Return of the Salish Sea Harbor Porpoise, Phocoena phocoena: Knowledge Gaps, Current Research, and What We Need to Do to Protect Their Future
- 1Pacific Mammal Research, Anacortes, WA, United States
- 2Porpoise Conservation Society, Victoria, BC, Canada
- 3Sea View Marine Sciences, Victoria, BC, Canada
The harbor porpoise (Phocoena phocoena) is one of the most abundant coastal cetacean species in the Northern Hemisphere with differential levels of regional knowledge. Gaps are particularly evident for the Pacific subspecies Phocoena phocoena vomerina. In the Salish Sea (a transboundary body of water spanning between Washington, United States and British Columbia (BC), Canada), there is a dearth of information on many aspects of the biology, ecology, behavior, sociality, and regionally specific threats. Here we present a case study of the Salish Sea harbor porpoise, combining historical and current research, from both BC and Washington, to provide a more holistic view of this species’ status, the knowledge continuum and gaps, risks from identified threats and what current research and collaborations are revealing about this enigmatic species. The Salish Sea harbor porpoise was abundant to the 1940s and 1950s, but by the 1990s their numbers were greatly reduced, and all but absent in some areas. By the early 2000s, numbers had resurged, and harbor porpoise are now once again found throughout much of the Salish Sea. Despite this, studies focused on Salish Sea harbor porpoises have been limited until recently. Current long-term research has been conducted from vessels and land in both Canada and the United States. Multi-faceted work using techniques including photo-identification (photo-ID), behavioral visual observations, acoustics, commercial fishery surveys, sighting reports, citizen science and other ecological data have provided insight into the seasonal variation in density and abundance, site fidelity, reproduction, by-catch rates, foraging and the identification of important habitats that are used intra- and inter-annually in this region. These may represent culturally and biologically significant habitats for Salish Sea harbor porpoise. Collaborations within and outside of the Salish Sea have revealed consistencies and dissimilarities between different communities or populations; indicating that some aspects are more uniform for the species, while others may be community or population specific. The importance of long-term broad and fine-scale research is highlighted, as well as recommendations to further close the knowledge gaps and reduce the known human threats within the Salish Sea.
Introduction
The harbor porpoise (Phocoena phocoena) has a wide geographic distribution throughout the Northern Hemisphere (Gaskin, 1984; Fontaine et al., 2017). In many regions, this species is considered to be one of the most abundant marine mammals with decades of multi-faceted research programs (e.g., European, Atlantic Canadian and Atlantic United States waters). However, in some locations, like the Salish Sea [the inland waters of Washington, United States and British Columbia (BC), Canada] (Figure 1), there is a dearth of knowledge, both temporally and spatially, regarding many aspects of harbor porpoise biology, ecology, behavior, sociality and regionally specific threats. The primary reasons for this lack of knowledge stems from the difficulties inherent in studying this often enigmatic species, and a historical, regional emphasis on larger cetaceans. The Salish Sea is well known for the long-term research that has focused on the more readily observable Southern Resident and transient killer whales (Orcinus orca) (e.g., Olesiuk et al., 1990; Ellis et al., 2017; Towers et al., 2019), humpback whales (Megaptera novaeangliae) (e.g., Calambokidis et al., 2009) and gray whales (Eschrichtius robustus) (e.g., Calambokidis et al., 2012), with far fewer studies focused on harbor porpoises–even if they are a more frequently encountered species.
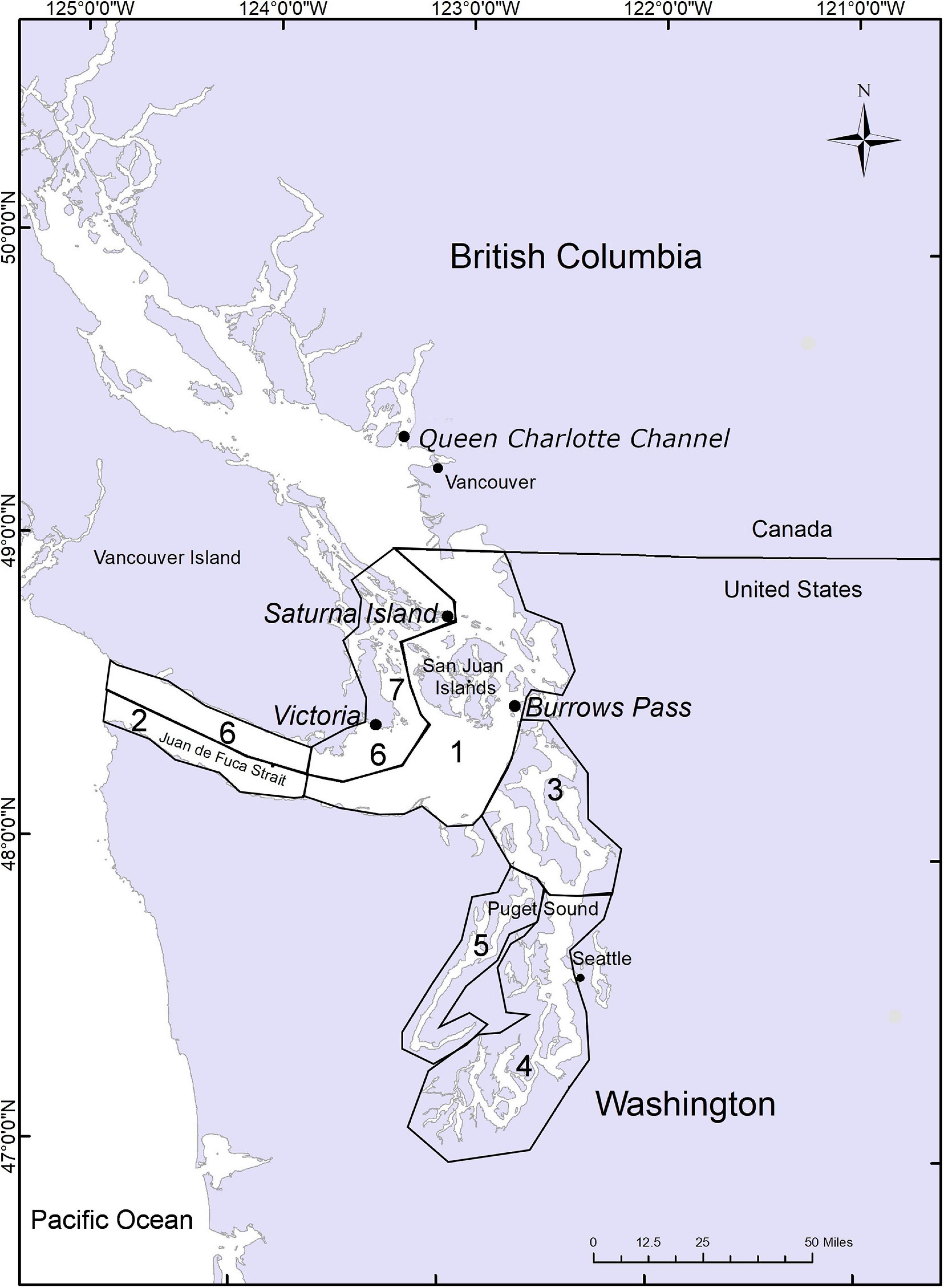
Figure 1. Map of the Salish Sea (inland waters of Washington, United States and British Columbia (BC), Canada) with italicized locations marking current harbor porpoise study areas discussed in the text. Numbers correspond to abundance estimates in Table 1. Collaborations are also occurring with D. Anderson in southern Puget Sound and whale watch vessels departing from Victoria, Fidalgo Island and the San Juan Islands.
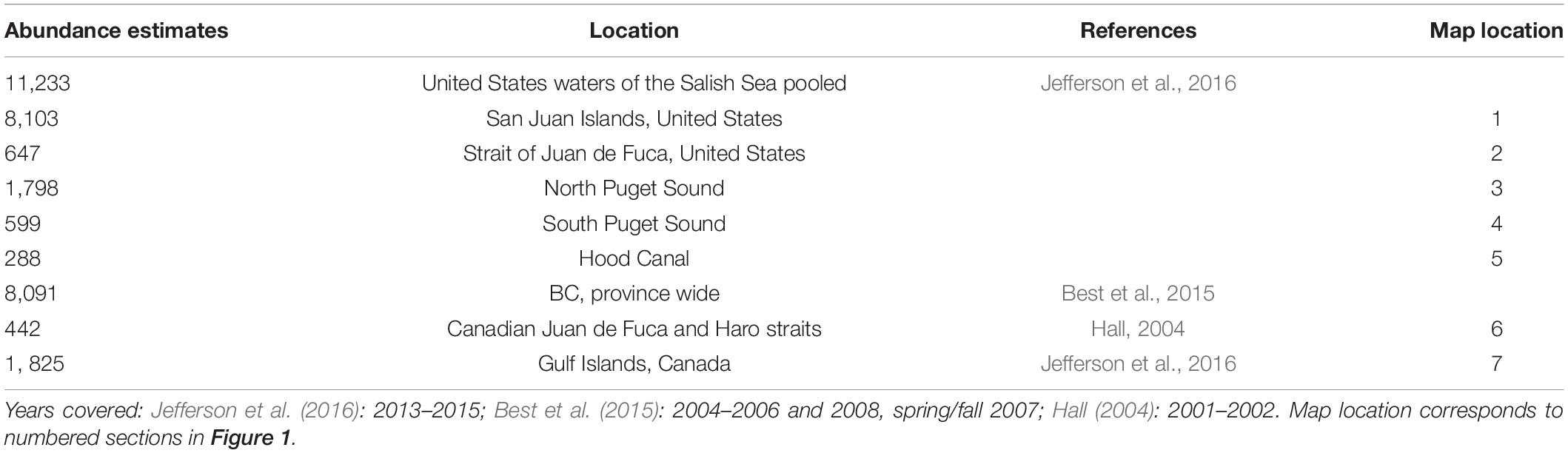
Table 1. Current abundance estimates for overall United States and BC with sub section abundance estimates when available.
Harbor porpoise are known for their small size, cryptic behavior, and few readily observable distinguishing marks (e.g., Flaherty and Stark, 1982; Gaskin and Watson, 1985; Koopman and Gaskin, 1994; Baird, 2003). Despite these observational challenges, research on harbor porpoises can provide valuable data on ecosystem health because they are top predators and are a recognized indicator species (Hammond et al., 2013; Andreasen et al., 2017). In fact, their shorter life span (Read and Hohn, 1995) and yearly or biyearly reproductive cycle (Hohn and Brownell, 1990; Norman et al., 2018), may allow for monitoring of harbor porpoise populations that provides more timely information on the health of an ecosystem compared to sympatric but longer lived and more slowly reproducing species like killer whales. However, until more is known about the Salish Sea harbor porpoise population(s), this potential benefit of knowledge and conservation will remain unrealized.
Successful conservation actions require an understanding of fundamental components of the biology and ecology of the species or population of interest, and concurrent knowledge of the threats or risks that may affect them. A key aspect of this is the quantification of the variability in abundance and distribution of wildlife at a variety of spatial and temporal scales (Gilles et al., 2016; Waggitt et al., 2020). In the Salish Sea, larger scale understanding of the species is restricted because Canada and the United States manage their populations separately, even though there are no obvious restrictions for harbor porpoise movement between the two jurisdictions. Fine-scale research in this region has been localized to only a handful of locations in both Canada and the United States, and knowledge of the ecological connectivity between the individual study sites remains unknown. In the broad geographic context of the Salish Sea, fine-scale research is largely lacking. This is troubling because harbor porpoise behavior and foraging habitat usage have been found to vary at smaller spatial scales compared to other marine megafauna (Benjamins et al., 2015, 2016). Not accounting for such fine-scale variation and potential structure could result in local declines or extirpations going unnoticed or the inadvertent loss or the degradation of locally important habitats. Compared to other more well-studied populations (like those in Europe), there are significant knowledge gaps for Salish Sea harbor porpoises that must be addressed in order for conservation measures to be successful–thereby securing the best insurance for future populations.
Here we review the story of harbor porpoises in the Salish Sea with a transboundary perspective, combining historical and current research from both BC and Washington providing a more holistic view of the status of this species and the knowledge gaps for this region. This story may serve as a model for the conservation of this species and other small coastal cetaceans. We present the lessons learned from the return of the harbor porpoise after disappearance from parts of their Salish Sea historic range, how current research reveals the power of long-term, fine-scale monitoring that complements larger scale abundance studies and how collaboration (local, regional, and international) is key to providing greater ecological insight over single-site studies. From this, we draw upon current research to help us close the knowledge gaps for the Salish Sea harbor porpoise and provide tools and recommendations for research and protection that have broad spatial applicability. Long-term research on harbor porpoises, which are highly susceptible to human impacts, is vital for the survival of populations where large knowledge gaps exist, allowing the prevention of potential future population declines–from which recovery is uncertain.
