Comparison of Reproductive Performance of Domesticated Litopenaeus vannamei Females Reared in Recirculating Tanks and Earthen Ponds: An Evaluation of Reproductive Quality of Spawns in Relation to Female Body Size and Spawning Order
- 1Science and Engineering Faculty, Queensland University of Technology, Brisbane, QLD, Australia
- 2Australian Rivers Institute, Griffith University, Brisbane, QLD, Australia
- 3Beijing Shuishiji Biotechnology Co. Ltd., Beijing, China
Optimizing broodstock reproductive performance quality in aquaculture is crucial for planning long-term genetic improvement programs to facilitate the development of an effective seed dissemination strategy. In the current study, we investigated the relative reproductive performance of female Litopenaeus vannamei broodstock reared under two common rearing systems: (i) recirculating tanks (RT) and (ii) earthen ponds (EP), and evaluated relative individual female reproductive performance (RT vs EP), quality of reproductive females in relation to individual body size of spawners, and female reproductive quality relative to spawning order (number of spawning events per individual). No significant difference (P > 0.05) was observed between RT-reared vs EP-reared females for: (i) number of eggs per spawn (RT = 23.34 ± 0.72 × 104, EP = 22.45 ± 0.67 × 104), (ii) number of nauplii per spawn (RT = 19.85 ± 0.85 × 104, EP = 19.53 ± 0.83 × 104), (iii) hatch rate of eggs per spawn (RT = 0.83 ± 0.02, EP = 0.85 ± 0.02), or (iv) relative fecundity – number of eggs per gram of female (RT = 5.51 ± 0.15 × 103, EP = 5.78 ± 0.19 × 103). We recorded 136 and 101 spawning events for RT and ER females, respectively. EP-reared females (1.93 ± 0.23) showed a significantly higher (P < 0.01) spawn frequency compared with RT-reared females (1.34 ± 0.12). Females under the two treatments showed a similar pattern for body size with larger body size spawners producing higher (P < 0.01) numbers of eggs and nauplii per spawn than smaller spawning females. Of interest, we observed that while large-sized RT-reared females recorded a higher mean spawn frequency, medium-sized females from the EP treatment showed double the spawn frequency compared with small or large sized females of EP. No compromise was evident in the quality of individual female reproductive performance for multiple spawning individuals compared with first or second spawning order events for all reproductive parameters evaluated (P > 0.05). The data generated here will be used to optimize a genetic breeding strategy for our broodstock line and to develop a seed distribution strategy for the local production sector in China.
Introduction
In most breed improvement programs for farmed aquatic species, the stage following development of a breeding nucleus is to identify the best breeding candidates and to optimize their reproductive performance prior to implementing a selection regime (Gjedrem, 2005). Specific tasks associated with this step in penaeid shrimp breeding include: (a) rearing offspring of individuals from the nucleus to sexual maturation; (b) providing the best broodstock to multipliers; and (c) supplying nauplii or postlarvae (usually PL5 or PL10) to the nursery sector – or if a nursery stage is not included then juvenile shrimp are supplied directly to growout farmers. In part, this sequence of events requires that broodstock used to produce juveniles show good individual reproductive performance as this is essential, not only to preserve genetic resources in the nucleus and to accumulate optimal breeding traits in live animals across generations, but also to facilitate dissemination of quality seed to growout farms. This can allow the majority of profit generated from sale of quality offspring to be fed back into investment in the breeding program. From a seed multiplier’s perspective, important parameters that determine relative individual female reproductive quality include; the number of eggs per spawn (NE), the number of nauplii per spawn (NN), the hatch rate of eggs (HR), and the proportion of females in the broodstock population that spawn per night (this also equates to female spawn frequency, SF), total nauplii numbers produced and the associated profit that is possible.
Nearly all Litopenaeus vannamei culture around the world, currently relies on domesticated strains that have many advantages (e.g., biosecurity concerns) over use of wild caught broodstock (Ibarra et al., 2007; Benzie, 2009; Ceballos-Vázquez et al., 2010; Andriantahina et al., 2012). In contrast, most other penaeid culture industries generally rely on sourcing broodstock from wild stock (Boucard et al., 2004; Preston et al., 2004; Peixoto et al., 2008, 2011; Jiang et al., 2009; Arnold et al., 2013; Marsden et al., 2013). Relative reproductive performance of domesticated stock, however, needs to be evaluated appropriately before broodstock are released to the seed production sector. There has been significant controversy about the relative reproductive performance of domesticated lines over the past 40 years, resulting from variation in a range of factors including impact of age, size, and/or genetic background (Aquacop, 1979; Primavera and Posadas, 1981; Menasveta et al., 1993; Medina et al., 1996; Browdy, 1998; Preston et al., 1999; Arcos et al., 2005a; Coman et al., 2006; Peixoto et al., 2008; Arnold et al., 2013; Marsden et al., 2013; Wen et al., 2015).
Female reproductive performance in penaeids can be impacted by a number of factors including; additive genetic composition, individual physical status, nutrition status, and culture water environmental factors (Benzie, 1997; Ibarra et al., 2007). Well managed recirculating tank (RT) systems provide a stable high quality water environment with bio-secure conditions that should result in lower mortality and minimum water pollution. For these reasons, they have been considered to be an ideal rearing system for closing the life cycle of penaeid shrimp in genetic improvement programs, and also for producing mature SPF broodstock for the industry (Chen et al., 1991; Crocos and Coman, 1997; Otoshi et al., 2003; Duy et al., 2012). Tank-reared broodstock, however, often do not show the same or similar reproductive performance compared with broodstock sourced from wild populations or even culture stock reared in earthen ponds (EP) (Otoshi et al., 2003; Coman et al., 2006; Andriantahina et al., 2012; Arnold et al., 2013). This issue needs to be further investigated to help meet demands from the seed production sector.
Earthen ponds are widely used for rearing domesticated L. vannamei stocks in the shrimp farming industry (Briggs et al., 2004). Small entrepreneurial family holders in China first learned about the maturation of L. vannamei broodstock in EP after unilateral eyestalk ablation was introduced by a Taiwanese technician in the late 20th century (A.B. Gao, personal communication), and this development pioneered shrimp farming in China. For decades, this method for nauplii production has contributed more than 50% to total nauplii supply in the seed sector. The practice has been used widely in China, where an annual production of more than one million tons of L. vannamei has been maintained for decades (FAO, 2016). Anecdotal stories about nauplii production using EP broodstock, however, have indicated that problems still exist. Farmers in China using this system prefer small- and medium-sized female broodstock rather than large individuals because they believed that smaller-sized mature females show better reproductive performance. In contrast, results of scientific studies on the relationship between body size and individual reproductive performance in penaeid shrimps, have suggested a reverse relationship (Arcos et al., 2003a; Peixoto et al., 2003; Ibarra et al., 2007; Ceballos-Vázquez et al., 2010; Andriantahina et al., 2012; Arnold et al., 2013). Moreover, technicians running hatcheries often claimed that stocks raised in EP are easier to bring to maturity (personal communication Anonymous) and showed higher mating rate compared with imported SPF stocks (generally reared in tank systems; Otoshi et al., 2003). To date, only two comparative studies have been conducted on the relative reproductive performance of L. vannamei broodstock reared in RT vs EP systems (Otoshi et al., 2003; Andriantahina et al., 2012). Notwithstanding, estimated reproductive parameters in these study were also collected following artificial insemination that produced significantly lower NE, NN, and HR rates compared with natural mating designs of data available from current commercial nauplii production.
