Stimulated Respiration and Net Photosynthesis in Cassiopeia sp. during Glucose Enrichment Suggests in hospite CO2 Limitation of Algal Endosymbionts
- 1Biological and Environmental Sciences and Engineering Division, Red Sea Research Center, King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia
- 2Marine Ecology Working Group, Faculty of Biology and Chemistry (FB 2), University of Bremen, Bremen, Germany
The endosymbiosis between cnidarians and dinoflagellates of the genus Symbiodinium is key to the high productivity of tropical coral reefs. In this endosymbiosis, Symbiodinium translocate most of their photosynthates to their animal host in exchange for inorganic nutrients. Among these, carbon dioxide (CO2) derived from host respiration helps to meet the carbon requirements to sustain photosynthesis of the dinoflagellates. Nonetheless, recent studies suggest that productivity in symbiotic cnidarians such as corals is CO2-limited. Here we show that glucose enrichment stimulates respiration and gross photosynthesis rates by 80 and 140%, respectively, in the symbiotic upside-down jellyfish Cassiopeia sp. from the Central Red Sea. Our findings show that glucose was rapidly consumed and respired within the Cassiopeia sp. holobiont. The resulting increase of CO2 availability in hospite in turn likely stimulated photosynthesis in Symbiodinium. Hence, the increase of photosynthesis under these conditions suggests that CO2 limitation of Symbiodinium is a common feature of stable cnidarian holobionts and that the stimulation of holobiont metabolism may attenuate this CO2 limitation.
Introduction
Despite being surrounded by highly nutrient-poor (oligotrophic) waters, tropical coral reefs are among the most productive marine ecosystems (Hatcher, 1988). Reef ecosystems are sustained by an efficient uptake, retention, and reuse of nutrients on all levels of biological organization (Hatcher, 1990; Wild et al., 2004). In particular, the symbiosis between cnidarian hosts and endosymbiotic algae of the genus Symbiodinium facilitates the recycling of nutrients as it sustains primary productivity in the absence of major nutrient sources (Muscatine and Porter, 1977; Rädecker et al., 2015). In this symbiosis, Symbiodinium translocate most of their photosynthates to the cnidarian host that in turn provides inorganic nutrients derived from its metabolism (Muscatine et al., 1989). Thereby, this tight nutrient-exchange relationship, particularly in stony corals, is the functional basis for the ecological success of tropical coral reefs over millions of years.
Being surrounded by host membranes, Symbiodinium rely on their host to fulfill their photosynthetic carbon dioxide (CO2) requirements. The supply of CO2 to the symbiont is controlled by two major processes: (1) CO2 is produced during holobiont respiration (Muscatine et al., 1989). (2) Active carbon concentrating mechanisms (CCMs) by the host facilitate the uptake of dissolved inorganic carbon from surrounding seawater (Furla et al., 2000).
Despite these processes, several studies suggest that productivity in Symbiodinium may be carbon-limited even in stable symbiotic systems (Muscatine et al., 1989; Herfort et al., 2008; Klein et al., 2017). Hence, understanding the processes and environmental controls of in hospite CO2 availability is crucial for our understanding of the cnidarian—alga symbiosis.
To address this issue, we experimentally tested whether photosynthesis of Symbiodinium in hospite is carbon-limited. Specifically, we investigated photosynthetic activity during glucose-stimulated holobiont respiration in the upside-down jellyfish Cassiopeia sp. Unlike most other Scyphozoa, Cassiopeia spp. are mixotrophic, i.e. draw energy and nutrients from both heterotrophic and autotrophic sources (Rahav et al., 1989; Muscatine, 1990), as they form a close endosymbiotic relationship with Symbiodinium. Thereby, Cassiopeia spp. offer distinct advantages for the study of the cnidarian—alga symbiosis, similar to the Aiptasia model system (Baumgarten et al., 2015). For instance, they are easy to rear in aquaria cultures, are non-calcifying, have motile medusa stages and can be infected with various algal symbionts (Klein et al., 2017). Using this emerging cnidarian model system allowed us to tackle the issue of CO2 limitation in the cnidarian—Symbiodinium symbiosis in a straightforward experiment.