Background
Along the west coast of the United States and Canada, genetic studies reveal that harbor porpoises are not panmictic and movement patterns along the west coast of North America are sufficiently restricted that genetic differences have evolved, currently resulting in eight different recognized stocks between California and Alaska (see Carretta et al., 2016). The harbor porpoises that inhabit the Salish Sea are recognized by the United States National Oceanic and Atmospheric Administration (NOAA) Fisheries as a single stock (Washington Inland Water Stock, Carretta et al., 2016) and their range includes the United States waters of the Juan de Fuca Strait, San Juan Islands and Puget Sound (Jefferson et al., 2016). On Canada’s west coast, there is no recognized stock structure, with the species assessed as Pacific harbor porpoise for the entire province of British Columbia. However, this scale may be too large for this species in the Pacific. Research in this region indicates that at least some harbor porpoise may have a tendency to live in relatively small and restricted geographic areas (Flaherty and Stark, 1982; Calambokidis and Barlow, 1991; Calambokidis and Baird, 1994; Walton, 1997; Hanson et al., 1999; Rosel et al., 1999; Chivers et al., 2002; Baird, 2003; Hall, 2011; Elliser et al., 2018). While individual preference for specific locations is not evidence in itself of population structuring, it does provide a basis to initiate enquires into Salish Sea harbor porpoise dispersal patterns. A little farther north in Alaska, recent genetic work has shown there to be significant genetic differentiation within a currently recognized management stock (Parsons et al., 2018), and more research is required to determine whether the United States and Canadian harbor porpoise stock structure and boundaries within the Salish Sea should be adjusted as there may be little dispersal or genetic exchange over small geographic scales (Chivers et al., 2002, 2007).
Little is known of the Salish Sea harbor porpoise biology and ecology, but progress is slowly being made in understanding this cryptic aquatic mammal. In April 1991, the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) reviewed the available information about the Pacific harbor porpoise in British Columbia and determined there was insufficient information to be able to recommend a conservation status and the species was considered Data Deficient (Gaskin, 1991). Within a few years of this designation, research was underway, in what is now referred to as the Salish Sea, evaluating harbor porpoise habitat selection, niche differentiation with Dall’s porpoise (Phocoenoides dalli), strandings, prey species and incidental mortality (Hall, 1996, 2004; Hall et al., 2002). Gradually progress was made in discovering the ecological role of the Salish Sea harbor porpoise.
With this progress, came an increased awareness of the sensitivity of this small cetacean to the anthropogenic pressures that are so prevalent within the shared transboundary waters of Canada and the United States. By 2003, research efforts had provided enough new information that COSEWIC was able to conduct a second review of the species. This resulted in a recommendation to increase the conservation status to Special Concern and the Pacific harbor porpoise was officially added to the Canadian Federal Species At Risk register (COSEWIC, 2003).
Nearly two decades later, the Pacific harbor porpoise is still listed as Special Concern in Canada (SARA, 2021). This conservation classification remains in place due to the known risks to harbor porpoise from incidental mortality in fishing gear, the species’ particular sensitivity to noise, the ongoing deterioration of the habitat quality by coastal developments, increasing noise, and other factors that were deemed unlikely to be reversed (COSEWIC, 2016). Despite the same threats to harbor porpoises in United States waters, they are not considered a species of concern. They are not listed as “depleted” under the Marine Mammal Protection Act, or listed as “threatened” or “endangered” under the Endangered Species Act (Carretta et al., 2016). However, the Puget Sound Ecosystem Monitoring Program in Washington State, a collaborative network of regional experts that track ecosystem conditions, has noted the need for systematic surveys of harbor porpoises in the inland waters of Washington as a high priority monitoring gap (PSEMP, 2018), yet to date little work has been done.
Fundamental to understanding the contemporary status of any species requires knowledge, insight and comparison with historical information. The lack of current systematic survey data from either Pacific Canada or the United States further complicates the assessment of the health of the Salish Sea harbor porpoise population(s). Assessment of abundance trends or trajectories over the last half century is hindered by this lack of quantitative information and the jurisdiction of two nations. Human boundaries are likely not significant to mobile animals like harbor porpoises, and it is recognized that animals in United States waters may also use waters of at least southern BC, though the extent of cross-border movements remains unknown (Jefferson et al., 2016). While the socio-political boundaries are likely not significant, human actions are. It is probable that the harbor porpoises of the Salish Sea have been, and continue to be, affected by the human activities on land and at sea from both nations. Whilst definitive evaluation cannot be conducted, the available information suggests that in the past, Salish Sea harbor porpoise abundance plummeted and the species was essentially absent from the southern regions (Puget Sound, United States), and experienced a range contraction in the central waters (near Victoria, Canada), with the latter likely continuing to the present day (detailed below). The anthropogenic activities of each nation may have had synergistic, negative and undocumented effects on the harbor porpoise population(s) of the Salish Sea.
Historically, the United States waters of the Salish Sea were considered to have had high abundance and year-round presence of harbor porpoises (Scheffer and Slipp, 1948; Barlow, 1988; Calambokidis et al., 1997; Raum-Suryan and Harvey, 1998; Gaydos and Pearson, 2011), and in British Columbia historical records appear to indicate that harbor porpoise were commonly sighted from shore in the Victoria region of Juan de Fuca Strait (Baird, 2003). However, observational data suggest a decline in numbers and regions used within United States waters since the 1940s (Scheffer and Slipp, 1948; Flaherty and Stark, 1982; Cowan, 1988; Gaskin, 1992; Calambokidis and Baird, 1994; Baird, 2003). From the 1970s through to the 1990s, research and observations revealed that harbor porpoises were virtually absent from Puget Sound, and greatly reduced in numbers in Juan de Fuca Strait and San Juan Islands (Evenson et al., 2016). While harbor porpoises are still present in Juan de Fuca Strait (and sometimes in large numbers–see Hall, 2011), they no longer regularly occupy the nearshore Canadian waters near Victoria and a shore-based sighting in this region would now be a rare occurrence (Hall, 2004 and unpublished data).
Despite the continued absence of harbor porpoise in the Canadian nearshore waters of the Victoria region, by the early 2000s opportunistic sightings, strandings and fisheries bycatch data indicated that the harbor porpoise had returned to the more southerly Salish Sea waters and the most recent United States assessments confirm this return and numerical recovery (Evenson et al., 2016; Jefferson et al., 2016). This is reflected in the stranding records as well. A relatively high number of strandings occurred in 2006–2007 prompting the declaration of an unusual mortality event; however later analyses showed that the increase in strandings was actually due to a growing harbor porpoise population, expansion of their range into previously sparsely populated areas and increased reporting and response of the stranding network (Huggins et al., 2015). The latest harbor porpoise abundance estimate (Table 1) for the United States waters of the Salish Sea is 11,233 harbor porpoises (in contrast there were only 3,509 harbor porpoises in 1996, Calambokidis et al., 1997), with the highest densities in the San Juan Island area with 8,103 harbor porpoises (Jefferson et al., 2016). Less effort was expended in evaluating British Columbian waters due to primary survey goals and funding limitations. However, a reliable abundance estimate of 1,825 harbor porpoises was determined for the Gulf Islands area, whereas the abundance estimate of 277 harbor porpoises in the Canadian Juan de Fuca Strait was not considered reliable (Jefferson et al., 2016). In comparison, a 2001–2002 systematic study conducted in the Canadian waters of Juan de Fuca and Haro Straits determined a corrected annual mean abundance estimate of 442 (CV = 18.6%, 95% CI 308–634) harbor porpoise (Hall, 2004). A multi-species systematic survey was conducted along much of the BC coastline which provided province-wide summer estimates of abundance for seven cetacean species including harbor porpoise (Williams and Thomas, 2007). These estimates were updated 6 years ago to yield a province-wide summer estimate of 8,091 (CV = 24.3%, 95 CI 4,885–13,401) harbor porpoise (Best et al., 2015). However, this estimate encompassed a much larger geographical extent than just the Salish Sea. There are no current population abundance estimates for the Canadian Salish Sea waters, but in 2018 the Pacific Region International Survey of Marine Megafauna (PRISMM) was conducted by Fisheries and Oceans Canada with line transects conducted throughout BC waters, including inshore and offshore regions spanning from the Salish Sea, to the Alaska border, and westward to Gwaii Haanas (formerly the Queen Charlotte Islands). The results of this survey (not available yet but anticipated by the end of 2021) are expected to provide a more reliable estimate of the abundance and distribution of harbor porpoise throughout BC, including the Canadian waters of the Salish Sea. While these large-scale studies are useful in terms of broad numbers, more fine-scale work is required to understand regional variation and potential stock boundaries.
The contemporary harbor porpoise population inhabits the Salish Sea year-round (Keple, 2002; Hall, 2004; Jefferson et al., 2016; Elliser et al., 2018) with differential spatial and temporal patterns revealed by systematic, fine-scale studies. In the central Strait of Georgia (northern Salish Sea), Keple (2002) found low density and abundance of harbor porpoise with no detectable seasonal variation. In contrast, in the central Salish Sea waters of the southern Strait of Georgia and central Juan de Fuca Strait, denser localized aggregations have been observed (Calambokidis et al., 1997; Hall, 2004, 2011). Quantitative evaluation of year-round survey sightings data in the central Salish Sea found harbor porpoise density and abundance changed significantly depending on season. A marked increase in the local abundance occurred from April to October with a high of 673 porpoises (CV = 20.5%, 95%, CI 450–1006) (Hall, 2004). This was followed by a decline to 208 porpoises from November to March (CV = 37.5%, 95% CI 101–429) (Hall, 2004). This is similar to the San Juan Islands where sightings were higher in the summer (June–August) (Raum-Suryan and Harvey, 1998). However, the opposite is seen in waters a little farther south near Fidalgo Island, Washington where sightings increased in the spring (March–May) and where harbor porpoises were significantly more likely to be seen in the fall (September–November) and winter (December–February) than in the summer (Elliser et al., 2018). A similar pattern has also been observed in Queen Charlotte Channel, BC, with a seasonal increase in usage patterns from the fall through to the spring (PCS unpublished data 2021).
Habitat use and seasonal abundance patterns are also reflected in the stranding data. Harbor porpoise are one of the most commonly reported stranded marine mammals in southern BC (Baird and Guenther, 1995), and evaluation of stranding frequencies found that the greatest proportion occurred in the late spring (Hall, 2004). Comparison of stranding data with local abundance data found the two data sets temporally aligned and the increase in strandings corresponded with the increase in local density and abundance (Hall, 2004). Similarly, in the United States the majority of stranded animals were recovered in the spring and summer, with fewer animals in the fall and winter (Norman et al., 2004; Huggins et al., 2015).
Collectively, these studies show that differences in seasonal habitat use may vary between locations (as seen in many European studies), but it is unknown whether this is due to larger-scale seasonal movement patterns, or more localized differences in population-specific habitat use patterns. The variation in abundance estimates throughout the United States waters of the Salish Sea between 2002–2003 and 2013–2015 suggest that there may have been a redistribution of porpoises throughout the inland waters (Jefferson et al., 2016), resulting in the habitat use patterns currently seen. The long-term (1940s to 2000s) use of the central Salish Sea waters near Victoria suggests that this represents previously undocumented important habitat occupied for at least the last half century with a possible reduction in habitat quality of the nearshore environment that contributed to the decline of harbor porpoise abundance in the area. Without regular dedicated surveys across the Salish Sea it will remain uncertain as to whether these changes represent snapshots of a larger cycle, or are anomalous occurrences that signal ephemeral events. The regional variation in habitat use patterns within the Salish Sea highlights the importance and the need for fine-scale evaluations for this phocoenid species.
Fine-scale behavioral studies on harbor porpoises in the Salish Sea are limited, but have begun to shed insight into important foraging and reproductive habitats in both BC and Washington waters. In the central Salish Sea, two harbor porpoise foraging habitats were identified in Juan de Fuca Strait, near Discovery Island and Race Rocks, that were selected during particular tidal and lunar phases (Hall, 2011). This study also evaluated high density aggregations in the Salish Sea, and demonstrated biological/social importance of conspecifics in foraging harbor porpoise habitat selection (Hall, 2011). Another biophysical evaluation of Salish Sea habitat use encompassing 37,648 km2, resulted in the identification of a small area (150 km2) in the central Salish Sea that was the first identified reproductively important habitat for harbor porpoise (Hall, 2011). On-going research is indicating that harbor porpoise continue to persist in these identified important habitats in the Canadian waters of the central Salish Sea (Hall, unpublished data). Land based, long-term behavioral and photo-ID work of harbor porpoises in United States waters of the Salish Sea (off Fidalgo Island) has documented site fidelity of recognizable individual harbor porpoises, habitat use in relation to rip tide strength and tidal cycle (but not lunar phases), and variations in group size (due to season, behavior and calf presence) (Elliser et al., 2018). In the southern Puget Sound, short-term (March–May) acoustic data using a C-POD has shown a relationship between harbor porpoise presence and rate of tidal change, hour of the day and vessel presence (Anderson, 2014). Long-term work at two study sites in the northern Salish Sea (Queen Charlotte Channel and Saturna Island, BC) confirms that in some regions harbor porpoise continue to use the nearshore environment such that shore-based sightings are still possible (PCS unpublished data). The same is true for more remote regions of Juan de Fuca Strait (e.g., west coast Vancouver Island near Jordan River) where harbor porpoise can be observed from shore and within the nearshore environment on a regular basis (Hall, unpublished data). These nearshore locations may represent habitats that are biologically or socially important for the survival of Salish Sea harbor porpoise. These areas may also serve as indicator habitats for the nearshore region from which to compare levels of anthropogenic activities.
Furthering the understanding of a species habitat use patterns can help to improve assessments of population size, trends and distribution (Gilles et al., 2016). This is important for developing biologically meaningful conservation measures for harbor porpoises (and other species) that face increasing human impacts. It is also important to recognize urbanized from non-urbanized habitats, and the differences or similarities that may exist, as these could indicate adaptations to, or potential effects from, the levels of human activity. Although knowledge of harbor porpoises in the Salish Sea has increased in the last 10–15 years, it is evident that there is much we do not yet know. Identification and recognition of these knowledge gaps are critical to guide future research studies and provide information to create successful conservation strategies.