Body size is the principal criterion for selecting female broodstock in penaeid shrimps because it is non-invasive and easy to measure with respect to labor requirements and the costs involved (Arcos et al., 2003a; Ibarra et al., 2007). In general, large female penaeids are considered better quality spawners because there is evidence for a positive correlation between individual size and NE (Emmerson, 1980; Ottogalli et al., 1988; Palacios et al., 1998; Arcos et al., 2003a) and SF (Menasveta et al., 1994; Hansford and Marsden, 1995; Palacios et al., 2000; Arnold et al., 2013; Wen et al., 2015). In hatcheries, the recommended choice for female L. vannamei broodstock currently, is to use 30–45 g individuals (Aquacop, 1983; Bray and Lawrence, 1991; Wyban and Sweeney, 1991; Otoshi et al., 2003). In small family hatcheries in China, however, farmers usually select females that range from 25 to 35 g for nauplii production. Choice of individual body size of broodstock, however, can be related to the rearing systems used due to effects of culture density, physical environmental factors in the water used, and nutritional factors (Ibarra et al., 2007; Peixoto et al., 2011). As a result, no clear advice is currently available for new producers, so selection of broodstock based on individual size requires further investigation to determine potential impacts of different rearing systems. In addition, results of aforementioned studies that investigated the relationship between body size and reproductive parameters have varied widely (in some cases they have produced contrasting results) in particular, in terms of HR. Of interest, however, is that most experimental tests of the above parameters have produced estimates much lower than those achieved currently under commercial production conditions. This highlights a need to standardize broodstock maturation environments and nutrition. Therefore, a starting point for this is to develop optimal management in experimental test tanks and then later, to trial the procedures at larger production scales.
A primary goal in penaeid shrimp reproductive biology study is to understand the mechanism(s) determining why a large proportion of mature females reproduce infrequently or may never spawn, while at the same time a very small proportion of mature females spawn multiple times and hence contribute to the majority of nauplii in each hatchery cycle (see reviews by Ibarra et al., 2007; Arcos et al., 2003a). For more than 40 years, studies have tried to manipulate a variety of factors to improve the rate of multiple spawning in penaeid species. Factors that have been considered include; correlation with phenotypic traits (Menasveta et al., 1994; Palacios et al., 1999a; Hoang et al., 2002; Arcos et al., 2003a; Palacios and Racotta, 2003); physiology and biochemistry (Arcos et al., 2003b; Palacios and Racotta, 2003; Peixoto et al., 2004); nutrition (Coman et al., 2007; Hoa et al., 2009; Goodall et al., 2016); additive genetic components (Arcos et al., 2005b; Macbeth et al., 2007; Ibarra et al., 2009) and hormonal levels and functional gene expression patterns (Tsutsui et al., 2005; Treerattrakool et al., 2014; Huerlimann et al., 2018). In L. vannamei farming, the two culture systems (RT and EP) widely used for domesticated stocks, may potentially impact SF. Most studies that have investigated the impact of different culture systems on SF in penaeid shrimps have focused on comparisons between wild stocks and earthen pond cultured populations (Menasveta et al., 1993; Palacios et al., 1999a; Wen et al., 2015).
While multiple spawning is considered a desirable trait, the premise behind this concept is no compromise on reproductive performance of multiple spawners. In particular, the egg quality of multiple spawners should not necessarily deteriorate from first to subsequent spawns (Palacios and Racotta, 2003; Arcos et al., 2004; Ibarra et al., 2007). Reproductive exhaustion of broodstock during nauplii production in penaeid shrimps is recognized to be a relatively common phenomenon (Wyban, 1997; Palacios et al., 1998, 1999b), if maturation conditions have not been managed well. This issue highlights a need for considering how optimal were the maturation conditions employed during any experimental tests of relative individual reproductive quality.
In the current study we reared L. vannamei broodstock under two culture conditions (RT and EP) using nauplii produced from the spawning of a single batch to eliminate any potential effects from the genetic resources used or different ages, and compared (a) differences in individual mature female reproductive performance under RT vs EP culture treatments, (b) the relationship between females size and the quality of their individual reproductive parameters, and (c) female reproductive quality relative to spawning order under commercial hatchery conditions.
Materials and Methods
Experimental Animal
Shrimp nauplii used in our study came from a single mass spawning on a single night in a commercial hatchery owned by the Beijing Shuishiji Biotech Ltd. at Wanning, Hainan Province, China. Genetic background of broodstock had been described in previous study (Ren S. J. et al., 2020). Larval culture and the nursery phase occurred from 1st July to 20th July, 2017. Post larvae (PL10 stage) were sampled randomly and transferred to either EP or RT for growout.
Broodstock Rearing Procedure in Earthen Ponds
Individuals were stocked into 0.8 ha EP at a commercial shrimp farm at Wanning, Hainan. Initially, PLs were stocked at a density of 25 individuals per m2 (200,000 PLs per pond) and fed with a commercially formulated diet (EVERGREEN AQUATIC & Ltd.) containing 40% dietary crude protein. Feeding ratio over the first 5 months of growout was initially ∼10% of biomass, steady decrease to ∼2% of biomass. At the end of the 5 month culture period, shrimp were collected at random and transferred to another earthen pond and supplied with enhanced nutrition for 3 months to reach pre-maturation stage. Management and feeding strategies during this time were almost identical to that used over the growout stage, except that shrimp were also supplied with fresh squid meal twice per week.
Broodstock Rearing Procedure in Recirculating Tanks
Shrimp were stocked into RT using the same standard procedure used in the family growth parameter study as described in Ren S. J. et al. (2020). RT tanks were circular polypropylene fiber tanks (3.5 m diameter, 0.9 m depth), with the water column depth maintained at 0.5 m. A biological recirculating culture system was used to maintain water quality at an exchange rate of 600 to 800% per day. The biological filter was constructed of four layers consisting of; filter biological cotton, silica sand, crushed coral stone, and volcanic rock, with a total approximate volume of 30% marine water in each tank. During the 3 to 4 months pre-maturation stage, tested shrimp were fed with a combined diet (2:1) of commercial pellets (containing 35 to 40% crude protein) and fresh squid, with water temperature maintained at 22 to 27°C.
Design for Experimental Comparisons
When individuals had reached 8 months of age, broodstock from EP were collected at random and transferred to the hatchery for acclimation in maturation tanks. Trials used 4 × 10 m2 RT and males and females were reared separately at a stocking rate of eight individuals per m2. Broodstock maturation management was the same as a previous study (Ren S. J. et al., 2020). Mature females from EP and RT were tagged with individual numbered silicon eye rings for source identification and then reared communally in two tanks. At 10 months of age, test females were subjected to unilateral eyestalk ablation. Reproductive parameters for females in both the RT and EP treatments were collected 1 month after eyestalk ablation, and data recorded for 30 days. Females with mature ovaries (stage IV) were collected daily at 10:00 am and transferred to tanks with mature male broodstock. At 19:00 h, successfully mated females with attached spermatophores were placed into individual 500 L fiberglass tanks filled with 300 L of clean seawater. Spawning environmental conditions were maintained at 28 ± 0.5°C and salinity at 32–36 ppt. At 24:00 h, all females in the spawning tanks were returned to their maturation tank and then released eggs were incubated with gentle aeration. In total, 107 RT females and 49 EP females broodstock were used for estimating individual reproductive parameters.
Evaluation of Reproductive Parameters
Reproductive performance was assessed for a series of standardized parameters over 30 days. Rate of survival of females under the two treatments (RT vs EP) was recorded at the end of the experiment (i.e., at 30 days). After successful spawning, body weight after spawning (BW) was measured. Individual female fecundity was measured using two methods: (i) number of eggs (NE) per spawn was calculated using a 200 mL beaker sub-sampling method with three replicates after eggs had been thoroughly homogenized in the spawning tanks with 300 L volume seawater; and (ii) relative fecundity data was estimated as the number of eggs per unit body weight after spawning. The number of nauplii per spawn (NN) was measured using the approach as used for NE on the second day at 11:30 h after nauplii had hatched. Hatchability (HR) was measured as the percentage of hatching nauplii per spawning event as (NN/NE) × 100%. SF of each female was examined after 1 month when the experiment had ended. Relative fecundity – measured as the number of eggs per unit body weight (FE) – calculated by dividing the number of eggs per spawn by BW. Finally, the number of successful spawning events was recorded for each surviving broodstock female at the end of the experiment.