Methods
Collection and Maintenance
A total of 14 individuals of Cassiopeia sp. (mean bell diameter of 6.9 ± 0.3 cm) were collected with a dip net in the KAUST Harbor Lagoon, Saudi Arabia (N22°18′18.63″, E39°6′10.45″) in the Central Red Sea in September 2014. After collection, animals were immediately transferred to 2 recirculation aquaria (each filled with 20 L of ambient seawater) and acclimated to aquaria conditions for 7 days (salinity of 40, 28°C, 12:12 h light/dark cycle with ~100 μmol m−2 s−1). Stability of water parameters was ensured by exchanging 50% of aquaria seawater daily.
Incubations and Glucose Enrichment
Following acclimation, net photosynthesis and respiration rates of animals were directly assessed from oxygen (O2) evolution/depletion measurements in 2 h light and dark incubations in 1 L gas-tight glass chambers, respectively. During these incubations, half of the animals were incubated in ambient seawater freshly enriched with glucose (500 mg L−1). The other half of the animals served as a control and were incubated in ambient seawater. To correct jellyfish O2 fluxes for planktonic background metabolism, two seawater controls (i.e., ambient seawater without jellyfish) were included for each treatment. Importantly, the dissolved organic carbon concentrations used here do not reflect naturally occurring ambient reef water conditions (Vaccaro et al., 1968; Kline et al., 2006). Rather, the level of enrichment was chosen to avoid glucose depletion over the course of the incubation and to ensure that effects of increased carbon availability were not buffered within the holobiont framework, in order to gain mechanistic insights into the cnidarian—alga symbiosis.
O2 fluxes were assessed based on differences in O2 concentrations before and after the incubation using an optical oxygen multiprobe (WTW, Germany). O2 production/consumption rates were corrected for seawater controls and normalized to bell surface area of animals and incubation time. Gross photosynthesis rates were calculated based on differences in O2 fluxes during light and dark incubations (gross photosynthesis = net photosynthesis + |respiration|). Differences between treatments for the individual response parameters were tested for significance using an unpaired Student's t-test with a significance level (α) of 0.05.
Results and Discussion
Glucose enrichment stimulated respiration rates in seawater during both light and dark incubations (Supplementary Table S1). Still, seawater respiration rates were ~5-fold below Cassiopeia sp. respiration rates at all times. Holobiont respiration rates of Cassiopeia sp. increased by ~80% under glucose-enriched conditions compared to untreated controls [t(13) = 5.27, P < 0.001, Figure 1]. Despite this increase in respiratory O2 consumption, net photosynthesis rates during glucose-enriched conditions showed a significant increase of nearly 400% compared to controls [t(13) = 3.08, P = 0.008]. Consequently, gross photosynthesis rates increased by ~140% under glucose-enriched conditions compared to untreated controls [t(13) = 4.94, P < 0.001].
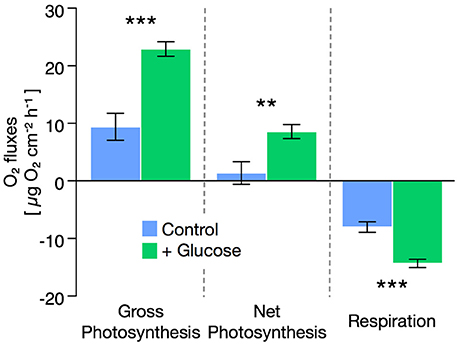
Figure 1. Effect of glucose enrichment (500 mg L−1) on gross and net photosynthesis as well as respiration rates in Cassiopeia sp. from the Central Red Sea. Net photosynthesis and respiration rates were derived from oxygen (O2) flux measurements in light and dark incubations, respectively. Gross photosynthesis was calculated based on the differences in O2 fluxes during light and dark incubations. All data are shown as mean ± SE. Asterisks indicate significant differences between groups (**p < 0.01; ***p < 0.001).
Glucose enrichment, hence, not only stimulated respiration rates but also caused a stark increase in photosynthetic activity in the mixotrophic cnidarian holobiont Cassiopeia sp. The increase in respiration rates indicates that glucose was rapidly taken up and consumed (i.e., respired) within the holobiont (Pogoreutz et al., 2017). Given our current understanding of cnidarian holobionts, there is no reason to assume that glucose enrichment directly affected photosynthetic activity in Symbiodinium. Rather, the observed increase in net and gross photosynthesis can be attributed to an increase in CO2 availability in hospite, stemming from increased respiration in the Cassiopeia holobiont and seawater planktonic communities within the incubation chamber. In the case of Cassiopeia sp. this CO2 limitation may be potentially attenuated by their continuous pumping motion facilitating increased gas exchange with the surrounding seawater (Wild and Naumann, 2013).