Knowledge Gaps and Threats
Knowledge gaps for the Salish Sea harbor porpoise are further complicated because this species inhabits the transboundary waters of Canada and the United States, where the arbitrary socio-political boundaries that transect the extensive waterways, are unlikely to be biologically meaningful to the animals. In the United States, stock assessments include an overall view of the stocks’ status including their geographic range, minimum population estimate, current population trends, current and maximum net productivity rates, potential biological removal (PBR) levels, status of the stock, estimates of annual human-caused mortality and serious injury by source and descriptions of other factors that may be causing a decline or impeding the recovery of strategic stocks (NOAA, 2020). Species stock assessments are reviewed every 3 years or when new information becomes available. However, the harbor porpoise Washington Inland Waters stock, for example, did not have any updated information since the 2002–2003 stock assessment, until anecdotal evidence of their return to the Salish Sea prompted new research on their abundance in 2013–2015 (Jefferson et al., 2016) and many of the parameters listed above are not well understood and/or have the necessary data to effectively evaluate. Thus, while there is general information about the stock structure and general population parameters, the information available is somewhat dated as most of the references in the assessment are from the 1980s, 1990s, and early 2000s (Carretta et al., 2016).
In Canada, the COSEWIC species assessments are similarly comprehensive and include wildlife species description and significance, distribution, habitat, biology, population size and trends, threats and limiting factors, and protection, status and ranks (COSEWIC, 2020). In Canada, species that have previously been designated as At Risk, have the status reports updated at least every 10 years by COSEWIC. However, species that are not considered At Risk can go considerably longer without updates being conducted (e.g., Dall’s porpoise–assessed in Canada as Not At Risk in 1989 with no subsequent publicly available review). On the other hand, if a species, like Pacific harbor porpoise, is listed as Special Concern under the Species At Risk Act, a Management Plan, which sets goals and objectives for maintaining sustainable population levels of one or more species that are particularly sensitive to environmental factors, but which are not in danger of becoming extinct, must be prepared within 3 years of listing (GOC, 2020). This plan is described as an “action-oriented planning document” that has an ultimate aim to “alleviate human threats and remove the species from the List of Wildlife Species at Risk” (DFO, 2009). Following this, progress reports are prepared on the implementation of the Management Plans (e.g., DFO, 2018 for harbor porpoise). Despite this, what remains to be learned about this species biology and ecology continues to outweigh the current knowledge base. Similar to in the United States, even the most recent assessments (e.g., COSEWIC, 2016) have to rely on the best available information which is spatially and temporally patchy and often discontinuous.
Information on species’ distribution and abundance patterns is critical for effective conservation, but challenges exist for mobile and cryptic species (like harbor porpoises) that are affected by many anthropogenic stressors and that may travel across international boundaries (Gilles et al., 2016; Nielsen et al., 2018). The ability to quantitatively analyze population trends in the Salish Sea is confounded by the separate research programs and projects occurring in United States and Canadian waters, with little overlap (Jefferson et al., 2016), and the lack of coordination to address knowledge gaps. Further, even the larger scale programs, such as population census efforts on both sides of the border, have yet to determine fundamental aspects such as holistic population(s) abundance estimate(s). These studies provide information on the abundance of harbor porpoises in each nation’s waters, but do not provide a more robust, more ecologically appropriate (and potentially biologically meaningful), abundance estimate for the entire Salish Sea. This complication to harbor porpoise conservation is not isolated, but in other locations it has been better addressed. In the North Sea for example, large scale surveys have been conducted (e.g., Hammond et al., 2002, 2013) and recent work has combined available comparable data sets from national monitoring programs to produce accurate fine-scale maps, providing a more cohesive picture of harbor porpoise distribution (e.g., Gilles et al., 2016; Waggitt et al., 2020). For the harbor porpoise population(s) that inhabit the Pacific transboundary waters of Canada and the United States, it is essential to enhance international collaborations to coordinate a more comprehensive understanding of the species distribution patterns and population structure. It is only this type of coordinated effort that will provide the basis for holistic ecological threat assessments that are relevant to the Salish Sea ecosystem irrespective of the international border that socially and politically intersects the natural world.
The decline and recovery of harbor porpoise in the southern waters of the Salish Sea have not been systematically studied and so are not fully understood, but some proposed causes include changes in fisheries bycatch and entanglement rates, habitat loss and degradation, pollution, and disturbance from vessels and noise (reviewed in relation to being correlated with the decline and recovery in Jefferson et al., 2016), all of which have also been identified as threats to harbor porpoise populations in general. However, due to the lack of historical systematic research on Salish Sea harbor porpoises, little data are available to assess the potential effects on past harbor porpoise population(s). This challenge continues to the present and determination of the threat-specific risks to the extant Salish Sea harbor porpoise compromises the ability to predict potential effects and outcomes of human activities. In addition, for species like harbor porpoise, that may occur in small ranges or exist in restricted habitats, the cumulative effect of any combination of factors may result in more deleterious consequences than any single threat alone (DFO, 2009).
It is also imperative to consider the past. As with other cetaceans in the Salish Sea (e.g., Southern Resident killer whales), the present day harbor porpoise population may have been significantly affected by historical events such as unquantified and undocumented fisheries-related incidental mortality that, if extensive, could have led to regional extirpations and population fragmentation. Events such as these remain beyond our collective, current knowledge base despite situational awareness of occurrence. Due to the lack of knowledge for this species, it is unknown how each of these potential threats have affected, or are currently affecting, the status of the Salish Sea harbor porpoise.
Fisheries-related porpoise entanglement and mortality have been sporadically reported for decades. In BC, historical mortality was reported from the dogfish drift gillnet, salmon troll and hake trawl fisheries (Pike and MacAskie, 1969; Baird and Guenther, 1991, 1995; Stacey et al., 1997). However, only the commercial salmon fishing industry has been quantitatively assessed for small cetacean bycatch (Hall et al., 2002) and these results are now nearly two decades out of date. Despite this, these data indicated that highest levels of entanglement and mortality occurred within the salmon gillnet fleet (Hall et al., 2002). These data also showed that the rates of entanglement and mortality in BC were low compared to many other regions throughout the harbor porpoise global distribution. There are no present or past estimates of incidental mortality in other fisheries in BC, including Indigenous gillnet and non-gillnet fisheries.
Incidental mortality of harbor porpoises in commercial and tribal fisheries in the United States waters of the Salish Sea was last observed in 1993–1994 and there are no observed fisheries in Washington’s inland waters. The more recent data from fisheries interactions were obtained from examination of stranded individuals between 2010 and 2014, but an estimated mortality rate was not able to be determined (Carretta et al., 2016). Correlation between a decrease in gillnet fishing and increase in harbor porpoise numbers in recent years indicates there is circumstantial evidence for a link between harbor porpoise status and bycatch (Jefferson et al., 2016). Although drift gillnet fishing has declined in the Salish Sea since 1994, it is likely that entanglements of harbor porpoises continue to occur, though the extent is unknown, and it is important to determine if the current take level is different from that of 1994 when the fishery was last observed (Carretta et al., 2016). It is also important to evaluate whether fishing gear injury or mortality is indiscriminate, or differentially affects males, females, or different age classes (Hall et al., 2002). The effect of separation of mothers and calves if only one is entangled could also be important because it was found to be a common belief amongst the salmon gillnet fishing community of BC that calves become entangled more frequently than adults (Hall et al., 2002). Review of Salish Sea fisheries (both Canada and United States) over the past century may provide insight into the potential for contribution to the perceived or real historic population declines and any link to their recovery, as was seen in analyses of harbor porpoise populations relative to past fishery bycatch off California, United States (Forney et al., 2021). Transboundary quantification of more recent mortality events and holistic evaluation of the significance, in comparison to a contemporary metric such as PBR, remains a significant knowledge gap. Further to this, the potential for past or present fisheries to temporarily degrade particular habitats through vessel and/or gillnet presence as well as the associated noise must also not be forgotten, and should be evaluated.
Habitat deterioration is a particularly noteworthy, and perhaps overlooked, threat to species that spend the majority of their lives within a few miles of land. The last century has seen significant changes to the Northern Hemisphere shorelines and coastal waterways, including in the Salish Sea. These changes are largely negative in terms of the habitat quality and quantity available for harbor porpoise (and other coastal marine mammals) and their prey, and are often a result of increasing human populations, and the recreational and commercial use of coastal waters. For example, about 40% of the shoreline of Puget Sound has been altered for human use (Fresh et al., 2011). The issues of habitat loss and degradation include physical, biological and chemical perturbations over many decades and have likely been overshadowed by other, sometimes more tangible, problems such as bycatch, pollution and disturbance affecting harbor porpoise populations, but have the potential to substantially add to the effects from these problems (Jefferson et al., 2016). Similarly, human-caused alterations and the resulting cumulative impacts are one of the factors that caused the disappearance of harbor porpoises in the early 1940s from San Francisco Bay, another heavily altered for-human-use waterway, and one where the species ultimately returned starting in 2008 (Stern et al., 2017). The scale to which specific events or the cumulative effects have impacted the Salish Sea harbor porpoise is not known. Thus recognition of the historical and present-day degradation and deterioration of the nearshore habitat quality from pollution, vessel presence, underwater noise levels and competition with fisheries for prey species are vital for the future of the Salish Sea harbor porpoise.
Pollution and bioaccumulation of contaminants can cause serious adverse effects on marine mammals, including harbor porpoises (Reijnders et al., 2009). Based on the correlation between the changes in pollutant levels and harbor porpoise numbers, a link between pollution and the decline and subsequent recovery of Salish Sea harbor porpoises was supported (Jefferson et al., 2016). Although environmental restrictions and regulations have resulted in a potentially “cleaner” Salish Sea, there are still pollutants that remain in the local waters from both local and international sources. These chemicals can adversely affect marine mammals. Harbor porpoises and harbor seals were found to have relatively high and disparate prevalence of antibiotic resistant bacteria, with harbor porpoises significantly more likely to host resistant organisms (Norman et al., 2021). High levels of organochlorine contaminants and increasing levels of emerging contaminants have been found in Southern Resident killer whales. These can cause adverse health effects including reduced immune system function, and can be influenced or exacerbated by other stressors such as prey availability (Mongillo et al., 2016). A compromised immune system can be particularly devastating to marine mammals where infectious diseases can be a high source of morbidity and mortality (Mongillo et al., 2016). Infectious disease (related to common parasitic, bacterial and viral infections) and trauma (asphyxiation on large fish, interspecific trauma, subcutaneous hemorrhage attributed to agonal death, dystocia in neonates and fishery-related human interaction) were the most commonly diagnosed causes of death for harbor porpoises in 2006–2007 in the Pacific Northwest, United States (Huggins et al., 2015). Research has revealed additional pathological stressors for harbor porpoises in this region and the Salish Sea was recognized as having the first recorded multi-species outbreak of Cryptococcus gattii with affected species including both harbor and Dall’s porpoise (Raverty et al., 2007; Norman et al., 2011). More recently there was emergence of the fungal disease mucormycosis which was found predominantly in harbor porpoises in both United States and Canadian waters of the Salish Sea (Huggins et al., 2020). Despite their status as a top predator, and indicator species, little is known about the potential effects from environmental pollutants on Salish Sea harbor porpoise ontogeny, physiology and longevity.
Insight into the potential for accumulation and metabolization of environmental contaminants by Salish Sea harbor porpoise can be gained from long-term research conducted in other regions such as the North Sea. For example, polychlorinated biphenyls (PCBs) have been found to bioaccumulate in harbor porpoise tissues and are correlated with increased risk of infectious disease mortality (Jepson et al., 2005, 2016; Hall et al., 2006), even though mean PCB blubber concentrations have fallen below the threshold for toxic effects (Williams et al., 2020). Differences in the types of PCBs present (and thus their effects on the individuals) varied between age, sex (particularly females and juveniles), and location (Williams et al., 2020a), and higher risks of exposure-related effects were found for calves (Weijs et al., 2010), likely related to pollutant offloading during lactation. Declines in reproductive ability for both females (Murphy et al., 2015; Jepson et al., 2016) and males (Williams et al., 2020b) and have also been reported. Reproductive effects are particularly critical to understand because they will impact the health of the population for years beyond the acute loss of individuals from associated infectious disease mortality. In the Salish Sea, it has been recognized that contaminants such as PCBs and dichlorodiphenyltrichloroethane (DDT) have decreased in the region, but that newly emerging contaminants like the flame-retardant polybrominated diphenyl ethers (PBDEs) are of concern (SSHPW, 2013; DFO, 2018). Monitoring contaminant ratios could be an ancillary technique to elucidating population structure (see Calambokidis, 1986) within the Salish Sea and that Central Puget Sound may be an ideal location to investigate the relationship between environmental contaminants and infectious disease in harbor porpoise (SSHPW, 2013). Other contaminants, including metals and trace minerals, have been detected in above normal levels of some stranded harbor porpoises (e.g., calcium, copper, mercury, manganese, selenium, and zinc), though none were considered clinically significant (Huggins et al., 2015). However, trace elements have been identified in Salish Sea harbor seals (Phoca vitulina richardsi) and regionally specific contaminant exposure levels were found (Akmajian et al., 2014). Thus, different regions of the Salish Sea likely have varying contaminant loads that could have differential effects on individuals and local marine mammal communities/populations. This may have cascading effects throughout the Salish Sea population as a whole. In addition to the persistent pollutants within the Salish Sea, there is also the risk of acute exposure during a toxic spill event. Whilst this has been ranked as a Low Impact event, it has been noted that high densities of vessel traffic increase the likelihood of a spill event and that this has the potential to reduce habitat quality, kill prey species and directly affect individuals through contact or inhalation of toxic vapors (COSEWIC, 2016). This threat may be exacerbated if localized stock structure exists within the Salish Sea (COSEWIC, 2016) and has been recognized in Canada as a moderate to high (depending on spill location and timing) population level risk factor (DFO, 2018). Whilst this threat inconspicuously exists throughout the Salish Sea, a more perceptible potential threat of disturbance from vessel traffic and other anthropogenic noise sources can lead to acoustic and physical degradation of the coastal habitats of harbor porpoise.