Statistical Analysis
As the data for HR are presented as percentages, values were arcsine transformed prior to further analysis (Zar, 1996). Both percentage data (HR) and arcsine-transformed data (HRat) were included in further statistical analyses. Single factor one-way ANOVAs were performed to compare reproductive parameters between treatments (RT vs EP). Variables evaluated included: BW, NE, NN, HR, HRat, and SF. Prior to performing ANOVAs, tests for assumptions of normality and homogeneity of variance were undertaken. There was no significant P values for any of the reproductive traits assessed here. In addition, the interaction between SF and rearing environment was investigated using a chi-square test of independence. To evaluate the effect of BW in relation to reproductive parameters, BW was first divided into three individual female size classes namely: small (BW < 38 g), medium (38–48 g), and large (>48 g) sized individuals [threshold for the three size categories was based on the criteria of body size for broodstock selection (see earlier), with the objective to introduce a variance for body weight that represented statistically significant differences among three size groups within two treatments]. Following this, BW group data were introduced as a covariate in a two-way factorial ANOVA analysis. To assess the quality of reproductive performance in relation to “spawning order,” spawning events were also divided into three groups, namely: first spawning event over the experimental period, second spawning event over the experimental period, and multiple spawning events (three or more) over the experimental period. The three groups for “spawning order” were set as a covariate in a two-way ANOVA analysis. Tukey’s post hoc means comparison was used to assess significance differences between means after ANOVA analyses. Level of statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS 23 (IBM).
Results
Reproductive Performance in Relation to Treatment (RT vs EP)
Means for reproductive parameters from broodstock females reared in the two culture environments (RT and EP) over a 1 month test period are presented in Table 1. No statistically significant difference was evident for female survival rate between the two culture environments, 94% – RT vs 92% – EP, respectively. A total of 136 spawning events for females were recorded in the RT treatment vs 101 spawning events in the EP environment. There was no significant difference between treatments (RT vs EP) for most reproductive quality parameters (NE, NN, HR, HRat, and FE). It should be noted that the mean values for NE, NN, and HR for females recorded from either RT or EP treatments were all within the ranges reported for optimal commercial nauplii production in China (personal observation). Females in the EP treatment however, showed significantly higher SF than females in the RT treatment (P < 0.01).
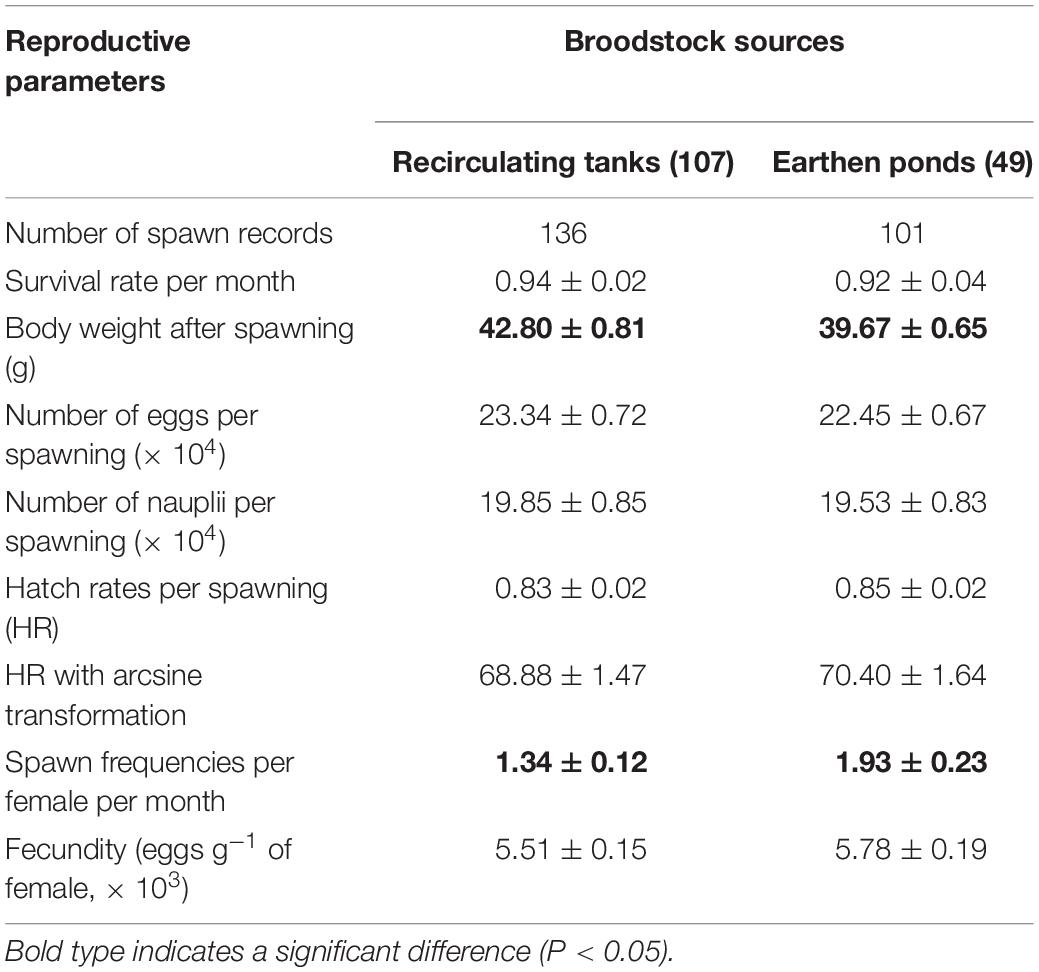
Table 1. Comparison of reproductive performance (±1 SE) of L. vannamei broodstock reared in two different treatments: earthen ponds (EP) vs recirculating tanks (RT).
Of the 101 females in the RT treatment over the 30 day experimental trial period, approximately 30% of individuals did not spawn at all, while another 30% spawned only once. A total of 23% spawned twice and 17% spawned three or more times (Figure 1A). In the EP treatment, approximately 20, 30, and 13% of females (N = 45) did not spawn, spawned once or twice, respectively. The proportion, however, of multiple spawners (38%) in the EP treatment was significantly higher ( = 8.392, P = 0.039) than that in the RT treatment (17%) (Figure 1B).
