surface protein); (NANP)5, immunodominant icosapeptide repeats of Pf circumsporozoite protein; CRR, central repeat region of circumsporozoite protein; MSP1-3, merozoite surface proteins 1-3; AMA1, apical membrane antigen-1; EBA-175, erythrocyte binding antigen 175.
References
1. World Health Organization. World Malaria Report 2016. Geneva: World Health Organization (2016). Available from: http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?ua=1 (accessed April 17, 2017).
2. Rosenberg R, Wirtz RA, Schneider I, Burge R. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg (1990) 84(2):209–12. doi: 10.1016/0035-9203(90)90258-G
3. Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis (2002) 185(8):1155–64. doi:10.1086/339409
4. Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med (2009) 361(5):468–77. doi:10.1056/NEJMoa0805832
5. Bijker EM, Bastiaens GJ, Teirlinck AC, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A (2013) 110(19):7862–7. doi:10.1073/pnas.1220360110
6. Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol (2011) 11(1):57–64. doi:10.1038/nri2902
7. Borrmann S, Matuschewski K. Targeting Plasmodium liver stages: better late than never. Trends Mol Med (2011) 17(9):527–36. doi:10.1016/j.molmed.2011.05.008
8. Ménard R, Tavares J, Cockburn I, Markus M, Zavala F, Amino R. Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol (2013) 11(10):701–12. doi:10.1038/nrmicro3111
9. Nardin EH, Nussenzweig RS, McGregor IA, Bryan JH. Antibodies to sporozoites: their frequent occurrence in individuals living in an area of hyperendemic malaria. Science (1979) 206(4418):597–9. doi:10.1126/science.386511
10. Tapchaisri P, Chomcharn Y, Poonthong C, Asavanich A, Limsuwan S, Maleevan O, et al. Anti-sporozoite antibodies induced by natural infection. Am J Trop Med Hyg (1983) 32(6):1203–8. doi:10.4269/ajtmh.1983.32.1203
11. Druilhe P, Pradier O, Marc JP, Miltgen F, Mazier D, Parent G. Levels of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfection. Infect Immun (1986) 53(2):393–7.
12. Campbell GH, Brandling-Bennett AD, Roberts JM, Collins FH, Kaseje DC, Barber AM, et al. Detection of antibodies in human sera to the repeating epitope of the circumsporozoite protein of Plasmodium falciparum using the synthetic peptide (NANP)3 in an enzyme-linked immunosorbent assay (ELISA). Am J Trop Med Hyg (1987) 37(1):17–21. doi:10.4269/ajtmh.1987.37.17
13. Campbell GH, Collins FH, Brandling-Bennett AD, Schwartz IK, Roberts JM. Age-specific prevalence of antibody to a synthetic peptide of the circumsporozoite protein of Plasmodium falciparum in children from three villages in Kenya. Am J Trop Med Hyg (1987) 37(2):220–4. doi:10.4269/ajtmh.1987.37.220
14. Del Giudice G, Engers HD, Tougne C, Biro SS, Weiss N, Verdini AS, et al. Antibodies to the repetitive epitope of Plasmodium falciparum circumsporozoite protein in a rural Tanzanian community: a longitudinal study of 132 children. Am J Trop Med Hyg (1987) 36(2):203–12. doi:10.4269/ajtmh.1987.36.203
15. Del Giudice G, Verdini AS, Pinori M, Pessi A, Verhave JP, Tougne C, et al. Detection of human antibodies against Plasmodium falciparum sporozoites using synthetic peptides. J Clin Microbiol (1987) 25(1):91–6.
16. Esposito F, Lombardi S, Modiano D, Zavala F, Reeme J, Lamizana L, et al. Prevalence and levels of antibodies to the circumsporozoite protein of Plasmodium falciparum in an endemic area and their relationship to resistance against malaria infection. Trans R Soc Trop Med Hyg (1988) 82(6):827–32. doi:10.1016/0035-9203(88)90007-7
17. Marsh K, Hayes RH, Carson DC, Otoo L, Shenton F, Byass P, et al. Anti-sporozoite antibodies and immunity to malaria in a rural Gambian population. Trans R Soc Trop Med Hyg (1988) 82(4):532–7. doi:10.1016/0035-9203(88)90495-6
18. Wijesundera MD, Peiris JS, Ariyaratne YG, Verdini AS, Pessi A, Del Giudice G. Antibodies to Plasmodium falciparum sporozoites following a malarial outbreak in a non-endemic area of Sri Lanka. Trans R Soc Trop Med Hyg (1990) 84(1):35–9. doi:10.1016/0035-9203(90)90372-L
19. Offeddu V, Thathy V, Marsh K, Matuschewski K. Naturally acquired immune responses against Plasmodium falciparum sporozoites and liver infection. Int J Parasitol (2012) 42(6):535–48. doi:10.1016/j.ijpara.2012.03.011
20. Del Giudice G, Cheng Q, Mazier D, Berbiguier N, Cooper JA, Engers HD, et al. Immunogenicity of a non-repetitive sequence of Plasmodium falciparum circumsporozoite protein in man and mice. Immunology (1988) 63(2):187–91.