Urbanization of the Salish Sea is likely one of the greatest contemporary threats to harbor porpoise in this region, as is the case in other areas. For example, declines in harbor porpoise abundance in parts of the well-studied North Sea over the last 20 years have been correlated with human high-use areas including activities like shipping, pollution, fisheries and offshore construction (Nachtsheim et al., 2021). Similarly, associated with urbanization of the Salish Sea is a myriad of vessel types ranging from recreational traffic, to essential services like ferries and the United States and Canadian Coast Guards, to the commercial economic livelihood sector including fishing, tourism (e.g., wildlife viewing and cruise ships), transportation of goods by ocean-going vessels, tug boats and barges, United States and Canadian national border services, research vessels, specialty services vessels (e.g., cable laying etc.) and military readiness training by both nations–including aircraft, submarines, surface vessels, live ammunition and tactical active sonar involving both vessel-based and sonobuoy sound emitting sources. This is in addition to the shore-based activities that can also influence the marine environment through the transmission of noise from coastal construction (e.g., terrestrial blasting), associated in-water construction machinery such as impact and vibratory pile installation equipment, dredging, and submarine blasting. Without knowledge of the harbor porpoise stock structure, important habitats and movement patterns, it is difficult to accurately quantify what the effects of the potential physical and acoustic disturbance may be on the long-term survival of the population(s). Nevertheless, this source of habitat degradation has been ranked as a potentially high population-level risk factor for Salish Sea harbor porpoise (DFO, 2018). This is because, in addition to displacement, physical and acoustic disturbance could lead to disruptions in foraging and reproductive success, and the poorly understood social behaviors of harbor porpoise (DFO, 2018). Despite the awareness of the potential consequences of these activities, there remains a significant knowledge gap of the cumulative effects of these anthropogenic activities as well as standardized mitigative actions to effectively manage this threat risk to both the harbor porpoise and their prey species.
Competition with fisheries may also be a spatially and temporally discontinuous threat to harbor porpoise populations, despite the uncertainty regarding their full prey spectrum. There have been only four dedicated diet studies in the Salish Sea (Baird et al., 1994; Walker et al., 1998; Hall, 2004; Nichol et al., 2013), along with other historical and current accounts of various prey items from stranded or bycaught individuals (Scheffer and Slipp, 1948; Wilke and Kenyon, 1952; Scheffer, 1953; Treacy, 1985; Gearin et al., 1994; D’Alessandro and Duffield, 2019, and observation of wild harbor porpoises Elliser et al., 2020a,b) (Table 2). Recent research has shown that salmon are being captured by harbor porpoises in the Salish Sea (Elliser et al., 2020b), and that salmon and American shad (Alosa sapidissima) are being ingested by harbor porpoises along the United States West Coast (D’Alessandro and Duffield, 2019; Elliser et al., 2020a). It is evident that the full spectrum of the diet of harbor porpoises is not fully understood, and updated large-sample diet studies are needed (Elliser et al., 2020a), which is critical to understand in order to quantify the effects of their competition with fisheries, and the potential consequences to harbor porpoise population survival.
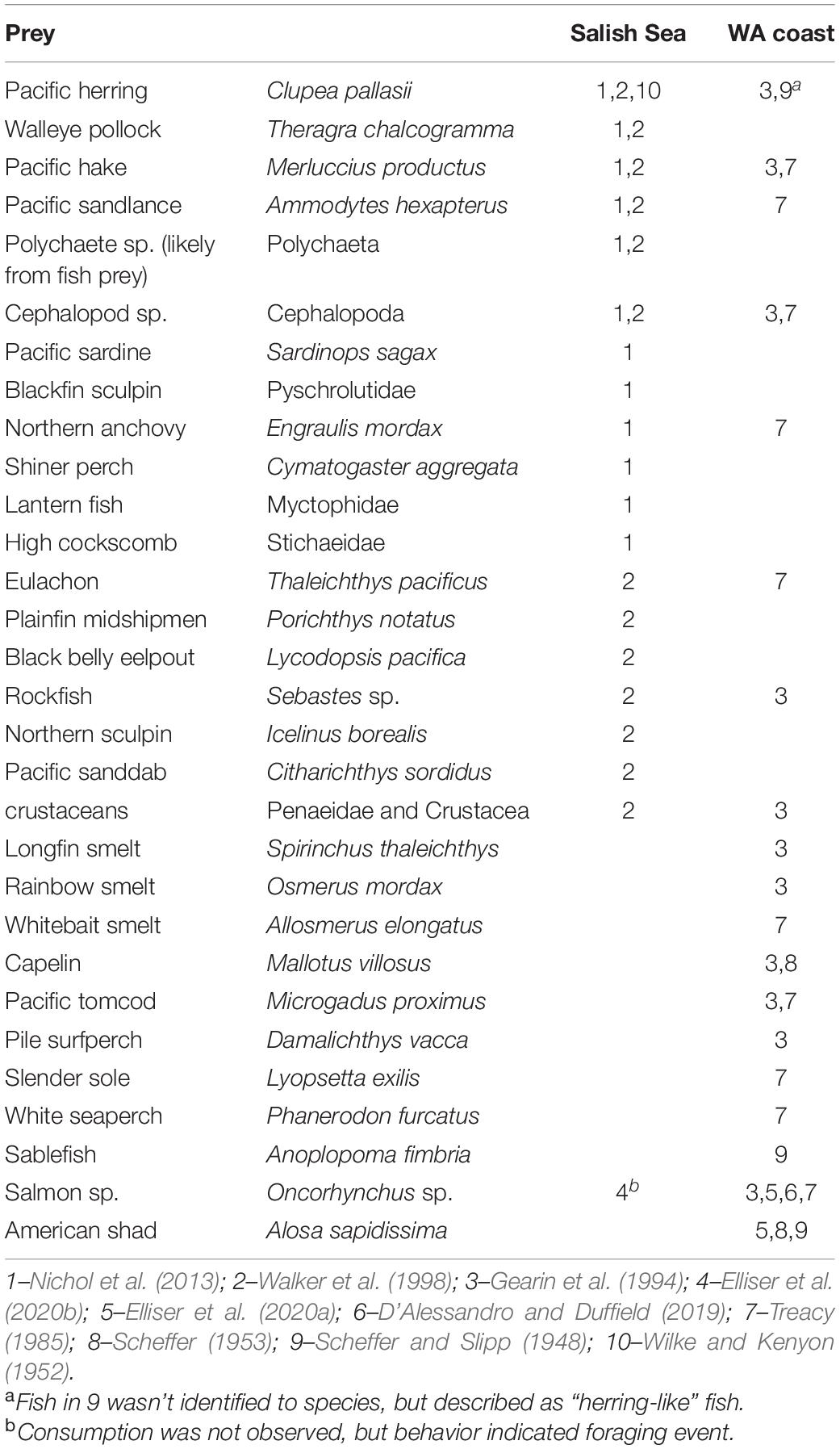
Table 2. Prey identified (family or species name given) in diet and observation studies in the Salish Sea and outer coast of Washington.
In addition, other knowledge gaps remain with regard to fundamental aspects of the biology, ecology, behavior and sociality of harbor porpoise and much work remains to be done. Understanding fundamental life history traits such as longevity, reproductive rate, habitat preferences, important or essential habitats for breeding, calving or foraging, prey species, predation rates by transient killer whales, predation rates by other predators (e.g., sharks), effects of disease and parasites, dispersal, trophic level competition, hybridization rates and social structure remains overall fairly low for the Salish Sea. Further significant gaps also exist with regard to anthropogenic effects such as vessel strike risk, climate change, gear entanglement survival, shootings, effects of plastic pollution, and the consequences to harbor porpoise population fitness, reproductive success, foraging ability, and compensatory behaviors that may result in habitat shifts or reduced foraging or social interaction. Much research is needed to help fill these gaps because there may be other, yet unidentified, factors contributing to, or detracting from, the survival of Salish Sea harbor porpoise.
Increased resolution of population parameters like ranging patterns, behavior, ecology, social and population structure, and site fidelity is required at both local, national and international levels, as is collaboration between nations for an ecosystem-based perspective. Distribution and abundance of wildlife must be understood at different spatial and temporal scales (Gilles et al., 2016; Waggitt et al., 2020). Thus an integrated, bi-national approach focused through a Salish Sea lens with complementary regional and local initiatives will work to reduce the spatial and temporal patchiness that diminishes the current conservation capacity by providing the much needed large-scale evaluations enhanced by the details of site-specific data, like that seen in European waters in the collaborative agreement among many countries on the conservation of small cetaceans of the Baltic, North East Atlantic, Irish and North Seas (ASCOBANS, Evans, 2020).
Current Status/Current Research
Cognizant of these knowledge gaps, researchers have recognized the importance of international collaboration and fine-scale research on harbor porpoise populations along the west coast of the United States and Canada. Collectively these efforts have provided substantial new information in just the last 4–5 years.
Harbor porpoises have previously been overlooked as candidates for photo-ID due to their small size, brief surface time, cryptic behavior, and few readily observable distinguishing marks (Flaherty and Stark, 1982; Gaskin and Watson, 1985; Koopman and Gaskin, 1994; Baird, 2003). However, a long-term behavioral and photo-ID study of Salish Sea harbor porpoises began in 2014 off Fidalgo Island, Washington (Elliser et al., 2018) and continues today. This was one of the few published studies of harbor porpoise photo-ID, along with a long-term study in San Francisco (Keener et al., 2011), that show the natural markings on harbor porpoises can be used to track individuals over long periods of time. A key finding in the Salish Sea was that of the 53 individually identified harbor porpoises, 35.8% were re-sighted over multiple months, with 15.1% seen on an inter-annual basis, indicating some degree of site fidelity (Elliser et al., 2018). This trend continues through 2018, with 72 identified individuals, 37.5% re-sighted over multiple months and 23.6% seen inter-annually (C. Elliser unpublished data). Site fidelity analysis is also being conducted in the northern Salish Sea (BC waters), with preliminary results supporting the findings at Fidalgo Island (PCS, unpublished data). These studies are important as individual site fidelity has been questioned for harbor porpoises in the Salish Sea. Elsewhere telemetry studies have shown that different populations can have varying degrees of movements and site fidelity (e.g., Greenland vs. the North Sea, Nielsen et al., 2018), and thus it is important to investigate this parameter for specific regions and/or populations. Further, as it is unknown if the Salish Sea harbor porpoise population is one large group, or made up of smaller, more discrete communities (based on social, genetic or geographic differences), international collaboration and comparison of results may provide insight into regional differences or similarities. Few telemetry studies have been conducted with harbor porpoises in the Salish Sea. One study using VHF tags found limited movements to within relatively small areas (e.g., within the central area of Juan de Fuca Strait, the northern San Juan Islands) (Hanson, 2007). The Marine Mammal Rescue Centre, Vancouver, Canada, rescued, rehabilitated and released a young male harbor porpoise, named Levi, with a telemetry tag. This individual essentially remained within the central Strait of Georgia after being released near southeastern Vancouver Island until the tag ceased transmitting after 71 days (DFO, 2018, M. Haulena, personal communication). The limited telemetry data provide further support for the notion that the Salish Sea harbor porpoise remain in relatively small geographic areas and that continued photo-ID efforts will provide useful data over time.
The continued success of the Fidalgo Island photo-ID study has led to a transboundary collaboration (C. Elliser, Pacific Mammal Research) with researchers in Canada (A. Hall, Porpoise Conservation Society) to expand the study into the northern Salish Sea. The aim is to determine if the matrix of markings used in the protocol developed by Elliser et al. (2018) can be applied, with or without modifications, to harbor porpoises in other parts of the Salish Sea. This regional protocol could then be used directly, or with modifications, in other harbor porpoise populations worldwide. This collaboration is also the beginning of a photo-ID regional database, where photographs, along with ancillary data, of individual harbor porpoises from both areas are stored in a collaborative database. A successful example of this type of collaboration is the Mid-Atlantic Bottlenose Dolphin Photo-ID Catalog (MABDC) that was created to help define stock structure of bottlenose dolphins in the Western North Atlantic and is contributed by researchers conducting photo-ID studies along the mid-Atlantic States (Urian et al., 1999). Having a centralized location for researchers to contribute images and data can greatly increase the ability to find matches between locations (and in this case transboundary) and thus better understand their movement patterns, site fidelity and population/stock structure. With the continued addition of more identifiable individuals to the harbor porpoise photo-ID catalog, collaborations between research groups sharing photographs and building a Salish Sea catalog (vs. single study site catalogs) will contribute to filling some of the existing data gaps. This type of transboundary collaboration between neighboring countries, like that seen in Europe (ASCOBANS, Evans, 2020), is critical in progressing appropriate conservation plans for populations that live in internationally connected waters. These photo-ID data, despite being from populations that include a high proportion of unmarked animals and low levels of distinctiveness, could potentially produce plausible estimates of survival, capture probabilities, information on movements and possibly even abundance estimates (Hupman et al., 2018), and it may be possible to use the collaborative photo-ID database to help elucidate ranging patterns, social/population structure and abundance for Salish Sea harbor porpoises.