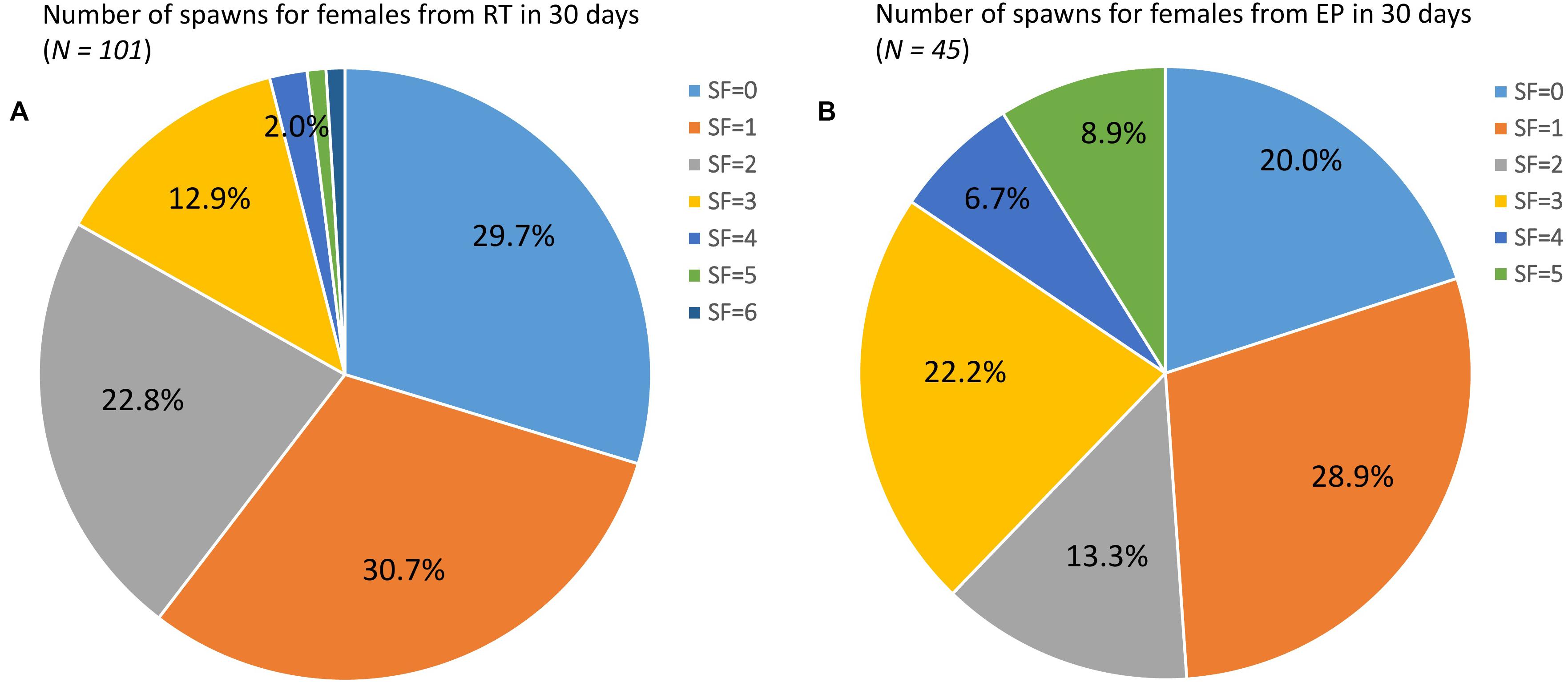
Figure 1. Pie charts showing the number of spawns for (A) 101 female L. vannamei broodstock in the recirculating tank treatment (RT) and (B) 45 females in the earthen pond treatment (EP), over a 1 month trial.
A significant interaction was also evident for females undergoing multiple spawning events between treatments (RT vs EP) and female body weight (BW) with RT (42.80 ± 0.81 g) vs EP (39.67 ± 0.65 g) (Table 2).
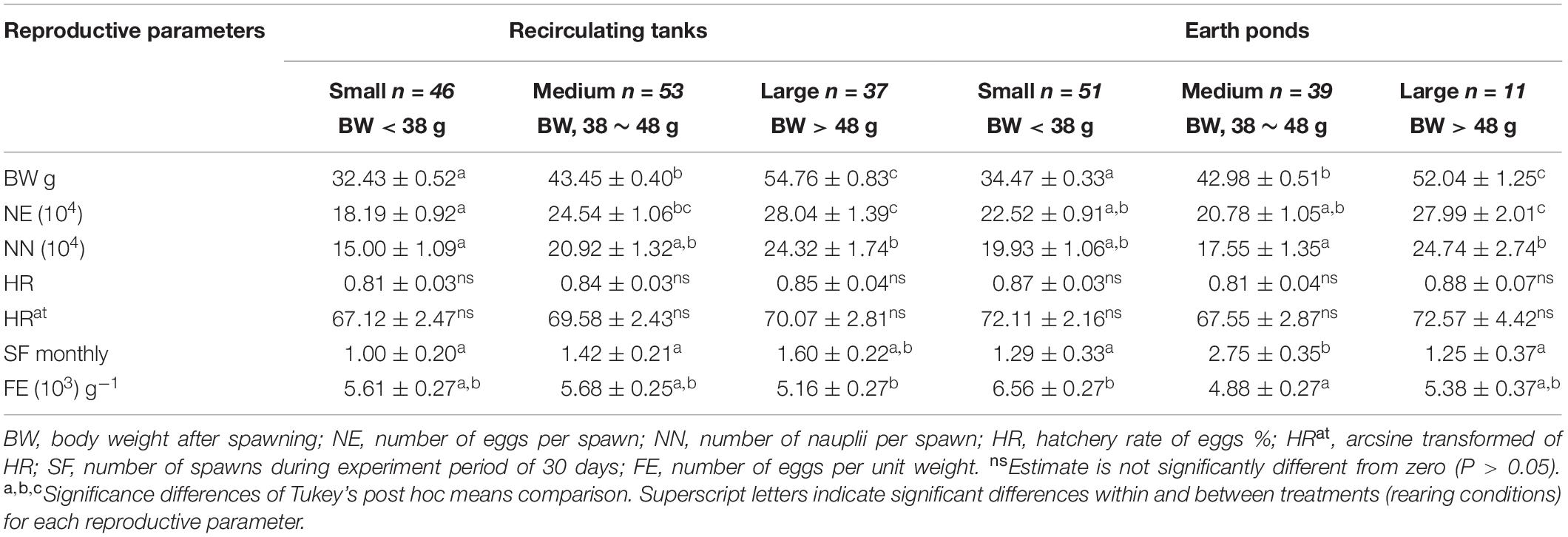
Table 2. Comparison of mean reproductive performance (± 1 SE) of different size classes of female broodstock reared in earthen ponds (EP) vs recirculating tanks (RT).
Correlation of Body Size With Individual Reproductive Performance
Hatchery managers in China in general, currently recommend that body weight of female L. vannamei broodstock should be in range between 30 and 45 g (Aquacop, 1983; Wyban and Sweeney, 1991; Robertson et al., 1993). Mature female bodyweight after spawning (BW) was divided into three bodyweight classes in the current study with 38 and 48 g used as cut-off weights for dividing broodstock females into the three size groups represented by small (<38 g), medium (38–48 g) and large size broodstock female bodyweight classes (>48 g). A statistically significant interaction (F(2, 0.05) = 6.71, P = 0.019) was evident for BW with multiple spawns among the three size classes in both the RT and EP treatments (Table 2). Differences within the same body size classes (small, medium, and large) between treatments (RT and EP), however, were not different (Table 2).
Large size class females in both treatments (RT and EP) produced significantly more eggs per spawn than did females in either the small or medium size classes (P < 0.01). No statistically significant differences were evident for NN in either the small or medium female size classes between treatments. In parallel, no significant differences were evident for either HR or HRat comparisons, a result indicating that egg hatchability was not associated with individual size class of broodstock female used (Table 2). Even given a relatively higher mean BW for females in the RT treatment and a tendency for a higher SF, no statistically significant difference was observed. It should be noted, however, that females in the medium size class for BW in the EP treatment produced more than twice the number of total spawning events (SF) compared with small and large size classes in the same treatment (P < 0.01). While no significant difference (P > 0.05) was observed for the effect of body size on FE for females in the RT treatment, small class females did show a significantly higher FE (P < 0.01) than medium class females in the EP treatment (Table 2).
Reproductive Parameters in Relation to Spawning Order
A summary of reproductive parameters in relation to spawning order were presented in Table 3. There were no statistically significant outcomes in these analyses of reproductive parameters among three groups for spawning order. This result indicated that reproductive performance was not negatively impacted by spawn order for a variety of key traits assessed in the current study.