On a broader scale, these results could have implications for our understanding of the mechanisms underlying the cnidarian—alga symbiosis. The observation of glucose-stimulated photosynthesis implies that productivity of Symbiodinium in hospite may be tightly limited by CO2 derived from holobiont metabolism.
Wooldridge (2009) proposed that a failure of coral CCMs during heat stress may ultimately result in a CO2 limitation of photosynthetic dark reactions in Symbiodinium, ultimately leading to coral bleaching. Direct empirical evidence for this theory is missing to date. Our results, therefore, add to a growing emerging body of work suggesting that Symbiodinium may be CO2-limited even in stable symbiotic systems (Muscatine et al., 1989; Herfort et al., 2008; Buxton et al., 2009; Klein et al., 2017). Hence, environmental stressors which alter metabolic processes in the holobiont may indeed lead to severe CO2 limitation as predicted by Wooldridge (2009). Furthermore, we could show that the stimulation of host heterotrophy may attenuate CO2 limitation in Symbiodinium. In this context, several studies reported that increased heterotrophic feeding may mitigate the effects of thermal stress in reef-building corals, resulting in increased bleaching resilience (Grottoli et al., 2006; Baird et al., 2009; Houlbrèque and Ferrier-Pagès, 2009; Ezzat et al., 2016). While this effect was mostly attributed to a compensation of autotrophic with heterotrophic energy sources by the host, here we show that heterotrophy may also increase bleaching resilience by increasing CO2 availability in hospite.
Taken together, our study highlights that the role of CO2 availability within the cnidarian—algae symbiosis deserves further in-depth assessment. Further work will be necessary to understand the effects of environmental conditions on CO2 availability in hospite, along with their implications for the cnidarian—alga symbiosis.
Author Contributions
NR and CP designed and conducted the experiment. All authors analyzed the data and wrote and revised the manuscript.
Funding
Research reported in this publication was supported by KAUST baseline funding to CRV and grant Wi 2677/9-1 awarded to CW by German Research Foundation (DFG).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Paul Müller and Zenon Batang for allocation of workspace and their assistance with the aquarium facilities at the Coastal and Marine Resources Core Lab (CMOR). We further thank the two reviewers for their constructive feedback and helpful comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmars.2017.00267/full#supplementary-material
References
Baird, A. H., Bhagooli, R., Ralph, P. J., and Takahashi, S. (2009). Coral bleaching: the role of the host. Trends Ecol. Evol. 24, 16–20. doi: 10.1016/j.tree.2008.09.005
Baumgarten, S., Simakov, O., Esherick, L. Y., Jin, Y., Lehnert, E. M., Michell, C. T., et al. (2015). The genome of Aiptasia, a sea anemone model for coral symbiosis. Proc. Natl. Acad. Sci. U.S.A. 112, 11893–11898. doi: 10.1073/pnas.1513318112
Buxton, L., Badger, M., and Ralph, P. (2009). Effects of moderate heat stress and dissolved inorganic carbon concentration on photosynthesis and respiration of Symbiodinium sp. (Dinophycae) in culture and in symbiosis. J. Phycol. 45, 357–365. doi: 10.1111/j.1529-8817.2009.00659.x
Ezzat, L., Towle, E., Irisson, J.-O., Langdon, C., and Ferrier-Pagès, C. (2016). The relationship between heterotrophic feeding and inorganic nutrient availability in the scleractinian coral T. reniformis under a short-term temperature increase. Limnol. Oceanogr. 61, 89–102. doi: 10.1002/lno.10200
Furla, P., Allemand, D., and Orsenigo, M. N. (2000). Involvement of H+-ATPase and carbonic anhydrase in inorganic carbon uptake for endosymbiont photosynthesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, 870–881.