Another long-term study was also initiated in 2015 in Queen Charlotte Channel, Canada, that builds on previous work conducted by Hall (1996, 2004, 2011) throughout the Salish Sea. This year-round study is both the first land-based harbor porpoise study in British Columbia, and the first to evaluate the nearshore habitat use in a region proximal to an urban area with significantly fluctuating vessel traffic levels. The study utilizes a modified transect design for systematic data collection and was developed to provide the basis for long-term monitoring of harbor porpoise in British Columbia. The intent was also that it could be used as a framework for future studies. The objectives were three-fold: (1) determine whether harbor porpoise used the nearshore waters, (2) determine whether usage varied seasonally, and (3) examine the group size, composition and spatial distribution within the study area for insight into the sociality of the species (PCS, unpublished data). Results indicate year-round presence, with a marked increase in winter abundance (PCS, unpublished data). This study also demonstrates site fidelity by at least some individuals (n = 10–analyses still in progress)–most notably a recognizable female present on an inter-annual basis (PCS, unpublished data). In the spring of 2017, the female was documented in the study area with a calf. She was re-sighted the following year, without a calf. Both data collection and photo-ID analyses are continuing to determine whether this female is exhibiting a generalized habitat selection pattern that further supports Salish Sea harbor porpoise site fidelity, or whether these preliminary results are anomalous. In addition, the current analyses will be evaluating whether this females’ calf presence/absence pattern data is seen with other individuals and whether these data provide support for a biennial calving interval in BC. This study has provided new information on harbor porpoise habitat use in a relatively urbanized region of BC where there is the potential for future developments that could lead to increases in vessel traffic and underwater noise. Recognition of the presence, relative abundance and behaviors of harbor porpoise provides a new reference point for this region of the Salish Sea. This is important for overall harbor porpoise conservation but also has applied implications such as the Environmental Assessment evaluations for proposed and future developments and marine mammal monitoring during anthropogenic activities that may degrade habitat quality.
Realizing the objective of providing a framework for future studies, the Queen Charlotte Channel project was subsequently adapted for a foraging behavior study based on Saturna Island (central Salish Sea) and a nearshore habitat use study on western Vancouver Island (PCS, unpublished data). The Saturna Island study commenced in 2019 and builds upon the long-term work conducted by Hall (2011) in nearby Juan de Fuca Strait and is aimed at evaluating foraging behaviors in predictable, but ephemeral, tidal features within the nearshore environment. Key findings thus far include the repeated use of the study area over consecutive days by multiple cow-calf pairs, seasonal variation in use with increased sighting rates during the summer and presence of Dall’s porpoise indicating a potentially shared foraging arena (PCS, unpublished data). This site fidelity by cow-calf pairs over the short term provides new information on habitat use, behavioral patterns and potentially the recognition of essential foraging habitat characteristics (PCS, unpublished data). This is the first time this has been identified in Pacific Canada, and has important implications for evaluating the potential effects of anthropogenic activities. It is also likely that it is not limited to this one location within the Salish Sea, and provides additional support for the notion that at least some harbor porpoise in the Salish Sea inhabit relatively small or restricted habitats. Whilst it is uncertain whether there are habitat selection differences based on age class, reproductive status or gender, this preliminary result may have applied importance in the assessment of potential effects by coastal developments, infrastructure projects or other anthropogenic actions that may degrade habitats or create a localized environment that could result in disturbance or displacement of harbor porpoise.
Information about harbor porpoise foraging ecology has also been elucidated from the long-term study on Fidalgo Island along with collaborations with other research groups. During photo-ID/behavioral surveys harbor porpoises were photographed capturing large salmonid species (Oncorhynchus sp.) in the Salish Sea and American shad in San Francisco Bay (Elliser et al., 2020b). Neither salmonidae species or American shad have previously been described as prey for harbor porpoises along the United States West Coast (except for a recent study showing an individual with tags from juvenile salmon in its forestomach, D’Alessandro and Duffield, 2019). However, salmonidae species have been found as prey species in Atlantic harbor porpoises (Fontaine et al., 1994; Aarefjord et al., 1996; Heide-Jørgensen et al., 2011; Andreasen et al., 2017). The fish that were observed being caught in the Salish Sea and San Francisco Bay are much larger than the typical harbor porpoise prey item (usually <30 cm), and in particular American shad (an introduced species) may pose a unique danger (Elliser et al., 2020a,b). Prey-related asphyxiation of harbor porpoises along the outer coasts of California, Oregon and Washington due to attempted ingestion of large fish was found to be caused by American shad in 87% of the cases where the prey was identified (Elliser et al., 2020a). Further, there was a strong bias toward females (92%), and in particular reproductively active females (at least 83.3%), as they may be more likely to attempt potentially risky behavior to compensate for their increased energetic needs (Elliser et al., 2020a). Collaboration was key in the development of these two studies and provided data that revealed two new prey species for harbor porpoises in this region and indicates that there is likely an interaction between location, age and reproductive status on their diet composition and foraging strategies that needs to be better understood (Elliser et al., 2020a).
A recent study has also revealed more about the mating behavior of harbor porpoises. In San Francisco lateralized aerial mating behavior was documented, where the male always approaches the females’ left side and often leaps into the air (contrary to their reputation for shy/inconspicuous behavior) (Keener et al., 2018) and this lateralized approach may be driven by anatomical co-evolution with females (Orbach et al., 2017). This stereotyped behavior is also seen in behavioral studies in different areas of the Salish Sea (PCS and PacMam, unpublished data) as well as other locations worldwide (Webber et al., 2019), indicating that it is not a population specific behavior. Collaborations with researchers in the Salish Sea (including D. Anderson with Cascadia Research Collective studying harbor porpoise behavior, abundance and distribution in the southern Puget Sound) and researchers in California on this and other behavioral topics are continuing.
Collaborations are also occurring between a professional wildlife tour operator (Eagle Wing Whale & Wildlife Tours) and researchers in British Columbia (A. Hall, Porpoise Conservation Society/Sea View Marine Sciences). A study was commenced in 2020 to collect data on large aggregations of harbor porpoise throughout the Salish Sea. This study is continuing through 2021, and is being expanded to also focus on Dall’s porpoise distribution and habitat use. These data will provide a reference point for distributional and group size data on harbor and Dall’s porpoise throughout the transboundary waters of the Salish Sea and will contribute to the identification of essential or important habitats for both species of porpoise. This study will also provide photographs that will be included in the photo-ID regional database. Similarly, various whale watching vessels in United States waters of the Salish Sea have begun sharing photographs and sightings data with researchers (C. Elliser, Pacific Mammal Research). Photographs from these platforms of opportunity are limited (as photographs are not always taken and/or may not be usable for ID), but at least seven individual harbor porpoises have been identified from whale watching tours, two of which were matched to individuals in Pacific Mammal Research’s ID catalog. Although opportunistic, contributions like this provide valuable data, further adding to the regional photo-ID database and knowledge of harbor porpoise movement patterns and habitat use. From this there is the potential for additional collaboration and communication amongst different sectors within the Salish Sea.
The focus on harbor porpoises is also being seen in stranding related research studies. The recent emergence of the fungal disease mucormycosis is a concern for all marine mammals in the Salish Sea, but more so for harbor porpoises that were disproportionately vulnerable (Huggins et al., 2020). A larger-scale epizootic may have severe negative consequences for this recently recovered population, thus stranded harbor porpoises are prioritized for collection and necropsy and efforts are underway to identify risk factors that may be associated with mucormycosis for harbor porpoises in Washington (Huggins et al., 2020). Monitoring of stranded animals has resulted in new information on the growth and development of harbor porpoise (Norman et al., 2018), parasites and disease (Stephen et al., 2002; Gibson et al., 2011; Norman et al., 2011; Kersh et al., 2012; Barbosa et al., 2015; Huggins et al., 2020), causes of mortality (Moore et al., 2013; Huggins et al., 2015; Fenton et al., 2017), and morphology (Piscitelli et al., 2013). In addition, local stranding networks in Washington are also beginning to share photographs of stranded individuals with researchers (C. Elliser, Pacific Mammal Research). Although no matches have been made as of yet, if a stranded individual is in the photo-ID database, then sightings and life history data will be shared with the stranding network. In addition, the stranding network may be able to provide data such as age and reproductive status for identified individuals, improving the data analyses that can be conducted with the photo-ID catalog. Other data such as disease prevalence, pollutant loads and other causes of death or co-morbidities may be better understood within the population when sighting histories are available for an individual. Understanding the life of the individual may provide context for why the animal died, and inform researchers of possible increased risks for individuals in certain areas and/or populations.
This focus on harbor porpoises isn’t limited to researchers and wildlife professionals; citizen science programs are becoming an integral part of harbor porpoise research in this region. Due to their nearshore habitat, harbor porpoises are ideal monitoring subjects for citizen scientists because they can easily conduct sightings from more accessible land-based study sites (as opposed to boat-based research). Trained volunteers contribute sightings data and amateur photographers are able to contribute photographs to the regional photo-ID database (both new individuals and re-sightings of known individuals have been documented by volunteer photographs). These volunteers are providing more information than could be obtained by researchers alone, and on a larger geographic scale. Being able to collect reliable data from various places around the Salish Sea (whether that is different citizen scientists, or different research groups, or both) increases the scope of the data, how quickly it can be obtained, and how representative the information will be for the whole population.
Collectively these are vital steps to reducing the human impact on the harbor porpoise of the Salish Sea which inhabit waters heavily influenced by anthropogenic activities.
Human Impact
Information on human activities, their effects, and knowledge of species abundance and distribution are critical in order to fully understand the impacts of any specific threat (Hammond et al., 2013; ASCOBANS, Evans, 2020). While there are still major gaps in our understanding of the relationships and interactions between the biological, ecological and anthropogenic variables there is an immediate need to reduce the human impact on the harbor porpoise population(s) of the Salish Sea because it has been recognized that continued development, use of prime habitat by humans, displacement by underwater noise, and contaminants in the food chain are some of the key threats (as discussed above) to the long term survival of this species (DFO, 2009). Using the International Union for the Conservation of Nature (IUCN) Threats Calculator, habitat degradation due to acoustic disturbance, entanglement in fishing gear and fisheries were identified as the principal known anthropogenic threats to Pacific harbor porpoise in Canada (COSEWIC, 2016). In both Pacific Canada and the United States, coastal areas continue to be urbanized for development of marinas, docks, ferry terminals, tanker ports, and log dumps. These actions could inadvertently result in the physical exclusion of harbor porpoise from these shallow water habitats and this in turn could affect their potential to feed, reproduce and socialize, if specific habitats are important for specific behaviors or life stages (DFO, 2018). As in Canada, fisheries are also a threat in the United States, and some stranded harbor porpoises have shown signs of human interaction/entanglement (7 out of 114 stranded harbor porpoises, Huggins et al., 2015) and fisheries bycatch (especially those using gillnets) is often cited as the likely major reason for their past decline in abundance (see Osmek et al., 1996; Carretta et al., 2016). However, fishing effort has decreased in recent years, while harbor porpoise abundance has increased (Carretta et al., 2016; Jefferson et al., 2016), indicating this threat may be of less concern than other factors discussed for Salish Sea harbor porpoises (at least in the United States portion of the Salish Sea), particularly compared to European populations where fisheries interactions and bycatch are of primary concern (Dolman et al., 2016). Regardless of the amount, reduction in fisheries bycatch can have profound effects on population levels. In California, United States, long-term analyses have shown that elimination or reduction in bycatch allowed three harbor porpoise populations to recover (Forney et al., 2021), emphasizing how management actions can make a difference in the conservation of harbor porpoises. In addition, a diverse array of vessel traffic continues to ply the waters that add to the underwater soundscape and potential for vessel strike. There are also military training ranges that include live fire, active sonar and torpedo ranges, and then there is the ubiquitous threat of plastic pollution that upon ingestion can result in death (Baird and Hooker, 2000). There is also the chemical pollution (past and present) that may contribute to the risk of immunosuppression, reduced reproductive capabilities and reduced neonate survival (DFO, 2018).
Recognition of human impacts is not enough to secure species survival, as it is clear what can happen to small cetacean populations that are subject to intense human activities or pressures that continue unchecked. Porpoises around the world are facing an uncertain future. In particular is the Vaquita (Phocoena sinus), which now has the dubious distinction of being on the brink of extinction with a declining population and an IUCN status of Critically Endangered (Rojas-Bracho and Taylor, 2017) and the narrow-ridged finless porpoise (Neophocaena asiaeorientalis) which is listed as Endangered and also has a negative population trajectory (Wang and Reeves, 2017). We are at risk of losing both species and it is entirely because of the human pressures put on the natural environment. For both the Vaquita and the narrow-ridged finless porpoise, the risk factors have been identified and articulated with conservation efforts expended, but still these species may not survive.
Whilst the reasons why the harbor porpoise of the southern Salish Sea have returned are unknown, the fact remains that they have made a recovery in numbers. This provides a second chance for ensuring the long-term survival of the Salish Sea harbor porpoise through direct conservation actions that improve habitat quality and prevent further degradation over the next century and beyond.
Future Directions
One of the most important research priorities should be the delineation of stock structure based on biological evidence for the entire Salish Sea as a whole, not separated by United States and Canadian assessments. Governmental entities should work together with non-governmental organizations (NGOs) and non-profit organizations. One opportunity is to use the protocol for collection of environmental DNA (eDNA) developed by Parsons et al. (2018) to obtain harbor porpoise mitochondrial DNA (mtDNA) non-invasively in various areas of the Salish Sea. This will provide genetic information that will help elucidate stock and population structure. Due to the matrilineal mode of inheritance of mtDNA, the sequence data do not allow for the ability to identify individuals, therefore, simultaneously collected photo-ID data will inform the likelihood of genetic re-captures, particularly in areas with high site fidelity. Integrating photo-ID with eDNA collection is a powerful approach for quantifying the likelihood of genetically re-capturing individual porpoise within and between geographic regions. The combination of eDNA and photo-ID becomes a powerful tool to address this issue, and ensure a representative collection of samples capturing the true variety of mtDNA haplotypes in the population. Combining genetics, photo-ID and other characteristics (like morphology and pathological differences, variation in diet and life history parameters) is important to be able to elucidate genetic and/or demographically distinct management units (Evans and Teilmann, 2009). Another important initiative would be to conduct internationally collaborative aerial and/or boat-based surveys, such that the entire Salish Sea, including both nations, is covered in adequate amounts of effort per spatial area to provide accurate and representative data. This would provide a more accurate population estimate for the Salish Sea. Coordination of this effort would also provide insight into the occurrence and distribution of high-density aggregations.