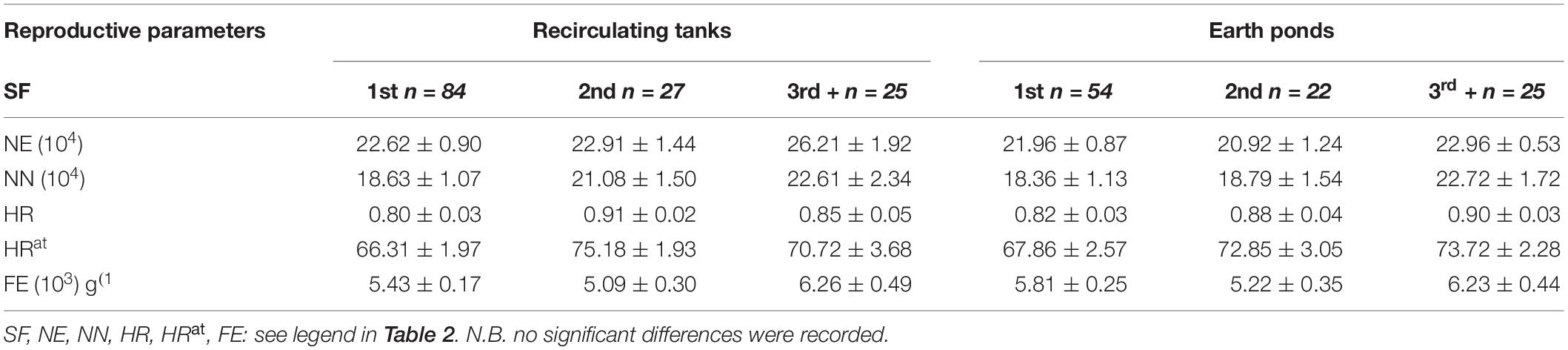
Table 3. Comparison of mean reproductive parameters (± 1 SE) for different spawn order [the first spawns (1st), the second spawns (2nd), or the third spawns or more (3rd+)] for female broodstock reared in earthen ponds (EP) vs recirculating tanks (RT).
Discussion
In the current study, all experimental animals originated from a single batch of nauplii produced from a single night. This approach was adopted to eliminate any potential impacts of nauplii being sourced from different genetic resources and/or age effect. Mean survival rate of reproductive parameters estimated for our broodstock were very similar to estimates reported by most commercial hatcheries in China.
Comparative Reproductive Performance of Broodstock in the RT and EP Treatments
Overall, we observed very similar results for NE, NN, and HR reproductive parameters in the two culture test environment treatments (RT vs EP). For SF, however, EP stocks spawned at a significantly higher rate than in the RT treatment. Results for NE, NN, and HR traits reported here were also much higher than the results reported in two earlier studies that compared reproductive parameters in domesticated L. vannamei broodstock in tanks vs EP (Otoshi et al., 2003; Andriantahina et al., 2012). These differences may largely reflect use of different breeding approaches of natural matings in the current study vs artificial insemination in the aforementioned published studies. Mean NE, NN, and HR estimates reported here are closer to optima proposed for L. vannamei broodstock performance standards for NE (20 × 104) and HR (85%) (Zeigler et al., 2015). Our results are also consistent with another earlier study that indicated that L. vannamei broodstock reared in RT do not show compromised reproductive parameters compared with pond reared females (Otoshi et al., 2003). A significant outcome of our study, however, was that we observed significant differences in SF with females spawning more often under EP than in RT culture conditions (Table 1). This difference was also reflected in reports of a shorter inter-spawn period for pond vs tank housed females by Andriantahina et al. (2012). Differences in SF for females in the RT vs EP treatments are also consistent with observations made by some hatchery technicians in China who report that in general, stocks reared in EP are easier to mature and show higher mating rates per night than their SPF counterparts (reared in tanks).
Studies of some other penaeid species have reported similar findings with culture stocks trialed in EP, generally showing better reproductive performance compared with those maintained in tank systems. For P. esculentus, while broodstock reared in ponds were sufficient for hatchery production in terms of reproductive performance at a commercial scale, tank-reared females showed significantly lower spawning rates and lower mean numbers of eggs per spawning event (NE) (Keys and Crocos, 2006). This meant that RT environments were unlikely to be favored by commercial scale hatcheries for nauplii production. For P. monodon, a significant improvement in key reproductive parameters (NE, NN, and HR) was observed in females reared over 5 months in EP then transferred to RT systems compared with females reared in RT systems over the whole rearing period (Coman et al., 2013).
Improving the proportion of multiple spawners in a broodstock population has been recognized as a key factor for optimizing nauplii production in penaeid species (Coman and Crocos, 2003; Racotta et al., 2003; Ibarra et al., 2007). In the current study over a 1 month trial, a third of the RT-reared females did not spawn, and a third spawned only a single time (Figure 1A). In contrast, EP-reared females showed a significantly higher SF than that observed in the RT treatment, with only 20% failing to spawn, and almost 40% spawning three times or more (Figure 1B). This result is similar to reports from a commercial L. vannamei nauplii hatchery in Mexico over a 36 day test period where 48% females did not spawn, 18% spawned once, 15% spawned twice, while 19% spawned three times or more (Arcos et al., 2003a). In another study over 29 days of 161 eyestalk-ablated females, 44% did not spawn and only 14% spawned four times or more (Arcos et al., 2004). These findings together highlight that multiple spawners are likely to represent only a relatively small proportion of the total female spawning population while importantly making a very significant contribution to total nauplii production. This is a very important factor to consider in any broodstock improvement program in penaeids because it has great potential to impact the rate of inbreeding if this issue is not actively managed. In fact, our previous work has demonstrated a moderate heritability (h2 = 0.15) for SF (Ren S. et al., 2020), suggesting that genetic improvement in culture for this trait is an achievable goal.
Impacts of Female Body Size on Reproduction Performance
Individual body size is the principle criterion widely used to select broodstock in penaeid shrimp hatcheries. Results of examining the relationships between reproductive parameters and individual female body size here show clearly that body size has a significant impact on reproductive performance for the following traits; NE, NN, SF, and FE, while there is little or no impact for HR or HRat.
There was a tendency for the large class females in both the EP and RT culture environments in our study to produce higher NE or NN than smaller females. This result is also consistent with earlier studies in other penaeid shrimps where fecundity (NE) has been correlated positively with individual spawner size (Emmerson, 1980; Ottogalli et al., 1988; Hansford and Marsden, 1995; Palacios et al., 1998; Peixoto et al., 2008; Andriantahina et al., 2012; Coman et al., 2013; Marsden et al., 2013; Wen et al., 2015).
It is relatively difficult, however, to directly compare our results for HR with other studies because reported HR ranges vary widely, particularly in early studies. Here worth noting, however, that the HR estimates reported in this study were all within the recognized current optimal range for commercial nauplii production of L. vannamei stocks in China. HR is known to be closely linked to the relative physiological condition of individual female broodstock and management of the maturation environment in test tanks. While larger females in general tended to show a higher SF rate, females in the medium size class group in the EP treatment had a SF mean of more than double that of small and large class females in the same treatment, respectively. Potentially, this phenomenon may be explained if females in different size classes employ different strategies when allocating energy to reproduction. We hypothesize that, after maturation, females in the EP medium size class likely directed more energy toward reproduction rather than to allocating resources to their own growth (i.e., growth rate slowed and individuals spawned multiple times while those individuals in the larger size class continued growing and spawned less frequently). It is likely therefore, that the observed differences for SF in interactions between body size group and treatment are also reflected in the different selection criteria used for L. vannamei broodstock currently in the shrimp farming industry in China, where large sized female SPF individuals (raised in tanks) are considered better stock while small entrepreneur hatcheries using their own culture lines prefer small and medium size class females as broodstock. Our FE results add support to this observation if relative hatchery production is measured based on total female biomass. This is because no relationship was evident between body size and FE for females raised in an RT environment whereas FE of small size females in the EP treatment was higher than that of the other two size groups in this treatment. SF has also been reported to be positively correlated with large female size and this size class for females also showed a higher SF (Menasveta et al., 1994; Palacios et al., 2000; Arcos et al., 2003a; Andriantahina et al., 2012).
The minimum size of adult SPF females currently supplied to farmers in China ranges from 35 to 45 g. Threshold body size (38 g) between small and medium size classes in our study in general, accords well with the recommended size for L. vannamei female stocks used as breeders. In general, 30–45 g individuals can be used for nauplii production in a hatchery (Aquacop, 1983), even though some animal breeders have advised use of even larger females of up to 45 g because they may perform better (Wyban and Sweeney, 1991; Robertson et al., 1993).
