- 1Institute of Food Research, Gut Health and Food Safety Programme, Norwich, UK
- 2Departamento Ingeniería de Alimentos y del Equipamiento Agrícola, Campus de Excelencia Internacional Regional “Campus Mare Nostrum,” Escuela Técnica Superior de Ingeniería Agronómica, Universidad Politécnica de Cartagena, Cartagena, Spain
- 3Instituto de Biotecnología Vegetal, Campus de Excelencia Internacional Regional “Campus Mare Nostrum,” Universidad Politécnica de Cartagena, Cartagena, Spain
- 4Cardiff School of Health Sciences, Cardiff Metropolitan University, Cardiff, UK
The bipolar flagella of the foodborne bacterial pathogen Campylobacter jejuni confer motility, which is essential for virulence. The flagella of C. jejuni are post-translationally modified, but how this process is controlled is not well understood. In this work, we have identified a novel PAS-domain containing regulatory system, which modulates flagella-flagella interactions in C. jejuni. Inactivation of the cj1387c gene, encoding a YheO-like PAS6 domain linked to a helix-turn-helix domain, resulted in the generation of a tightly associated “cell-train” morphotype, where up to four cells were connected by their flagella. The morphotype was fully motile, resistant to vortexing, accompanied by increased autoagglutination, and was not observed in aflagellated cells. The Δcj1387c mutant displayed increased expression of the adjacent Cj1388 protein, which comprises of a single endoribonuclease L-PSP domain. Comparative genomics showed that cj1387c (yheO) orthologs in bacterial genomes are commonly linked to an adjacent cj1388 ortholog, with some bacteria, including C. jejuni, containing another cj1388-like gene (cj0327). Inactivation of the cj1388 and cj0327 genes resulted in decreased autoagglutination in Tween-20-supplemented media. The Δcj1388 and Δcj0327 mutants were also attenuated in a Galleria larvae-based infection model. Finally, substituting the sole cysteine in Cj1388 for serine prevented Cj1388 dimerization in non-reducing conditions, and resulted in decreased autoagglutination in the presence of Tween-20. We hypothesize that Cj1388 and Cj0327 modulate post-translational modification of the flagella through yet unidentified mechanisms, and propose naming Cj1387 the Campylobacter Flagella Interaction Regulator CfiR, and the Cj1388 and Cj0327 protein as CfiP and CfiQ, respectively.
Introduction
The thermophilic Campylobacter species C. jejuni and C. coli are causative agents of human gastroenteritis, and are commonly transmitted via contaminated food, especially poultry meat. Diarrhoeal disease linked with Campylobacter spp. is prevalent in many countries in the Western world, with around half a million cases annually in the UK (Tam et al., 2012). Despite its importance as a human pathogen, much remains to be learned about Campylobacter virulence mechanisms. The role of the flagellar system in Campylobacter virulence cannot be understated. Firstly, flagellar motility confers the ability to swim toward intestinal epithelial cells, which is critical for subsequent cell invasion (Lee et al., 1986; Szymanski et al., 1995). Secondly, the flagellar Type III secretion system is utilized to secrete non-flagellar effector proteins, which have roles in virulence (Konkel et al., 2004; Song et al., 2004; Poly et al., 2007; Barrero-Tobon and Hendrixson, 2012, 2014; Neal-McKinney and Konkel, 2012). Thirdly, motility is essential for chemotaxis, and various chemotaxis-defective mutants are attenuated in animal models of disease (Takata et al., 1992; Yao et al., 1997) or show reduced immunopathology (Bereswill et al., 2011). Flagella are also required for autoagglutination [i.e., aggregation, AAG (Golden and Acheson, 2002)] in Campylobacter, which is thought to play a role in adherence to, and invasion of human embryonic intestinal cells (Misawa and Blaser, 2000). AAG activity is also associated with virulence in Escherichia coli, Helicobacter pylori, and Burkholderia pseudomallei (Cole et al., 2004; Boddey et al., 2006; Moreira et al., 2006).
The Campylobacter flagellum consists of two highly similar flagellin sub-units, FlaA, and FlaB (Guerry et al., 1991), and is heavily glycosylated, which plays an important role in flagella structure and function in Campylobacter. The flagellum is modified by covalent O-linked attachment of modified pseudaminic or legionaminic acid sugars (Thibault et al., 2001; Logan et al., 2002; McNally et al., 2007) and glycosylation has been shown to be essential for flagella assembly (Goon et al., 2003; Asakura et al., 2013). Moreover, subtle changes in glycosylation affect AAG as mutants unable to modify flagella with an acetamidino-derivative of pseudaminic acid, fail to autoagglutinate, suggesting that these sugar modifications are required for flagella interaction during AAG (Guerry et al., 2006). Genes encoding the biosynthetic pathway for glycan synthesis and transfer are highly variable among Campylobacter strains (Guerry et al., 2006), which likely gives rise to a high level of glycan heterogeneity within the genus that may have a role in antigenic variation and immune avoidance.