More information is needed on how underwater noise levels are impacting harbor porpoises. Short-term monitoring using a C-POD passive acoustic monitor (PAM) was shown to be a useful tool to complement visual observations in studying harbor porpoise behavior and distribution, however additional deployment of PAMs at long-term study sites throughout the Puget Sound are needed for a better understanding of harbor porpoise abundance, distribution and habitat usage (Anderson, 2014). In the near future, a Soundtrap ST500 HF PAM that can record high frequency sounds (20 Hz–150 kHz ± 3 dB) will be deployed at the Fidalgo Island study site. Acoustic monitoring is not reliant on daylight, good weather and sighting ability (required for visual observations) and assists in collecting data when researchers cannot (e.g., at night, and during the winter when weather is more of a challenge for field work). PAM click detectors have been useful for looking at harbor porpoise fine-scale changes in distribution, behavior and abundance (e.g., Simon et al., 2010; Nuuttila et al., 2017). The benefit over traditional PAM click detectors used in many studies is that the Soundtrap ST500 HF combines a click detector that runs in parallel with the normal recording process, providing data on the soundscape these animals are exposed to and their vocalizations, along with click detection. In addition, the ability to combine data from the PAM (including vocalizations) with the visual observations of behavior during daylight hours, will allow for correlating vocalizations with behavior and provide a greater wealth of information and insight into their daily lives than either technique would on its own. Locations throughout the Salish Sea will have differing levels of noise based on natural and anthropogenic sources. How these levels relate to harbor porpoise presence will be critical in understanding their overall distribution and habitat usage, thus more acoustic data are needed from different locations.
The identification of important foraging and reproductive habitats in BC waters (Hall, 2011) and a potentially biologically important habitat (BIA) in United States waters (Elliser et al., 2018) shows that there may be certain habitats that are more important than others to the daily lives of harbor porpoises, and that these may have a greater need for protection than other areas. Identifying these areas should be a high priority as they may be essential for population survival. Additionally, it is important to remember that due to their nearshore habitat selection, harbor porpoises are more at risk for effects of habitat degradation and loss from human impacts than many other marine mammals. Evaluation of altered coastlines with the potential for remediation and rewilding where possible offers the possibility of expanding harbor porpoise habitat. Restoration of shorelines and encouraging land owners to keep natural shorelines could have broad ecological effects that could result in ecosystem-wide positive consequences and improve habitat quality for untold species of the nearshore environment.
It is recommended that a Salish Sea harbor porpoise conservation team be implemented with membership from both Canada and the United States with an immediate agenda to review, rank, and enact human impact reduction actions for known spatial and/or temporal overlap between human activity and porpoise occurrence. It is also recommended that a transboundary review of mitigative actions, assessment metrics and standards be conducted to reduce anthropogenic effects during coastal construction and other temporarily disruptive human activities within the Salish Sea. Collaborations with those working for porpoise conservation in the international community will aid efforts here as lessons can be learned from their long-term research and management plans. Ultimately, conservation of harbor porpoises in the Salish Sea hinges upon the wide-spread recognition of the ecological importance of these elusive small cetaceans, resulting in dedicated long-term research, transboundary collaborations and reduction of human impacts.
Author Contributions
Both authors performed the conceptualization, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, and have read and agreed to the published version of the manuscript.
Conflict of Interest
AH was employed by Sea View Marine Sciences.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the staff, volunteers, and supporters of Pacific Mammal Research, especially S. Elliser and Marathon Petroleum Foundation. We also thank all the supporters and volunteers of the Porpoise Conservation Society without whom our work would not have been possible. We would especially like to acknowledge C. and M. Wernicke, A. Hall and Eagle Wing Whale and Wildlife Tours. Thank to D. Anderson for assistance in creating the map.
References
Aarefjord, H., Bjørge, A. J., Kinze, C. C., and Lindstedt, I. (1996). Diet of the harbour porpoise (Phocoena phocoena) in Scandinavian waters. Oceanogr. Lit. Rev. 10:1041.
Akmajian, A., Calambokidis, J., Huggins, J. L., and Lambourn, D. (2014). Age, region, and temporal patterns of trace elements measured in stranded harbor seals (Phoca vitulina richardii) from Washington inland waters. Northwest. Nat. 95, 83–91. doi: 10.1898/nwn13-26.1
Anderson, D. (2014). Harbor Porpoise Return to the SOUTH PUGET SOUND: Using Bioacoustics Methods to Monitor a Recovering Population. Master’s thesis. Olympia, WA: The Evergreen State College.
Andreasen, H., Ross, S. D., Siebert, U., Andersen, N. G., Ronnenberg, K., and Gilles, A. (2017). Diet composition and food consumption rate of harbor porpoises (Phocoena phocoena) in the western Baltic Sea. Mar. Mamm. Sci. 33, 1053–1079. doi: 10.1111/mms.12421
Baird, R. W. (2003). Update COSEWIC Status Report on The Harbor Porpoise Phocoena phocoena (Pacific Ocean Population) in Canada, in COSEWIC Assessment and Update Status Report on the Harbor Porpoise Phocoena phocoena (Pacific Ocean population) in Canada. Ottawa, ON: Committee on the Status of Endangered Wildlife in Canada.
Baird, R. W., and Guenther, T. J. (1991). Marine Mammals of the Southern Strait of Georgia; Compilations of Information for an Oil Spill Response Atlas. Unpublished Sub-Contract Report. Sidney, BC: LGL. Ltd.
Baird, R. W., and Guenther, T. J. (1995). Account of Harbor Porpoise (Phocoena phocoena) Strandings and Bycatches along the Coast of British Columbia. Report of the International Whaling Commission Biology of the Phocoenids. Sidney, BC: LGL Limited, 159–168.
Baird, R. W., Guenther, T. J., Lewis, R. J., McAdie, M. L., and Cornish, T. E. (1994). An Investigation into the Causes of an Unusual Porpoise (Phocoena phocoena and Phocoenoides dalli) Mortality Event in Southern British Columbia. Final Report Department of Fisheries and Oceans, Contract No. IIHS3-050. Victoria, BC: Department of Fisheries and Oceans.
Baird, R. W., and Hooker, S. K. (2000). Ingestion of plastic and unusual prey by a juvenile harbor porpoise. Mar. Pollut. Bull. 40, 719–720. doi: 10.1016/s0025-326x(00)00051-5
Barbosa, L., Johnson, C., Lambourn, D., Gibson, A., Haman, K., Huggins, S., et al. (2015). Parasite genotype is associated with severe disease and encephalitis in Sarcocystis neurona-infected marine mammals in the Northeastern Pacific Ocean. Int. J. Parasitol. 45, 595–603. doi: 10.1016/j.ijpara.2015.02.013
Barlow, J. (1988). Harbor porpoise, Phocoena phocoena, abundance estimation for California, Oregon, and Washington: I. Ship surveys. Fish. Bull. 86, 417–432.
Benjamins, S., Dale, A., Hastie, G., Waggitt, J. J., Lea, M. A., Scott, B. E., et al. (2015). “Confusion reigns? A review of marine megafauna interactions with tidal-stream environments,” in Oceanography and Marine Biology: An Annual Review, Vol. 53, eds R. N. Hughes, D. J. Hughes, I. P. Smith, and A. C. Dale (Boca Raton, FL: CRC Press), 1–54. doi: 10.1201/b18733-2
Benjamins, S., van Geel, N., Hastie, G., Elliott, J., and Wilson, B. (2016). Harbor porpoise distribution can vary at small spatiotemporal scales in energetic habitats. Deep Sea. Res. Part II Top. Stud. Oceanogr. 549, 275–288.
Best, B. D., Fox, C. H., Williams, R., Halpin, P. N., and Paquet, P. C. (2015). Updated marine mammal distribution and abundance estimates in British Columbia. J. Cetacean Res. Manag. 15, 9–26.
Calambokidis, J. (1986). Chlorinated Hydrocarbons in Harbor Porpoise from Washington, Oregon, and California: Regional Differences in Pollutant Ratios. Final report for Contract No. 40ABNF5 3875. La Jolla, CA: Southwest Fisheries Center, 29.
Calambokidis, J., and Baird, R. W. (1994). “Review of the marine environment and biota of Strait of Georgia, Puget Sound and Juan de Fuca Strait,” in Proceedings of the BC/Washington Symposium on the Marine Environment, January 13 and 14, 1994, eds R. C. H. Wilson, R. J. Beamish, F. Aitkens, and F. Bell (Ottawa, ON: Canadian Technical Report of Fisheries and Aquatic Sciences), 282–300.
Calambokidis, J., and Barlow, J. (1991). “Chlorinated hydrocarbon concentrations and their use of describing population discreteness in harbor porpoises from Washington, Oregon, and California,” in Proceedings of the Second Marine Mammal Stranding Workshop: Marine Mammal Strandings in the United States, eds J. I. Reynolds and D. Odell (Miami, FL: NOAA Technical Report NMFS 98), 101–110.
Calambokidis, J., Falcone, E., Douglas, A., Schlender, L., and Huggins, J. (2009). ). Photographic Identification of Humpback and Blue Whales off the US West Coast: Results and Updated Abundance Estimates from the 2008 Field Season. Final Report for Contract AB133F08SE2786 from Southwest Fisheries Science Center. Available online at: https://www.cascadiaresearch.org/files/Projects/MnBmPhotoID/Rep-BmMn-2008-SWFSC-Rev.pdf (accessed December 8, 2020).
Calambokidis, J., Laake, J. L., and Klimek, A. (2012). Updated Analysis of Abundance and Population Structure of Seasonal Gray Whales in the Pacific Northwest, 1998-2010. Paper SC/M12/AWMP2-Rev Submitted to the International Whaling Commission Scientific Committee. Available online at: https://www.cascadiaresearch.org/files/publications/Calambokidis_et_al_2012.pdf (accessed December 8, 2020).
Calambokidis, J., Osmek, S., and Laake, J. L. (1997). Aerial Surveys for Marine Mammals in Washington and British Columbia Inside Waters. Unpublished contract report for NOAA Contract 52ABNF-6-00092. Olympia, WA: Cascadia Research Collective.
Carretta, J. V., Oleson, E., Weller, D. W., Lang, A. R., Forney, K. A., Baker, J., et al. (2016). U.S. Pacific Marine Mammal Stock Assessments: 2014. NOAA Technical Memorandum. NMFS-SWFSC No. 549. La Jolla, CA: Southwest Fisheries Science Center (U.S.).
Chivers, S. J., Dizon, A. E., Gearin, P. J., and Robertson, K. M. (2002). Small-scale population structure of eastern North Pacific harbor porpoises (Phocoena phocoena) indicated by molecular genetic analyses. J. Cetacean Res. Manag. 4, 111–222.
Chivers, S. J., Hanson, B., Laake, J., Gearin, P., Muto, M. M., Calambokidis, J., et al. (2007). Additional Genetic Evidence for Population Structure of Phocoena phocoena off the Coasts of California, Oregon, and Washington. Southwest fisheries Science Center Administrative Report LJ-07-08. La Jolla, CA: Southwest Fisheries Science Center, 14.
COSEWIC (2003). COSEWIC Assessment and Update Status Report on the Harbor Porpoise Phocoena phocoena (Pacific Ocean population) in Canada. Ottawa, ON: Committee on the Status of Endangered Wildlife in Canada, 22.
COSEWIC (2016). COSEWIC Assessment and Status Report on the Harbour Porpoise Phocoena phocoena Vomerina, Pacific Ocean population in Canada, Vol. Xi. Ottawa, ON: Committee on the Status of Endangered Wildlife in Canada, 51.
COSEWIC (2020). Species at Risk Act: COSEWIC Assessments and Status Reports. Available online at: https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/cosewic-assessments-status-reports.html (accessed October 15, 2020).
Cowan, I. M. (1988). “The marine mammals of British Columbia, their status and distribution,” in The wildlife of northern British Columbia–Past, present and future, ed. R. J. Fox (Smithers, BC: Spatsizi Association for Biological Researc), 95–104. doi: 10.1525/9780520947313-005
D’Alessandro, D. N., and Duffield, D. A. (2019). Salmonid passive integrated transponder tags and codedwire tags found in the forestomach of a harbor porpoise (Phocoena phocoena) in Southwestern Washington. Fish. Bull. 117, 303–307. doi: 10.7755/fb.117.4.3
DFO (2009). Fisheries and Oceans Canada. Management Plan for the Pacific Harbor Porpoise (Phocoena phocoena) in Canada. Species at Risk Act Management Plan Series. Ottawa ON: Fisheries and Oceans Canada, 49.
DFO (2018). Fisheries and Oceans Canada. 2018. Report on the Progress of Management Plan Implementation for the Pacific Harbour Porpoise (Phocoena phocoena vomerina) in Canada for the Period 2010-2015. Species at Risk Act Management Plan Report Series. Ottawa ON: Fisheries and Ocean Canada, 38.