Quality of Reproductive Performance in Relation to Spawning Order
It was clear from our results that no compromise was evident for NE, NN, HR, or FE reproductive parameters in multiple spawners, or even that multiple spawners were better in terms of mean NN or FE. Our results also support some earlier studies that show offspring quality was not negatively impacted by spawning order for a variety of key reproductive parameters including fecundity, fertilization rate, hatchery, or biochemical variables that in general, reflect reproductive quality (Arcos et al., 2003a, 2004; Palacios and Racotta, 2003; Peixoto et al., 2004). In contrast, a series of earlier studies reported that a deterioration in the reproductive capacity of broodstock females can result from reproductive exhaustion and that this is correlated with spawning order in penaeids (Emmerson, 1980; Hansford and Marsden, 1995; Marsden et al., 1997; Mendoza et al., 1997; Palacios et al., 1999a,b). Differences between results from different studies however, may result from time factors. It is quite common for female penaeids to show a decline in reproductive capacity under captive maturation conditions after unilateral eyestalk ablation (Bray et al., 1990; Menasveta et al., 1993; Wyban, 1997; Palacios et al., 1998; Palacios et al., 1999b). In general, experiments that test spawning quality in relation to spawning order are undertaken over a relatively long time frame (30–40 days). Reproductive data on multiple spawns as a result, are often collected later over the experimental time period than are data for “first order” or “second order” spawns. As a consequence, the time factor for measuring “exhaustion” effects are very different and could significantly impact results between earlier studies and more recent ones that have used natural spawning. In particular, if maturation tank conditions were sub-optimal or diet had been insufficient to supply adequate nutritional requirements.
In our study, data were collected during the second month after a female had experienced unilateral eyestalk ablation, so production of nauplii occurred over a stable period. Furthermore, mortality rates of broodstock and estimates of reproductive parameters in the current study indicate that near optimal maturation conditions were provided to the broodstock tested. As a consequence, this would likely minimize any impacts of test time on potential for reproductive exhaustion. Again, this highlights the difficulties with dealing with domesticated penaeid broodstock studies and how to establish the best, uniform standard experimental conditions that will allow meaningful comparisons to be made between different studies.
Conclusion
In conclusion, results here indicate that no significant differences were evident for the majority of reproductive performance traits tested between female L. vannamei broodstock reared in RT vs EP environments. Females in the EP treatment however, produced more nauplii per individual than females raised in an RT environment and this resulted from a significantly higher SF rate. No evidence was observed for reproductive exhaustion related to the number of consecutive spawns. Nauplii production in hatcheries therefore, potentially can be optimized by employing different strategies in relation to female broodstock body size selection. When RT-reared stocks were used, selecting larger body size females should result in higher nauplii production levels, while for small-scale farmers who use EP-reared stocks, use of female broodstock in the medium size class range should maximize nauplii production.
Data Availability Statement
The raw data used to support the findings of this study are available from the corresponding author upon request.
Author Contributions
SR and DH developed the idea for this research. PM and BT contributed to the data analysis of manuscript. All authors contributed to the article, revised the final manuscript and approved the submitted version.
Conflict of Interest
BT was employed by the company Beijing Shuishiji Biotechnology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Tao Lu, Zhikai Xu, and Xuehua Pi for their valuable technical assistance during hatchery trials. We also acknowledge a QUT Postgraduate Research Award (QUTPRA) to SR to undertake his Ph.D. research in Australia.
References
Andriantahina, F., Liu, X., Huang, H., Xiang, J., and Yang, C. (2012). Comparison of reproductive performance and offspring quality of domesticated Pacific white shrimp. Litop. Vann. Aquacult. 324, 194–200. doi: 10.1016/j.aquaculture.2011.10.026
Aquacop. (1979). Penaeid reared brood stock: closing the cycle of P. monodon, P. stylirostris and P. vannamei. Proc. World. Maricult. Soc. 10, 445–452. doi: 10.1111/j.1749-7345.1979.tb00040.x
Aquacop, A. (1983). Constitution of Broodstock, Maturation, Spawning and Hatching Systems for Penaeid Shrimps in the Centre Océanologique du Pacifique. Boca Raton, FL: Crustacean Aquaculture, 105–121.
Arcos, F. G., Ibarra, A. M., Palacios, E., Vazquez-Boucard, C., and Racotta, I. S. (2003a). Feasible predictive criteria for reproductive performance of white shrimp Litopenaeus vannamei: egg quality and female physiological condition. Aquaculture 228, 335–349. doi: 10.1016/s0044-8486(03)00313-2
Arcos, G. F., Ibarra, A. M., Vazquez−Boucard, C., Palacios, E., and Racotta, I. S. (2003b). Haemolymph metabolic variables in relation to eyestalk ablation and gonad development of Pacific white shrimp Litopenaeus vannamei Boone. Aquac. Res. 34, 749–755. doi: 10.1046/j.1365-2109.2003.00878.x
Arcos, F. G., Racotta, I. S., and Ibarra, A. M. (2004). Genetic parameter estimates for reproductive traits and egg composition in Pacific white shrimp Penaeus (Litopenaeus) vannamei. Aquaculture 236, 151–165. doi: 10.1016/j.aquaculture.2004.03.003
Arcos, F. G., Racotta, I. S., Palacios, E., and Ibarra, A. M. (2005a). Ovary development at the onset of gametogenesis is genetically determined and correlated with reproductive traits at maturity in shrimp Litopenaeus (Penaeus) vannamei. Mar. Biol. 148, 339–346. doi: 10.1007/s00227-005-0087-1
Arcos, F. G., Palacios, E., Ibarra, A. M., and Racotta, I. S. (2005b). Larval quality in relation to consecutive spawnings in white shrimp Litopenaeus vannamei Boone. Aquac. Res. 36, 890–897. doi: 10.1111/j.1365-2109.2005.01298.x
Arnold, S. J., Coman, G. J., and Emerenciano, M. (2013). Constraints on seedstock production in eighth generation domesticated Penaeus monodon broodstock. Aquaculture 410, 95–100. doi: 10.1016/j.aquaculture.2013.06.023
Benzie, J. (1997). A review of the effect of genetics and environment on the maturation and larval quality of the giant tiger prawn Penaeus monodon. Aquaculture 155, 69–85. doi: 10.1016/s0044-8486(97)00110-5
Benzie, J. A. (2009). Use and exchange of genetic resources of penaeid shrimps for food and aquaculture. Rev. Aquacult. 1, 232–250. doi: 10.1111/j.1753-5131.2009.01018.x
Boucard, C. G. V., Patrois, J., and Ceccaldi, H. J. (2004). Exhaustion of lipid reserves in the hepatopancreas of Fenneropenaeus indicus broodstock in relation to successive spawnings. Aquaculture 236, 523–537. doi: 10.1016/j.aquaculture.2003.09.048
Bray, W., and Lawrence, A. (1991). “New concepts in seedstock production: learning to determine quality,” in Proceedings of the International Symposium on Commercial Production of Shrimp Larvae, Dec. 5, 1991, Mazatlan, 1–15.
Bray, W. A., Lawrence, A. L., and Lester, L. J. (1990). Reproduction of eyestalk−ablated Penaeus stylirostris fed various levels of total dietary lipid. J. World. Aquacult. Soc. 21, 41–52. doi: 10.1111/j.1749-7345.1990.tb00952.x
Briggs, M., Funge-Smith, S., Subasinghe, R., and Phillips, M. (2004). Introductions and movement of Penaeus vannamei and Penaeus stylirostris in Asia and the Pacific. RAP Publ. 10:92.