The whole flagellar apparatus involves the coordinated assembly of 40–100 proteins (Chen et al., 2011; Lertsethtakarn et al., 2011), and flagellar rotation imposes an energy demand on cells. Accordingly, expression of flagella genes is tightly regulated, with genes involved in the secretion apparatus subject to expression from σ54-dependent promoters, and the major flagellin and several effectors requiring σ28 (Nuijten et al., 1990; Guerry et al., 1991; Carrillo et al., 2004; Wosten et al., 2010). Other factors involved in regulating transcription of flagellar genes are the two-component FlgSR system (Hendrixson and DiRita, 2003; Wosten et al., 2004) and the FlgM anti-sigma factor (Wosten et al., 2010). Flagella may be further regulated via phase variation as a result of polymeric A/T tracts within the flgR gene (Hendrixson, 2006). Activation of the FlgS histidine kinase is thought to be dependent on interaction with components of the flagellar assembly apparatus (Boll and Hendrixson, 2013) and σ54-dependent flagella genes are activated by low pH (Le et al., 2012). Glycosylation may also be regulated at the metabolic level as pyridoxal-5′-phosphate production results in decreased flagellar glycosylation (Asakura et al., 2013).
The Campylobacter genome encodes other environmental sensing modules, including those involved in chemotactic sensing (Marchant et al., 2002) and gene regulation (Raphael et al., 2005). Among the repertoire of sensing modules, PAS domains have been linked to both chemotaxis sensors (Reuter and van Vliet, 2013) and two-component sensors (Luethy et al., 2015). PAS (Per, Arnt, Sim) domains are wide-spread in bacteria, archea, and eukaryotes, and have roles in sensing a wide range of stimuli including light, oxygen, redox potential, and even play a role in circadian regulation in higher eukaryotes (Taylor and Zhulin, 1999). In C. jejuni, three PAS-domain-containing proteins are involved in balancing energy and redox taxis (Elliott and Dirita, 2008; Reuter and van Vliet, 2013). Here we investigate the function of the previously uncharacterized PAS-domain protein Cj1387c in C. jejuni. Using a combination of mutational analysis, microscopy and proteomics, we have identified two proteins that have a role in mediating cell-cell contacts via flagella. The role of these proteins in virulence is also assessed using the Galleria mellonella invertebrate infection model.
Materials and Methods
C. jejuni Strains and Growth Conditions
Campylobacter jejuni strain NCTC 11168 and its isogenic mutants (Table 1) were routinely cultured in a MACS-MG-1000 controlled atmosphere cabinet (Don Whitley Scientific) under microaerobic conditions (85% N2, 5% O2, 10% CO2) at 37°C. For growth on plates, strains were either grown on Brucella agar, blood plates [Blood Agar Base 2 (BAB), 1% yeast extract, 5% horse blood (Oxoid)], or BAB with Skirrow supplements (10 μg ml−1 vancomycin, 5 μg ml−1 trimethoprim, 2.5 IU polymyxin-B). Broth culture was carried out in Brucella broth (Becton Dickinson).