21. Shi YP, Udhayakumar V, Alpers MP, Povoa MM, Oloo AJ, Ruebush TK II, et al. Natural antibody responses against the non-repeat-sequence-based B-cell epitopes of the Plasmodium falciparum circumsporozoite protein. Infect Immun (1993) 61(6):2425–33.
22. Lopez JA, Roggero MA, Duombo O, Gonzalez JM, Tolle R, Koita O, et al. Recognition of synthetic 104-mer and 102-mer peptides corresponding to N- and C-terminal nonrepeat regions of the Plasmodium falciparum circumsporozoite protein by sera from human donors. Am J Trop Med Hyg (1996) 55(4):424–9. doi:10.4269/ajtmh.1996.55.424
23. Scarselli E, Tolle R, Koita O, Diallo M, Muller HM, Fruh K, et al. Analysis of the human antibody response to thrombospondin-related anonymous protein of Plasmodium falciparum. Infect Immun (1993) 61(8):3490–5.
24. Charoenvit Y, Fallarme V, Rogers WO, Sacci JB Jr, Kaur M, Aguiar JC, et al. Development of two monoclonal antibodies against Plasmodium falciparum sporozoite surface protein 2 and mapping of B-cell epitopes. Infect Immun (1997) 65(8):3430–7.
25. Dolo A, Modiano D, Doumbo O, Bosman A, Sidibe T, Keita MM, et al. Thrombospondin related adhesive protein (TRAP), a potential malaria vaccine candidate. Parassitologia (1999) 41(1–3):425–8.
26. Fidock DA, Pasquetto V, Gras H, Badell E, Eling W, Ballou WR, et al. Plasmodium falciparum sporozoite invasion is inhibited by naturally acquired or experimentally induced polyclonal antibodies to the STARP antigen. Eur J Immunol (1997) 27(10):2502–13. doi:10.1002/eji.1830271007
27. Suwancharoen C, Putaporntip C, Rungruang T, Jongwutiwes S. Naturally acquired IgG antibodies against the C-terminal part of Plasmodium falciparum sporozoite threonine-asparagine-rich protein in a low endemic area. Parasitol Res (2011) 109(2):315–20. doi:10.1007/s00436-011-2257-z
28. John CC, Moormann AM, Pregibon DC, Sumba PO, McHugh MM, Narum DL, et al. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg (2005) 73(1):222–8. doi:10.4269/ajtmh.2005.73.222
29. John CC, Tande AJ, Moormann AM, Sumba PO, Lanar DE, Min XM, et al. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis (2008) 197(4):519–26. doi:10.1086/526787
30. Agak GW, Bejon P, Fegan G, Gicheru N, Villard V, Kajava AV, et al. Longitudinal analyses of immune responses to Plasmodium falciparum derived peptides corresponding to novel blood stage antigens in coastal Kenya. Vaccine (2008) 26(16):1963–71. doi:10.1016/j.vaccine.2008.02.020
31. Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun (2008) 76(5):2240–8. doi:10.1128/IAI.01585-07
32. Murungi LM, Kamuyu G, Lowe B, Bejon P, Theisen M, Kinyanjui SM, et al. A threshold concentration of anti-merozoite antibodies is required for protection from clinical episodes of malaria. Vaccine (2013) 31(37):3936–42. doi:10.1016/j.vaccine.2013.06.042
33. Tetteh KK, Osier FH, Salanti A, Kamuyu G, Drought L, Failly M, et al. Analysis of antibodies to newly described Plasmodium falciparum merozoite antigens supports MSPDBL2 as a predicted target of naturally acquired immunity. Infect Immun (2013) 81(10):3835–42. doi:10.1128/IAI.00301-13
34. Osier FH, Mackinnon MJ, Crosnier C, Fegan G, Kamuyu G, Wanaguru M, et al. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med (2014) 6(247):247ra102. doi:10.1126/scitranslmed.3008705
35. Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, Gunter JT, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg (2003) 68(6):734–42. doi:10.4269/ajtmh.2003.68.734
36. Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis (2005) 191(11):1932–9. doi:10.1086/430006
37. Zavala F, Cochrane AH, Nardin EH, Nussenzweig RS, Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med (1983) 157(6):1947–57. doi:10.1084/jem.157.6.1947
38. Olotu A, Fegan G, Wambua J, Nyangweso G, Ogada E, Drakeley C, et al. Estimating individual exposure to malaria using local prevalence of malaria infection in the field. PLoS One (2012) 7(3):e32929. doi:10.1371/journal.pone.0032929
39. Zavala F, Tam JP, Hollingdale MR, Cochrane AH, Quakyi I, Nussenzweig RS, et al. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science (1985) 228(4706):1436–40. doi:10.1126/science.2409595
40. Swearingen KE, Lindner SE, Shi L, Shears MJ, Harupa A, Hopp CS, et al. Interrogating the Plasmodium sporozoite surface: identification of surface-exposed proteins and demonstration of glycosylation on CSP and TRAP by mass spectrometry-based proteomics. PLoS Pathog (2016) 12(4):e1005606. doi:10.1371/journal.ppat.1005606
41. Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science (2013) 341(6152):1359–65. doi:10.1126/science.1241800
42. Del Giudice G, Lambert PH, Mendis K, Pessi A, Tanner M. Antibody responses to Plasmodium falciparum and P. vivax sporozoites in areas with stable and unstable malaria. Bull World Health Organ (1990) 68(Suppl):191–6.