Browdy, C. L. (1998). Recent developments in penaeid broodstock and seed production technologies: improving the outlook for superior captive stocks. Aquaculture 164, 3–21. doi: 10.1016/s0044-8486(98)00174-4
Ceballos-Vázquez, B. P., Palacios, E., Aguilar-Villavicencio, J., and Racotta, I. S. (2010). Gonadal development in male and female domesticated whiteleg shrimp, Litopenaeus vannamei, in relation to age and weight. Aquaculture 308, 116–123. doi: 10.1016/j.aquaculture.2010.08.020
Chen, F., Reid, B., and Arnold, C. (1991). Maturing, spawning and egg collecting of the white shrimp Penaeus vannamei Boone in a recirculating system. J. World. Aquacult. Soc. 22, 167–172. doi: 10.1111/j.1749-7345.1991.tb00729.x
Coman, G., Arnold, S., Callaghan, T., and Preston, N. (2007). Effect of two maturation diet combinations on reproductive performance of domesticated Penaeus monodon. Aquaculture 263, 75–83. doi: 10.1016/j.aquaculture.2006.10.016
Coman, G., Arnold, S., Peixoto, S., Crocos, P., Coman, F., and Preston, N. (2006). Reproductive performance of reciprocally crossed wild-caught and tank-reared Penaeus monodon broodstock. Aquaculture 252, 372–384. doi: 10.1016/j.aquaculture.2005.07.028
Coman, G. J., Arnold, S. J., Wood, A. T., and Preston, N. P. (2013). Evaluation of egg and nauplii production parameters of a single stock of domesticated Penaeus monodon (Giant Tiger Shrimp) across generations. Aquaculture 400, 125–128. doi: 10.1016/j.aquaculture.2013.03.015
Coman, G. J., and Crocos, P. J. (2003). Effect of age on the consecutive spawning of ablated Penaeus semisulcatus broodstock. Aquaculture 219, 445–456. doi: 10.1016/s0044-8486(03)00002-4
Crocos, P., and Coman, G. (1997). Seasonal and age variability in the reproductive performance of Penaeus semisulcatus broodstock: optimising broodstock selection. Aquaculture 155, 55–67. doi: 10.1016/s0044-8486(97)00109-9
Duy, H. N., Coman, G. J., Wille, M., Wouters, R., Quoc, H. N., Vu, T., et al. (2012). Effect of water exchange, salinity regime, stocking density and diets on growth and survival of domesticated black tiger shrimp Penaeus monodon (Fabricius, 1798) reared in sand-based recirculating systems. Aquaculture 338, 253–259. doi: 10.1016/j.aquaculture.2012.01.021
Emmerson, W. (1980). Induced maturation of prawn Penaeus indicus. Mar. Ecol. Prog. Ser. 2, 121–131. doi: 10.3354/meps002121
FAO (2016). Aquaculture Production (Quanlities and values) 1950-2013. FishStatJ–Software for Fishery Statistical Time Series. Rome: FAO Fisheries and Aquaculture Department.
Goodall, J. D., Wade, N. M., Merritt, D. J., Sellars, M. J., Salee, K., and Coman, G. J. (2016). The effects of adding microbial biomass to grow-out and maturation feeds on the reproductive performance of black tiger shrimp. Pen. Monod. Aquacult. 450, 206–212. doi: 10.1016/j.aquaculture.2015.07.036
Hansford, S., and Marsden, G. (1995). Temporal variation in egg and larval productivity of eyestalk ablated spawners of the prawn Penaeus monodon from Cook Bay. Australia. J. World Aquacult. Soc. 26, 396–405. doi: 10.1111/j.1749-7345.1995.tb00835.x
Hoa, N. D., Wouters, R., Wille, M., Thanh, V., Dong, T. K., Van Hao, N., et al. (2009). A fresh-food maturation diet with an adequate HUFA composition for broodstock nutrition studies in black tiger shrimp Penaeus monodon (Fabricius, 1798). Aquaculture 297, 116–121. doi: 10.1016/j.aquaculture.2009.09.005
Hoang, T., Lee, S. Y., Keenan, C. P., and Marsden, G. E. (2002). Observations on growth, sexual maturity and spawning performance of pond−reared Penaeus merguiensis. Aquac. Res. 33, 863–873. doi: 10.1046/j.1365-2109.2002.00726.x
Huerlimann, R., Wade, N. M., Gordon, L., Montenegro, J. D., Goodall, J., McWilliam, S., et al. (2018). De novo assembly, characterization, functional annotation and expression patterns of the black tiger shrimp (Penaeus monodon) transcriptome. Sci. Rep. 8:13553.
Ibarra, A. M., Famula, T. R., and Arcos, F. G. (2009). Heritability of vitellogenin in hemolymph, a pre-spawning selectable trait in Penaeus (Litopenaeus) vannamei, has a large genetic correlation with ovary maturity measured as oocytes mean diameter. Aquaculture 297, 64–69. doi: 10.1016/j.aquaculture.2009.09.015
Ibarra, A. M., Racotta, I. S., Arcos, F. G., and Palacios, E. (2007). Progress on the genetics of reproductive performance in penaeid shrimp. Aquaculture 268, 23–43. doi: 10.1016/j.aquaculture.2007.04.028
Jiang, S.-G., Huang, J.-H., Zhou, F.-L., Chen, X., Yang, Q.-B., Wen, W.-G., et al. (2009). Observations of reproductive development and maturation of male Penaeus monodon reared in tidal and earthen ponds. Aquaculture 292, 121–128. doi: 10.1016/j.aquaculture.2009.03.054
Keys, S., and Crocos, P. (2006). Domestication, growth and reproductive performance of wild, pond and tank-reared brown tiger shrimp Penaeus esculentus. Aquaculture 257, 232–240. doi: 10.1016/j.aquaculture.2006.02.044
Macbeth, M., Kenway, M., Salmon, M., Benzie, J., Knibb, W., and Wilson, K. (2007). Heritability of reproductive traits and genetic correlations with growth in the black tiger prawn Penaeus monodon reared in tanks. Aquaculture 270, 51–56. doi: 10.1016/j.aquaculture.2007.03.018
Marsden, G., Richardson, N., Mather, P., and Knibb, W. (2013). Reproductive behavioural differences between wild-caught and pond-reared Penaeus monodon prawn broodstock. Aquaculture 402, 141–145. doi: 10.1016/j.aquaculture.2013.03.019
Marsden, G. E., McGuren, J. J., Hansford, S. W., and Burke, M. J. (1997). A moist artificial diet for prawn broodstock: its effect on the variable reproductive performance of wild caught Penaeus monodon. Aquaculture 149, 145–156. doi: 10.1016/s0044-8486(96)01430-5
Medina, A., Vila, Y., Mourente, G., and Rodríguez, A. (1996). A comparative study of the ovarian development in wild and pond-reared shrimp, Penaeus kerathurus (Forskal, 1775). Aquaculture 148:63. doi: 10.1016/s0044-8486(96)01408-1
Menasveta, P., Piyatiratitivorakul, S., Rungsupa, S., Moree, N., and Fast, A. W. (1993). Gonadal maturation and reproductive performance of giant tiger prawn (Penaeus monodon Fabricius) from the Andaman Sea and pond-reared sources in Thailand. Aquaculture 116, 191–198. doi: 10.1016/0044-8486(93)90008-m
Menasveta, P., Sangpradub, S., Piyatiratitivorakul, S., and Fast, A. W. (1994). Effects of broodstock size and source on ovarian maturation and spawning of Penaeus monodon Fabricius from the Gulf of Thailand. J. World. Aquacult. Soc. 25, 41–49. doi: 10.1111/j.1749-7345.1994.tb00802.x
Mendoza, R., Revol, A., Fauvel, C., Patrois, J., and Guillaume, J. C. (1997). Influence of squid extracts on the triggering of secondary vitellogenesis in Penaeus vannamei. Aquac. Nutr. 3, 55–63. doi: 10.1046/j.1365-2095.1997.00075.x
Otoshi, C. A., Arce, S. M., and Moss, S. M. (2003). Growth and reproductive performance of broodstock shrimp reared in a biosecure recirculating aquaculture system versus a flow-through pond. Aquacult. Eng. 29, 93–107. doi: 10.1016/s0144-8609(03)00048-7
Ottogalli, L., Galinie, C., and Goxe, D. (1988). Reproduction in captivity of Penaeus stylirostris in New Caledonia. J. Aquac. Trop. 3, 111–125.