Construction of Δcj1387c, Δcj1388, Δcj0327, Δcj1388cj0327, and Δcj1387cflaAB Mutants
Plasmids used in this study are listed in Table 2, and primers used are listed in Supplementary Table 1. DNA fragments of the target gene and approximately 500 bp of flanking sequence on each side were PCR amplified using Phusion DNA polymerase (New England Biolabs) using the primers detailed in Supplementary Table 1. These amplified fragments were purified (PCR purification kit, QIAGEN, Germany), digested and ligated into pNEB193 (New England Biolabs) also digested with the corresponding restriction endonucleases (see Supplementary Table 1) and transformed into a chemically competent E. coli strain Top10 (Invitrogen). Constructs containing an insert were selected on LB agar plates containing 100 μg ml−1 carbenicillin and 20 μg ml−1 X-gal. Constructs containing an insert were confirmed by restriction digest analysis and sequencing (Eurofins MWG Operon, Ebersberg, Germany). To make the Δcj1388 and Δcj0327 insertional inactivation constructs, BamHI sites, were introduced at the 5′ and 3′ end of the target genes by inverse PCR using primers detailed in Supplementary Table 1. Inverse PCR products were purified (PCR-purification kit, QIAGEN) digested with BamHI, and ligated to either the kanamycin cassette (BamHI cohesive ends) from pMARKan9 (Δcj1388) or chloramphenicol cassette (BamHI cohesive ends) from pAV35 (Δcj0327). All ligation reactions were transformed into E. coli strain Top10 and positive transformants were selected for by plating on LB agar supplemented with either 30 μg ml−1 kanamycin or 30 μg ml−1 chloramphenicol as appropriate. Plasmids with insert, and insert orientation, were verified with restriction digest analysis and sequencing. Single C. jejuni mutant strains were isolated after transformation of the C. jejuni NCTC 11168 wild-type strain with plasmids by electroporation (Reuter and van Vliet, 2013), followed by selection on plates supplemented with either 50 μg ml−1 kanamycin or 10 μg ml−1 chloramphenicol. The Δcj1387c mutant (antibiotic cassette in the same orientation as cj1387c) was made by transforming wild-type cells with either pCj1387::KanC1 (Kanr) or pCj1387::CatC1 (Catr). To make the Δcj1388cj0327 double mutant, the Δcj1388 strain was transformed with the cj0327 insertional inactivation construct and transformants selected on Brucella plates supplemented with 10 μg ml-1 chloramphenicol. To make the 11168 Δcj1387c-flaAB strain (cj1387c inactivated in a non-motile background), the ΔflaAB strain (Kanr) was transformed with pCj1387::CatC1 and transformants selected on Brucella plates supplemented with 10 μg ml−1 chloramphenicol.
To confirm the position of the antibiotic cassette in antibiotic resistant clones, colonies were picked into 10 ml Brucella broth, supplemented with the appropriate antibiotic, and grown overnight. Genomic DNA was isolated from 4 ml overnight culture (DNeasy kit, QIAGEN). Diluted genomic DNA was used as template for PCR using primers that anneal outside of the cloned flanking regions in combination with antibiotic cassette-specific primers (Supplementary Table 1).
Construction of cj1387c, cj0327, and cj1388 Complementation Constructs
C. jejuni mutants were complemented by inserting the cj1387c, cj0327, or cj1388 gene in trans using the cj0046 pseudogene (Reuter and van Vliet, 2013). The genes were PCR-amplified from the NCTC 11168 genomic DNA (for primers see Supplementary Table 1), digested with NcoI or BspHI, and ligated into pKfdxA or pCfdxA digested with Esp3I (Fermentas) to make pCASO24 (cj1387c expressed from constitutive fdxA promoter), pCASO62 (cj0327 expressed from constitutive fdxA promoter), and pCASO41 (cj1388 expressed from constitutive fdxA promoter). These constructs were transformed into the relevant mutant backgrounds using standard electroporation methods. Complementation strains were confirmed by PCR using purified genomic DNA and primers that anneal outside of the cj0046 flanking regions in combination with gene-specific primers (see Supplementary Table 1). To make the cys70ser substitution, pCASO41 was used as template for inverse PCR using primers 1388Cys-SerDpnI1/2 (see Supplementary Table 1). The PCR product was digested with DpnI for 60 min at 37°C and then purified (PCR purification kit, QIAGEN). A 1/10 volume of the total purified DNA was transformed into E. coli. To confirm those constructs with the correct sequence, plasmid DNA was purified and sequenced using the T7 primer (Eurofins Genomics, Ebersberg, Germany).
Assessment of Growth
A 50 μl single-use glycerol stock, routinely stored at −80°C, was used to inoculate a BAB plate with Skirrow supplements and these cells were used to inoculate fresh Brucella broth. Cultures were grown in microaerobic conditions with shaking overnight at 37°C. The overnight culture was diluted to A600 ≈0.05 (~ 1 × 107 CFU ml−1) in either 50 ml fresh Brucella broth in a T75 filter screw cap tissue culture flask (TPP, Helena Biosciences) or 200 μl in a 96-well plate [flat bottom, non-treated, sterile, polystyrene, (Corning, NY, USA)]. Flasks were grown in microaerobic conditions with shaking at 37°C and the A600 was monitored every 60 min for up to 10 h. Growth in 96-well plates was assessed using an Omega plate reader [FLUOstar Omega (BMG Labtech, Germany)] linked to an atmospheric control unit in microaerobic conditions at 37°C. Omega assays were run for 24 h and A600 data was recorded every 60 min.