43. Burkot TR, Graves PM, Wirtz RA, Brabin BJ, Battistutta D, Cattani JA, et al. Differential antibody responses to Plasmodium falciparum and P. vivax circumsporozoite proteins in a human population. J Clin Microbiol (1989) 27(6):1346–51.
44. Kilombero Malaria Project. The level of anti-sporozoite antibodies in a highly endemic malaria area and its relationship with exposure to mosquitoes. Trans R Soc of Trop Med Hyg (1992) 86(5):499–504. doi:10.1016/0035-9203(92)90084-P
45. Bull PC, Lowe BS, Kaleli N, Njuga F, Kortok M, Ross A, et al. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J Infect Dis (2002) 185(11):1688–91. doi:10.1086/340420
46. Polley SD, Conway DJ, Cavanagh DR, McBride JS, Lowe BS, Williams TN, et al. High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine (2006) 24(19):4233–46. doi:10.1016/j.vaccine.2005.06.030
47. al-Yaman F, Genton B, Reeder JC, Anders RF, Smith T, Alpers MP. Reduced risk of clinical malaria in children infected with multiple clones of Plasmodium falciparum in a highly endemic area: a prospective community study. Trans R Soc Trop Med Hyg (1997) 91(5):602–5. doi:10.1016/S0035-9203(97)90046-8
48. Bereczky S, Liljander A, Rooth I, Faraja L, Granath F, Montgomery SM, et al. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect (2007) 9(1):103–10. doi:10.1016/j.micinf.2006.10.014
49. Sonden K, Doumbo S, Hammar U, Vafa Homann M, Ongoiba A, Traore B, et al. Asymptomatic multiclonal Plasmodium falciparum infections carried through the dry season predict protection against subsequent clinical malaria. J Infect Dis (2015) 212(4):608–16. doi:10.1093/infdis/jiv088
50. Farnert A, Williams TN, Mwangi TW, Ehlin A, Fegan G, Macharia A, et al. Transmission-dependent tolerance to multiclonal Plasmodium falciparum infection. J Infect Dis (2009) 200(7):1166–75. doi:10.1086/605652
51. Crompton PD, Traore B, Kayentao K, Doumbo S, Ongoiba A, Diakite SA, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis (2008) 198(9):1265–75. doi:10.1086/592224
52. Nahrendorf W, Scholzen A, Sauerwein RW, Langhorne J. Cross-stage immunity for malaria vaccine development. Vaccine (2015) 33(52):7513–7. doi:10.1016/j.vaccine.2015.09.098
53. Hollingdale MR, Nardin EH, Tharavanij S, Schwartz AL, Nussenzweig RS. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol (1984) 132(2):909–13.
54. Beier JC, Oster CN, Onyango FK, Bales JD, Sherwood JA, Perkins PV, et al. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am J Trop Med Hyg (1994) 50(5):529–36. doi:10.4269/ajtmh.1994.50.529
55. Sokhna CS, Rogier C, Dieye A, Trape JF. Host factors affecting the delay of reappearance of Plasmodium falciparum after radical treatment among a semi-immune population exposed to intense perennial transmission. Am J Trop Med Hyg (2000) 62(2):266–70. doi:10.4269/ajtmh.2000.62.266
Keywords: malaria, Plasmodium falciparum, sporozoites, humoral immunity, clinical malaria, protective immunity
Citation: Offeddu V, Olotu A, Osier F, Marsh K, Matuschewski K and Thathy V (2017) High Sporozoite Antibody Titers in Conjunction with Microscopically Detectable Blood Infection Display Signatures of Protection from Clinical Malaria. Front. Immunol. 8:488. doi: 10.3389/fimmu.2017.00488
Received: 08 January 2017; Accepted: 07 April 2017;
Published: 08 May 2017
Edited by:
José Roberto Mineo, Federal University of Uberlandia, BrazilReviewed by:
Miguel Prudêncio, Instituto de Medicina Molecular, PortugalV. Ann Stewart, Uniformed Services University of the Health Sciences, USA
Copyright: © 2017 Offeddu, Olotu, Osier, Marsh, Matuschewski and Thathy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vittoria Offeddu, vittoria.offeddu@nus.edu.sg;
Vandana Thathy, vandana.thathy@rdm.ox.ac.uk
†Present address: Vittoria Offeddu, Epidemiology Domain, Saw Swee Hock School of Public Health, National University of Singapore, Singapore;
Ally Olotu, Ifakara Health Institute, Bagamoyo, Tanzania;
Vandana Thathy, Radcliffe Department of Medicine, MRC Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford, UK
 Vittoria Offeddu
Vittoria Offeddu Ally Olotu
Ally Olotu Faith Osier
Faith Osier Kevin Marsh
Kevin Marsh Kai Matuschewski
Kai Matuschewski Vandana Thathy
Vandana Thathy