Grottoli, A. G., Rodrigues, L. J., and Palardy, J. E. (2006). Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186–1189. doi: 10.1038/nature04565
Hatcher, B. G. (1988). Coral reef primary productivity: a beggar's banquet. Trends Ecol. Evol. 3, 106–111. doi: 10.1016/0169-5347(88)90117-6
Hatcher, B. G. (1990). Coral reef primary productivity. A hierarchy of pattern and process. Trends Ecol. Evol. 5, 149–155. doi: 10.1016/0169-5347(90)90221-X
Herfort, L., Thake, B., and Taubner, I. (2008). Bicarbonate stimulation of calcification and photosynthesis in two hermatypic corals. J. Phycol. 44, 91–98. doi: 10.1111/j.1529-8817.2007.00445.x
Houlbrèque, F., and Ferrier-Pagès, C. (2009). Heterotrophy in tropical scleractinian corals. Biol. Rev. Camb. Philos. Soc. 84, 1–17. doi: 10.1111/j.1469-185X.2008.00058.x
Klein, S. G., Pitt, K. A., Nitschke, M. R., Goyen, S., Welsh, D. T., Suggett, D. J., et al. (2017). Symbiodinium mitigate the combined effects of hypoxia and acidification on a non-calcifying organism. Glob. Chang. Biol. 23, 3690–3703. doi: 10.1111/gcb.13718
Kline, D. I., Kuntz, N. M., Breitbart, M., Knowlton, N., and Rohwer, F. (2006). Role of elevated organic carbon levels and microbial activity in coral mortality. Mar. Ecol. Prog. Ser. 314, 119–125. doi: 10.3354/meps314119
Muscatine, L. (1990). “The role of symbiotic algae in carbon and energy flux in reef corals,” in Ecosystems of the World, Coral Reefs, ed Z. Dubinsky (Amsterdam: Elsevier), 75–87.
Muscatine, L., and Porter, J. W. (1977). Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience, 27, 454–460. doi: 10.2307/1297526
Muscatine, L., Porter, J. W., and Kaplan, I. R. (1989). Resource partitioning by reef corals as determined from stable isotope composition. Mar. Biol. 100, 185–193. doi: 10.1007/BF00391957
Pogoreutz, C., Rädecker, N., Cárdenas, A., Gärdes, A., Voolstra, C. R., and Wild, C. (2017). Sugar enrichment provides evidence for a role of nitrogen fixation in coral bleaching. Glob. Chang Biol. 23, 3838–3848. doi: 10.1111/gcb.13695
Rädecker, N., Pogoreutz, C., Voolstra, C. R., Wiedenmann, J., and Wild, C. (2015). Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends Microbiol. 23, 490–497. doi: 10.1016/j.tim.2015.03.008
Rahav, O., Dubinsky, Z., Achituv, Y., and Falkowski, P. G. (1989). Ammonium metabolism in the zooxanthellate coral, Stylophora pistillata. Proc. R. Soc. B Biol. Sci. 236, 325–337. doi: 10.1098/rspb.1989.0026
Vaccaro, R. F., Hicks, S. E., Jannasch, H. W., and Carey, F. G. (1968). The occurrence and role of glucose in seawater. Limnol. Oceanogr. 13, 356–360. doi: 10.4319/lo.1968.13.2.0356
Wild, C., Huettel, M., Klueter, A., Kremb, S. G., Rasheed, M. Y. M., and Jørgensen, B. B. (2004). Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428, 66–70. doi: 10.1038/nature02344
Wild, C., and Naumann, M. S. (2013). Effect of active water movement on energy and nutrient acquisition in coral reef-associated benthic organisms. Proc. Natl. Acad. Sci. U.S.A. 110, 8767–8768. doi: 10.1073/pnas.1306839110
Keywords: symbiosis, Symbiodinium, carbon limitation, heterotrophy, upside-down jellyfish
Citation: Rädecker N, Pogoreutz C, Wild C and Voolstra CR (2017) Stimulated Respiration and Net Photosynthesis in Cassiopeia sp. during Glucose Enrichment Suggests in hospite CO2 Limitation of Algal Endosymbionts. Front. Mar. Sci. 4:267. doi: 10.3389/fmars.2017.00267
Received: 21 June 2017; Accepted: 02 August 2017;
Published: 15 August 2017.
Edited by:
Stanley Chun Kwan Lau, Hong Kong University of Science and Technology, Hong KongReviewed by:
Luke Thompson, Southwest Fisheries Science Center (NOAA), United StatesAdam Michael Reitzel, University of North Carolina at Charlotte, United States
Copyright © 2017 Rädecker, Pogoreutz, Wild and Voolstra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian R. Voolstra, christian.voolstra@kaust.edu.sa
†These authors have contributed equally to this work.
 Nils Rädecker
Nils Rädecker Claudia Pogoreutz
Claudia Pogoreutz Christian Wild
Christian Wild Christian R. Voolstra
Christian R. Voolstra