Dolman, S., Baulch, S., Evans, P. G. H., Read, F., and Ritter, F. (2016). Towards an EU action plan on cetacean bycatch. Mar. Policy 72, 67–75. doi: 10.1016/j.marpol.2016.06.020
Ellis, S., Franks, D. W., Nattrass, S., Cant, M. A., Weiss, M. N., Giles, D., et al. (2017). Mortality risk and social network position in resident killer whales: sex differences and the importance of resource abundance. Proc. R. Soc.Lond. B Biol. Sci. 284:20171313. doi: 10.1098/rspb.2017.1313
Elliser, C. R., Calambokidis, J., D’Alessandro, D. N., Duffield, D. A., Huggins, J. L., and Rice, J. (2020a). Prey-related asphyxiation in harbor porpoises (Phocoena phocoena) along the U.S. West Coast: importance of American Shad (Alosa sapidissima) on adult female harbor porpoise mortality. Oceans 1, 94–113. doi: 10.3390/oceans1030008
Elliser, C. R., Hessing, S., MacIver, K. H., Webber, M. A., and Keener, W. (2020b). Harbor porpoises (Phocoena phocoena vomerina) catching and handling large fish on the U.S. West Coast. Aquat. Mamm. 46, 191–199. doi: 10.1578/AM.46.2.2020.191
Elliser, C. R., MacIver, K. H., and Green, M. (2018). Group characteristics, site fidelity and photo identification of harbor porpoises, Phocoena phocoena, in Burrows Pass, Fidalgo Island, Washington. Mar. Mamm. Sci. 34, 365–384. doi: 10.1111/mms.12459
Evans, P. G. H. (2020). European Whales, Dolphins and Porpoises: Maine Mammal Conservation in Practice. London: Academic Press, 306.
Evans, P. G. H., and Teilmann, J. (2009). ASCOBANS/HELCOM Small Cetacean Population Structure Workshop. Held on 8-10 October 2007 At UN Campus, Hermann-Ehlers-Str. 10, 53113 Bonn, Germany. Available online at: https://www.ascobans.org/sites/default/files/document/Report_PopulationStructureWorkshops2007_small_1.pdf (accessed November 28, 2020).
Evenson, J. R., Anderson, D., Murphie, B. L., Cyra, T. A., and Calambokidis, J. (2016). Disappearance and Return of Harbor Porpoise to Puget Sound: 20 Year Pattern Revealed from Winter aerial Surveys (Technical Report). Olympia, WA: Washington Department of Fish & Wildlife and Cascadia Research Collective.
Fenton, H., Daoust, P. Y., Forzán, M., Vanderstichel, R., Ford, J., Spaven, L., et al. (2017). Causes of mortality of harbor porpoise (Phocoena phocoena) along the Atlantic and Pacific Coasts of Canada. Dis. Aquat. Organ. 122, 171–183. doi: 10.3354/dao03080
Flaherty, C., and Stark, S. (1982). “Harbor porpoise (Phocoena phocoena) assessment,” in Washington Sound. Seattle, WA: Final report for Subcontract #80-ABA-3584 to the National Marine Mammal Laboratory, NMFS.
Fontaine, M. C., Thatcher, O., Ray, N., Piry, S., Brownlow, A., Davison, N. J., et al. (2017). Mixing of porpoise ecotypes in southwestern UK waters revealed by genetic profiling. R. Soc. Open Sci. 4:160992. doi: 10.1098/rsos.160992
Fontaine, P. M., Hammill, M. O., Barrette, C., and Kingsley, M. C. (1994). Summer diet of the harbour porpoise (Phocoena phocoena) in the estuary and the northern Gulf of St. Lawrence. Can. J. Fish. Aquat. Sci. 51, 172–178. doi: 10.1139/f94-019
Forney, K. A., Moore, J. E., Barlow, J., Carretta, J. V., and Benson, S. R. (2021). A multidecadal Bayesian trend analysis of harbor porpoise (Phocoena phocoena) populations off California relative to past fishery bycatch. Mar. Mamm. Sci. 37, 546–560. doi: 10.1111/mms.12764
Fresh, K., Dethier, M., Simenstad, C., Logsdon, M., Shipman, H., Tanner, C., et al. (2011). Implications of Observed Anthropogenic Changes to the Nearshore Ecosystems in Puget Sound. Prepared for the Puget Sound Nearshore Ecosystem Restoration Project. Tech. Rep. No. 2011-03. Olympia, WA: Washington Department of Fish and Wildlife.
Gaskin, D. E. (1984). The harbor porpoise Phocoena phocoena: regional populations, status, and information on direct and indirect catches. Rep. Int. Whal. Commn. 34, 569–586.
Gaskin, D. E. (1991). COSEWIC Status Report on the Harbour porpoise Phocoena phocoena (Northeast Pacific Ocean population) in Canada. Ottawa, ON: Committee on the Status of Endangered Wildlife in Canada, 60.
Gaskin, D. E. (1992). Status of the harbor porpoise, Phocoena phocoena, in Canada. Can. Field Nat. 106, 36–54.
Gaskin, D. E., and Watson, A. P. (1985). The harbor porpoise, Phocoena phocoena in Fish Harbor, New Brunswick, Canada: occupancy, distribution, and movements. Fish. Bull. 83, 427–442.
Gaydos, J. K., and Pearson, S. F. (2011). Birds and mammals that depend on the Salish Sea: a compilation. Northwest. Nat. (Olymp. Wash.) 92, 79–94. doi: 10.1898/10-04.1
Gearin, P. J., Melin, S. R., Delong, R. L., Kajimura, H., and Johnson, M. A. (1994). Harbor porpoise interactions with a chinook salmon set-net fishery in Washington State. Rep. Int. Whal. Commn. Special 15, 427–438.
Gibson, A., Raverty, S., Lambourn, D., Huggins, J., Magargal, S., and Grigg, M. (2011). Polyparasitism predicts virulence of Toxoplasma gondii in marine sentinel species. PLoS Negl. Trop. Dis 5:e1142. doi: 10.1371/journal.pntd.0001142.2011
Gilles, A., Viquerat, S., Becker, E. A., Forney, K. A., Geelhoed, S. C. V., Haelters, J., et al. (2016). Seasonal habitat- based density models for a marine top predator, the harbor porpoise, in a dynamic environment. Ecosphere 7:e01367. doi: 10.1002/ecs2.1367
GOC (2020). Government of Canada Species At Risk: management plans. Available online at: https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/management-plans.html (accessed: October 14, 2020).
Hall, A. (1996). Habitat use Related to Water Depth of Harbour Porpoise, Phocoena phocoena, and Dall’s Porpoise, Phocoenoides dalli, in the Inshore Waters of Southern Vancouver Island. B.Sc. Honours thesis. Victoria, BC: University of Victoria.
Hall, A. (2004). Seasonal Abundance, Distribution and Prey Species Of Harbor Porpoise (Phocoena phocoena) in southern Vancouver Island waters. Master’s thesis. Vancouver, BC: University of British Columbia.
Hall, A. (2011). Foraging Behavior and Reproductive Season Habitat Selection of Northeast Pacific Porpoise. Ph.D. dissertation. Vancouver, BC: Deptartment. of Zoology, University of British Columbia.
Hall, A., Ellis, G., and Trites, A. W. (2002). Harbor Porpoise Interactions with the 2001 Selective Salmon Fisheries in Southern British Columbia and License Holder Reported Small Cetacean By-Catch. Selective Salmon Fisheries Science Program. Victoria, BC: Fisheries and Oceans Canada.
Hall, A. J., Hugunin, K., Deaville, R., Law, R. J., Allchin, C. R., and Jepson, P. D. (2006). The risk of infection from polychlorinated biphenyl exposure in harbor porpoise (Phocoena phocoena) – a case control approach. Environ. Health Perspect. 114, 704–711. doi: 10.1289/ehp.8222
Hammond, P. S., Berggren, P., Benke, H., Borchers, D. L., Collet, A., Heide-Jorgensen, M. P., et al. (2002). Abundance of harbor porpoises and other cetaceans in the North Sea and adjacent waters. J. Appl. Ecol. 39, 361–376. doi: 10.1046/j.1365-2664.2002.00713.x
Hammond, P. S., Macleod, K., Berggren, P., Borchers, D. L., Burt, L., Cañadas, A., et al. (2013). Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biol. Conserv. 164, 107–122. doi: 10.1016/j.biocon.2013.04.010
Hanson, M. B. (2007). “Seasonal movements and habitat use of Dall’s and harbor porpoises in the inland and coastal waters of Washington State as determined by radiotelemetry,” in Report of the National Marine Fisheries Service workshop on Advancing Electronic Tag Technology and their use in Stock Assessments, eds P. Sheridan, J. W. Ferguson, and S. L. Downing (Washington, DC: U.S. Department of Commerce, NOAA Technical Memorandum NMFS-F/SPO-82), 53–54.
Hanson, M. B., Baird, R. W., and DeLong, R. L. (1999). Movements of a Tagged harbor Porpoise in Inland Washington Waters From June 1998 to January 1999. Seattle, WA: National Marine Mammal Laboratory.
Heide-Jørgensen, M. P., Iversen, M., Nielsen, N. H., Lockyer, C., Stern, H., and Ribergaard, M. H. (2011). Harbour porpoises respond to climate change. Ecol. Evol. 1, 579–585. doi: 10.1002/ece3.51
Hohn, A. A., and Brownell, J. R. L. Jr. (1990). Harbor Porpoise in Central California Waters: Life History and Incidental Catches; IWC Report SC/42/SM47. Cambridge: International Whaling Commission.
Huggins, J. L., Garner, M. M., Raverty, S. A., Lambourn, D. M., Norman, S. A., Rhodes, L. D., et al. (2020). The emergence of mucormycosis in free-ranging marine mammals of the Pacific Northwest. Front. Mar. Sci. 7:555. doi: 10.3389/fmars.2020.00555
Huggins, J. L., Raverty, S. A., Norman, S. A., Calambokidis, J., Gaydos, J. K., Dufffield, D. A., et al. (2015). Increased harbor porpoise mortality in the Pacific Northwest, USA: understanding when higher levels may be normal. Dis. Aquat. Org 115, 93–102. doi: 10.3354/dao02887
Hupman, K., Stockin, K. A., Pollock, K., Pawley, M. D. M., Dwyer, S. L., Lea, C., et al. (2018). Challenges of implementing Mark-recapture studies on poorly marked gregarious delphinids. PloS One 13:e0198167. doi: 10.1371/journal.pone.0198167
Jefferson, T. A., Smultea, M. A., Courbis, S. S., and Campbell, G. S. (2016). Harbor porpoise (Phocoena phocoena) recovery in the inland waters of Washington: estimates of density and abundance from aerial surveys, 2013-2015. Can. J. Zool. 94, 505–515. doi: 10.1139/cjz-2015-0236
Jepson, P. D., Bennett, P. M., Deaville, R., Allchin, C. R., Baker, J. R., and Law, R. J. (2005). Relationships between polychlorinated biphenyls and health status in harbour porpoises (Phocoena phocoena) stranded in the United Kingdom. Environ. Toxicol. Chem. 24, 238–248. doi: 10.1897/03-663.1
Jepson, P. D., Deaville, R., Barber, J. L., Aguilar, A., Borrell, A., Murphy, S., et al. (2016). PCB pollution continues to impact populations of orcas and other dolphins in European waters. Nat. Sci. Rep. 6:18573. doi: 10.1038/srep18573
Keener, W., Szczepaniak, I., Adam, U., Webber, M., and Stern, J. (2011). First records of anomalously white harbor porpoises (Phocoena phocoena) from the Pacific Ocean. J. Mar. Anim. Ecol. 4, 19–24.
Keener, W., Webber, M. A., Szczepaniak, I. D., Markowitz, T. M., and Orbach, D. N. (2018). The sex life of harbor porpoises (Phocoena phocoena): lateralized and aerial behavior. Aquat. Mamm. 44, 620–632. doi: 10.1578/AM.44.6.2018.620
Keple, A. (2002). Seasonal Abundance and Distribution of Marine Mammals in the Strait of Georgia, British Columbia. Master’s thesis. Vancouver, BC: University of British Columbia.
Kersh, G., Lambourn, D., Raverty, S., Fitzpatrick, K., Self, J., Akmajian, A. M., et al. (2012). Coxiella burnetii infection of marine mammals in the Pacific Northwest, 1997-2010. J. Wildl. Dis. 48, 201–206. doi: 10.7589/0090-3558-48.1.201
Koopman, H. N., and Gaskin, D. E. (1994). Individual and geographical variation in pigmentation patterns of the harbor porpoise, (Phocoena phocoena) (L.). Can. J. Zool. 72, 135–143. doi: 10.1139/z94-017
Mongillo, T. M., Ylitalo, G. M., Rhodes, L. D., O’Neill, S. M., Noren, D. P., and Hanson, M. B. (2016). Exposure to a Mixture of Toxic Chemicals: Implications for the Health of Endangered Southern Resident Killer Whales. Washington, DC: U.S. Department of Commerce, NOAA Technical Memorandum NMFS-NWFSC-135, 107. doi: 10.7289/V5/TM-NWFSC-135
Moore, M., Barco, S., Costidis, A., Gulland, F., Jepson, P., Moore, K., et al. (2013). Criteria and case definitions for serious injury and death of marine mammals caused by anthropogenic trauma: Underwater entrapment, chronic entanglement, sharp and blunt vessel, and gunshot. Dis. Aquat. Organ. 103, 230–235. doi: 10.3354/dao02566.2013
Murphy, S., Barber, J. L., Learmonth, J. A., Read, F. L., Deaville, R., Perkins, M. W., et al. (2015). Reproductive failure in UK harbour porpoises Phocoena phocoena: legacy of pollutant exposure? PLoS One 10:e0131085. doi: 10.1371/journal.pone.0131085
Nachtsheim, D. A., Viquerat, S., Ramirez-Martinez, N. C., Unger, B., Siebert, U., and Gilles, A. (2021). Small cetacean in a human high-use area: trends in harbor porpoise abundance in the North Sea over two decades. Front. Mar. Sci. 7:606609. doi: 10.3389/fmars.2020.606609
Nichol, L. M., Hall, A. M., Ellis, G. M., Stredulinsky, E., Boogaards, M., and Ford, J. K. B. (2013). Diet overlap and niche partitioning of sympatric harbor porpoises and Dall’s porpoises in the Salish Sea. Prog. Oceanogr. 115, 202–210. doi: 10.1016/j.pocean.2013.05.016
Nielsen, N. H., Teilmann, J., Sveegaard, S., Hansen, R. G., Sinding, M. S., Dietz, R., et al. (2018). Oceanic movements, site fidelity and deep diving in harbor porpoises from Greenland show limited similarities to animals from the North Sea. Mar. Ecol. Prog. Ser. 597, 259–272. doi: 10.3354/meps12588
NOAA (2020). Marine Mammal Stock Assessments. Available online at: https://www.fisheries.noaa.gov/national/marine-mammal-protection/marine-mammal-stock-assessments (accessed October 14, 2020).