Palacios, E., Ibarra, A., and Racotta, I. (2000). Tissue biochemical composition in relation to multiple spawning in wild and pond-reared Penaeus vannamei broodstock. Aquaculture 185, 353–371. doi: 10.1016/s0044-8486(99)00362-2
Palacios, E., Ibarra, A., Ramirez, J., Portillo, G., and Racotta, I. (1998). Biochemical composition of eggs and nauplii in white pacific shrimp, Penaeus vannamei (Boone), in relation to the physiological condition of spawners in a commercial hatchery. Aquac. Res. 29, 183–189. doi: 10.1046/j.1365-2109.1998.00953.x
Palacios, E., Racolta, I. S., and de La Paz, A. (1999a). Spawning frequency analysis of wild and pond−reared pacific white shrimp Penaeus vannamei broodstock under large−scale hatchery conditions. J. World. Aquacult. Soc. 30, 180–191. doi: 10.1111/j.1749-7345.1999.tb00865.x
Palacios, E., Perez-Rostro, C., Ramirez, J., Ibarra, A., and Racotta, I. (1999b). Reproductive exhaustion in shrimp (Penaeus vannamei) reflected in larval biochemical composition, survival and growth. Aquaculture 171, 309–321. doi: 10.1016/s0044-8486(98)00393-7
Palacios, E., and Racotta, I. S. (2003). Effect of number of spawns on the resulting spawn quality of 1−year−old pond−reared Penaeus vannamei (Boone) broodstock. Aquac. Res. 34, 427–435. doi: 10.1046/j.1365-2109.2003.00826.x
Peixoto, S., Cavalli, R. O., Krummenauer, D., Wasielesky, W., and D’Incao, F. (2004). Influence of artificial insemination on the reproductive performance of Farfantepenaeus paulensis in conventional and unisex maturation systems. Aquaculture 230, 197–204. doi: 10.1016/s0044-8486(03)00431-9
Peixoto, S., Wasielesky, W. Jr., D’Incao, F., and Cavalli, R. O. (2003). Comparison of the Reproductive Performance of Similarly−Sized Wild and Captive Farfantepenaeus paulensis. J. World. Aquacult. Soc. 34, 50–56. doi: 10.1111/j.1749-7345.2003.tb00038.x
Peixoto, S., Wasielesky, W. Jr., Martino, R. C., Milach, Â, Soares, R., and Cavalli, R. O. (2008). Comparison of reproductive output, offspring quality, ovarian histology and fatty acid composition between similarly-sized wild and domesticated Farfantepenaeus paulensis. Aquaculture 285, 201–206. doi: 10.1016/j.aquaculture.2008.08.021
Peixoto, S., Wasielesky, W., and Cavalli, R. O. (2011). Broodstock maturation and reproduction of the indigenous pink shrimp Farfantepenaeus paulensis in Brazil: an updated review on research and development. Aquaculture 315, 9–15. doi: 10.1016/j.aquaculture.2010.04.009
Preston, N., Brennan, D., and Crocos, P. (1999). Comparative costs of postlarval production from wild or domesticated Kuruma shrimp. Penaeus japonicus (Bate), broodstock. Aquac. Res. 30, 191–197. doi: 10.1046/j.1365-2109.1999.00306.x
Preston, N. P., Crocos, P. J., Keys, S. J., Coman, G. J., and Koenig, R. (2004). Comparative growth of selected and non-selected Kuruma shrimp Penaeus (Marsupenaeus) japonicus in commercial farm ponds; implications for broodstock production. Aquaculture 231, 73–82. doi: 10.1016/j.aquaculture.2003.09.039
Primavera, J. H., and Posadas, R. A. (1981). Studies on the egg quality of Penaeus monodon Fabricius, based on morphology and hatching rates. Aquaculture 22, 269–277. doi: 10.1016/0044-8486(81)90152-6
Racotta, I. S., Palacios, E., and Ibarra, A. M. (2003). Shrimp larval quality in relation to broodstock condition. Aquaculture 227, 107–130. doi: 10.1016/s0044-8486(03)00498-8
Ren, S., Mather, P. B., Prentis, P., Li, Y. T., Tang, B. G., and Hurwood, D. A. (2020). Quantitative genetic assessment of female reproductive traits in a domesticated pacific white shrimp (Penaeus vannamei) line in China. Sci Rep. 10:7840.
Ren, S. J., Prentis, P., Mather, P. B., Li, Y. T., Tang, B. G., and Hurwood, D. A. (2020). Genetic parameters for growth and survival traits in a base population of Pacific white shrimp (Litopenaeus vannamei) developed from domesticated strains in China. Aquaculture 523, 735148. doi: 10.1016/j.aquaculture.2020.735148
Robertson, L., Bray, B., Samocha, T., and Lawrence, A. (1993). Reproduction of penaeid shrimp: an operations guide. CRC Hanbook Maricult. Ed. 1, 107–132.
Treerattrakool, S., Boonchoy, C., Urtgam, S., Panyim, S., and Udomkit, A. (2014). Functional characterization of recombinant gonad-inhibiting hormone (GIH) and implication of antibody neutralization on induction of ovarian maturation in marine shrimp. Aquaculture 428, 166–173. doi: 10.1016/j.aquaculture.2014.03.009
Tsutsui, N., Katayama, H., Ohira, T., Nagasawa, H., Wilder, M. N., and Aida, K. (2005). The effects of crustacean hyperglycemic hormone-family peptides on vitellogenin gene expression in the kuruma prawn. Marsupenaeus japonicus. Gen. Comp. Endocr. 144, 232–239. doi: 10.1016/j.ygcen.2005.06.001
Wen, W., Yang, Q., Ma, Z., Jiang, S., Qiu, L., Huang, J., et al. (2015). Comparison of ovarian maturation and spawning after unilateral eyestalk ablation of wild-caught and pond-reared Penaeus monodon. Span. J. Agric. Res. 13:0402.
Wyban, J. (1997). Adding paprika to Penaeus vannamei maturation diet improves nauplii quality. World. Aquacult. 28, 59–62.
Wyban, J., and Sweeney, J. N. (1991). Intensive Shrimp Production Technology: the Oceanic Institute Shrimp Manual. Honolulu, HI: The Oceanic institute.
Zeigler, T. R., Browdy, C. L., and Wyk, P. V. (2015). Major League Performance Requires System Specific Performance Standards. Golbal Aquaculture Advocate. Available online at: https://www.aquaculturealliance.org/advocate/major-league-performance-requires-system-specific-performance-standards/ (accessed March 1, 2015).
Keywords: aquaculture, reproductive performance, broodstock, Litopenaeus vannamei, multiple spawning
Citation: Ren S, Mather PB, Tang B and Hurwood DA (2020) Comparison of Reproductive Performance of Domesticated Litopenaeus vannamei Females Reared in Recirculating Tanks and Earthen Ponds: An Evaluation of Reproductive Quality of Spawns in Relation to Female Body Size and Spawning Order. Front. Mar. Sci. 7:560. doi: 10.3389/fmars.2020.00560
Received: 01 November 2019; Accepted: 18 June 2020;
Published: 14 July 2020.
Edited by:
Liping Liu, Shanghai Ocean University, ChinaReviewed by:
Silvio Peixoto, Federal Rural University of Pernambuco, BrazilElena Palacios, Centro de Investigación Biológica del Noroeste (CIBNOR), Mexico
Copyright © 2020 Ren, Mather, Tang and Hurwood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengjie Ren, shengjieren@hotmail.com
 Shengjie Ren
Shengjie Ren Peter B. Mather2
Peter B. Mather2