Norman, S., Raverty, S., Zabek, E., Etheridge, S., Hanson, B., Ford, J., et al. (2011). Maternal fetal transmission of Cryptococcus gattii in a harbor porpoise (Phocoena phocoena). Emerg. Infect. Dis. 17, 304–305.
Norman, S. A., Bowlby, C. E., Brancato, M. S., Calambokidis, J., Duffield, D., Gearin, P. J., et al. (2004). Cetacean strandings in Oregon and Washington between 1930 and 2002. J. Cetacean Res. Manag. 6, 87–99.
Norman, S. A., Hanson, M. B., Huggins, J., Lambourn, D., Calambokidis, J., Cottrell, P., et al. (2018). Conception, fetal growth, and calving seasonality of harbor porpoise (Phocoena phocoena) in the Salish Sea waters of Washington. USA and southern British Columbia, Canada. Can. J. Zool. 96, 566–575. doi: 10.1139/cjz-2017-0155
Norman, S. A., Lambourn, D. M., Huggins, J. L., Gaydos, J. K., Dubpernell, S., Berta, S., et al. (2021). Antibiotic resistance of bacteria in two marine mammal species, harbor seals and harbor porpoises, living in an urban marine ecosystem, the Salish Sea. Washington State, USA. Oceans 2, 86–104. doi: 10.3390/oceans2010006
Nuuttila, H. K., Courtene-Jones, W., Baulch, S., Simon, M., and Evans, G. H. (2017). Don’t forget the porpoise: acoustic monitoring reveals fine scale temporal variation between bottlenose dolphin and harbour porpoise in Cardigan Bay SAC. Mar. Biol. 164:50.
Olesiuk, P. F., Bigg, M. A., and Ellis, G. M. (1990). Life history and population dynamics of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State. Rep. Int. Whal. Commn. Spec. Issue 12, 209–243.
Orbach, D. N., Kelly, D. A., Solano, M., and Brennan, P. L. R. (2017). Genital interactions during simulated copulation among marine mammals. Proc. R. Soc. Lond. B Biol. Sci. 284, 20171265. doi: 10.1098/rspb.2017.1265
Osmek, S., Calambokidis, J., Laake, J., Gearin, P., Delong, R., Scordino, J., et al. (1996). Assessment of the status of harbor porpoise (Phocoena phocoena) in Oregon and Washington Waters. NOAA Technical Memorandum NMFS-AFSC No. 76. Seattle, WA: Agencies and Staff of the U.S. Department of Commerce.
Parsons, K. M., Everett, M., Dahlheim, M., and Park, L. (2018). Water, water everywhere: environmental DNA can unlock population structure in elusive marine species. R. Soc. Open Sci. 5:180537. doi: 10.1098/rsos.180537
Pike, G. C., and MacAskie, I. B. (1969). Marine mammals of British Columbia. Fish. Res. Board Can. Bull. Num. 171:54.
Piscitelli, M., Raverty, S., Lillie, M., and Shadwick, R. (2013). A review of lung morphology and mechanics. J. Morphol. 274, 1425–1440. doi: 10.1002/jmor.20192
PSEMP (2018). Puget Sound Ecosystem Monitoring Program Strategic Plan 2018-2022. Available online at: https://pspwa.box.com/s/hjfgnvf7v8qvf66pbejzz9wby71uqsoi (accessed October 10, 2020).
Raum-Suryan, K. L., and Harvey, J. T. (1998). Distribution and abundance of habitat use by harbor porpoise, Phocoena phocoena, off the northern San Juan Islands, Washington. Fish. Bull. 96, 808–822.
Raverty, S., Hanson, B., Huggins, J., Calambokidis, J., Hall, A., Lamborn, D., et al. (2007). “Multispecies outbreak of Cryptococcosis (Cryptococcus gatti) in stranded harbor, Dall’s porpoises and a Pacific white sided dolphin in the northeastern Pacific,” in Proceedings of the Preconference Workshop: Conservation Medicine on Marine Mammals. Society of Marine Mammalogy, Cape Town, South Africa, November, 2007, Cape Town.
Read, A. J., and Hohn, A. A. (1995). Life in the fast lane: the life history of harbor porpoises from the Gulf of Maine. Mar. Mamm. Sci. 11, 423–440. doi: 10.1111/j.1748-7692.1995.tb00667.x
Reijnders, P. J. H., Aguilar, A., and Borrell, A. (2009). “Pollution and marine mammals,” in Encyclopedia of Marine Mammals, 2nd Edn, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (London: Academic Press), 890–898. doi: 10.1016/b978-0-12-373553-9.00205-4
Rojas-Bracho, L., and Taylor, B. L. (2017). Phocoena Sinus. The IUCN Red List of Threatened Species 2017: e.T17028A50370296. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2017-2.RLTS.T17028A50370296.en (accessed October 15, 2020).
Rosel, P. E., France, S. C., Wang, J. Y., and Kocher, T. D. (1999). Genetic structure of harbour porpoise Phocoena phocoena populations in the Northwest Atlantic based on mitochondrial and nuclear markers. Mol. Ecol. 8, 41–54.
SARA (2021). Species At Risk Act (SARA) Harbor Porpoise Pacific Ocean population – Species Profile. SARA Website. Government of Canada. Available online at: https://wildlife-species.canada.ca/species-risk-registry/species/speciesDetails_e.cfm?sid=493#ot18 (accessed May 7, 2021).
Scheffer, V. B. (1953). Measurements and stomach contents of eleven Delphinids from the Northeast Pacific. Murrelet 34, 27–30. doi: 10.2307/3535867
Scheffer, V. B., and Slipp, J. W. (1948). The whales and dolphins of Washington State with the key to the cetaceans of the west coast of North America. Am. Midl. Nat. 39, 257–337. doi: 10.2307/2421587
Simon, M., Nuuttila, H., Reyes-Zamudio, M. M., Ugarte, F., Verfub, U., and Evans, P. G. H. (2010). Passive acoustic monitoring of bottlenose dolphin and harbour porpoise, in Cardigan Bay, Wales, with implications for habitat use and portioning. J. Mar. Biol. Assoc. U. K. 90, 1539–1545. doi: 10.1017/s0025315409991226
SSHPW (2013). “Statement on Salish Sea Harbor Poroise Research and Management Needs,” in Proceedings of the Salish Sea Harbor Porpoise Workshop on 7 February 2013, Anacortes, WA, 9.
Stacey, P. J., Duffus, D. A., and Baird, R. W. (1997). A preliminary evaluation of incidental mortality of small cetaceans in coastal fisheries in British Columbia. Can. Mar. Mamm. Sci. 13, 321–326. doi: 10.1111/j.1748-7692.1997.tb00637.x
Stephen, C., Lester, S., Black, W., Fyfe, M., and Raverty, S. (2002). Multi-species outbreak of cryptococcosis on Southern Vancouver Island, British Columbia. Can. Vet. J. 42, 792–794.
Stern, S. J., Keener, W., Szczepaniak, I. D., and Webber, M. A. (2017). Return of harbor porpoises (Phocoena phocoena) to San Francisco Bay. Aquat. Mamm. 43, 691–702. doi: 10.1578/am.43.6.2017.691
Towers, J. R., Sutton, G. J., Shaw, T. J. H., Malleson, M., Matkin, D., Gisborne, B., et al. (2019). Photo-Identification Catalogue, Population Status, and Distribution of Bigg’s Killer Whales known from Coastal Waters of British Columbia, Canada. Canadian. Technical. Report Fisheries and Aquatic Science 3311. Nanaimo, BC: Fisheries and Oceans Canada, Pacific Biological Station, 299.
Treacy, S. D. (1985). Feeding Habits of Marine Mammals from Grays Harbor, Washington to Netarts Bay Oregon. Marine Mammals and their Interactions with Fisheries of the Columbia River and Adjacent Waters, 1980–1982. NOAA: Northwest Alaska Fisheries Science Center. NWAFC Processed Report 85-04. National Marine Fishery Service, 149–198. Available online at: https://archive.fisheries.noaa.gov/afsc/Publications/ProcRpt/NWAFCPR85-04.pdf (accessed May 2, 2020).
Urian, K. W., Hohn, A. A., and Hansen, L. J. (1999). Status of the Photo-Identification Catalog of Coastal Bottlenose Dolphins of the Western North Atlantic: Report of a Workshop of Catalog Contributors. NOAA Technical Memorandum NMFS-SEFSC-425 Beaufort, NC: National Marine Fisheries Service Beaufort Laboratory, 425, 22.
Waggitt, J. J., Evans, P. G. H., Andrade, J., Banks, A. N., Boisseau, O., Bolton, M., et al. (2020). Distribution maps of cetacean and seabird populations in the North-East Atlantic. J. Appl. Ecol 57, 253–269.
Walker, W. A., Hanson, M. B., Baird, R. W., and Guenther, T. J. (1998). Food Habits of the Harbor Porpoise, Phocoena phocoena, and Dall’s Porpoise, Phocoenoides dalli, in the Inland Waters of British Columbia and Washington. Alaska Fisheries Science Center Processed Report 98-10. Seattle, WA: Alaska Fisheries Science Center. 63–75.
Walton, M. J. (1997). Population structure of harbour porpoises Phocoena phocoena in the seas around the UK and adjacent waters. Proc. R. Soc. Lond. Ser. B 264, 89–94. doi: 10.1098/rspb.1997.0013
Wang, J. Y., and Reeves, R. (2017). Neophocaena Asiaeorientalis. The IUCN Red List of Threatened Species 2017: e.T41754A50381766. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T41754A50381766.en (accessed October 15, 2020).
Webber, M. A., Szczepaniak, I. D., Keener, W., Markowitz, T. M., Anderson, D., Elliser, C. R., et al. (2019). “Harbor porpoise sex life: are lateralized and aerial mating behaviors characteristic throughout the range?” in Poster presentation at the World Marine Mammal Conference, Barcelona, Spain December 9-12, 2019, Barcelona.
Weijs, L., van Elk, C., Das, K., Blust, R., and Covaci, A. (2010). Persistent organic pollutants and methoxylated PBDEs in harbour porpoise from the North Sea from 1990 until 2008: young wildlife at risk? Sci. Total Environ. 409, 228–237. doi: 10.1016/j.scitotenv.2010.09.035
Wilke, F., and Kenyon, K. W. (1952). Notes on the Food of fur Seal, Sea Lion, and Harbor Porpoise. J. Wildl. Manage. 16, 396–397. doi: 10.2307/3796662
Williams, R., Ten Doeschate, M., Curnick, D. J., Brownlow, A., Barber, J. L., Davison, N. J., et al. (2020). Levels of polychlorinated biphenyls are still associated with toxic effects in harbor porpoises (Phocoena phocoena) despite having fallen below proposed toxicity thresholds. Environ. Sci. Technol. 54, 2277–2286. doi: 10.1021/acs.est.9b05453
Williams, R., and Thomas, L. (2007). Distribution and abundance of marine mammals in the coastal waters of British Columbia. Can. J. Cetacean Res. Manag. 9, 15–28.
Williams, R. S., Curnick, D. J., Barber, J. L., Brownlow, A., Davison, N. J., Deaville, R., et al. (2020a). Juvenile harbor porpoises in the UK are exposed to a more neurotoxic mixture of polychlorinated biphenyls than adults. Sci. Total Environ. 708:134835. doi: 10.1016/j.scitotenv.2019.134835
Keywords: harbor porpoise, Phocoena phocoena, Salish Sea, conservation, knowledge gaps, current research
Citation: Elliser CR and Hall A (2021) Return of the Salish Sea Harbor Porpoise, Phocoena phocoena: Knowledge Gaps, Current Research, and What We Need to Do to Protect Their Future. Front. Mar. Sci. 8:618177. doi: 10.3389/fmars.2021.618177
Received: 16 October 2020; Accepted: 19 April 2021;
Published: 24 May 2021.
Edited by:
Jeremy Kiszka, Florida International University, United StatesReviewed by:
Thomas A. Jefferson, Clymene Enterprises, United StatesPeter G. H. Evans, Sea Watch Foundation, United Kingdom
Copyright © 2021 Elliser and Hall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cindy R. Elliser, cindy.elliser@pacmam.org
 Cindy R. Elliser
Cindy R. Elliser Anna Hall2,3
Anna Hall2,3