Autoagglutination Assays
Autoagglutination (AAG or cell aggregation and sedimentation) was measured by monitoring the A600 of a 1 ml overnight culture in a plastic cuvette, statically incubated at room temperature. All strains were assessed using at least three independent biological replicates. The percentage autoagglutination (% AAG) was calculated as the recorded A600 divided by the initial A600. Data was analyzed for statistical significance using unpaired t-tests (GraphPad Prism 6.01).
Light Microcopy and Flagella Staining Using the Ryu stain
Typically, 10 μl of an overnight culture was added to a microscope slide and covered with a coverslip. Cells were monitored using an Eclipse 50i microscope using the x100 lens (x1000 including ocular lens) to confirm either a motile or non-motile (ΔflaAB, Δcj1387c-flaAB) swimming phenotypes. When necessary, videos (15 frames second−1, 320 × 240 pixels) were captured using a Coolpix 4500 digital camera (Nikon). Video compilations were made by extracting appropriate frames using ImageJ (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2014) and combined using iMovie version 8.0.6.
To visualize flagella, cells were stained using the Ryu stain (Heimbrook et al., 1989). Ryu stain was prepared freshly on a weekly basis (as necessary) by mixing the two components and centrifuging (15,000 × g, 3 min, room temperature). Five ul of the stain was pipetted at the edge of the coverslip to draw the stain into the sample by capillarity. Ryu stained regions were photographed (2272 × 1704 pixels, 300 dpi) using a Coolpix 4500 digital camera. ImageJ was used to prepared montage images and apply a scale bar.
Swarm Plate Assays
All swarm plate assays were carried out using square 10 mm2 petri plates (Sterilin) inoculated with wild-type and three test strains. Swarming in rich media was assessed using Brucella broth supplemented with 0.01% TTC and 0.4% agar (Oxoid). Plates were inoculated with 5 μl of overnight culture, and incubated in microaerobic conditions at 37°C. Swarm plates were photographed using a CCD camera and gel documentation system (U:Genius, Syngene) after 24 and 48 h and halo area measured using ImageJ. For each plate, halo size was expressed as a percentage of the corresponding wild-type and each strain was tested for significance using a one-sample t-test, compared to a hypothetical value of 100 (GraphPad Prism 6.01).
Galleria Assays
The Galleria mellonella infection model (Champion et al., 2010) was used to assess virulence. G. mellonella larvae were obtained from Livefoods.co.uk (United Kingdom). C. jejuni strains were resuspended from Skirrow plates, centrifuged (15,000 × g, 3 min, room temperature), resuspended in PBS and adjusted to an A600 = 1.0. An aliquot of wild-type cells was heated at 70°C for 5 min using a MultiGene thermal cycler (Labnet International) to provide a heat-killed control. For each test strain, 10 larvae were inoculated in the right foremost pro-leg by microinjection (Hamilton, Switzerland) with a 10 μl cell suspension (~1 × 106 cells). Control groups, each containing 10 larvae, were prepared in each assay: mock injection, PBS, and heat-killed bacteria. The larvae were incubated at 37°C for 24 h and then scored for survival. Larvae that showed high levels of melanization, were unable to self-right, and showed an absence of mobility were scored as non-survivors, and percentage survival was calculated. Each strain was assessed using at least four independent experiments.
Construction of Cj1388 Over-expression constructs
The cj1388 gene was PCR amplified from the NCTC 11168 genomic DNA using primers cj1388pET_Fwd/_Rev (see Supplementary Table 1). The PCR product was digested with NdeI and BamHI and ligated into pET28a digested with NdeI and BamHI to make pCASO40. To make the cys70ser substitution, pCASO40 was used as template for inverse PCR using primers 1388Cys-SerDpnI1/2. The PCR product was digested with DpnI for 60 min at 37°C and then purified. A 1/10 volume of the total purified DNA was transformed into E. coli. To confirm those constructs with the correct sequence, plasmid DNA was purified and sequenced using the T7 primers.
Protein Expression
Expression plasmids were transformed into E. coli BL21 (DE3) cells (NEB). For induced protein expression, 50 ml of LB supplemented with 30 μg ml−1 kanamycin was inoculated with 50 μl overnight broth culture and grown at 37°C with 200 rpm shaking. The A600 of the cultures was monitored; when the culture reached an A600 of ~0.5, IPTG was added to a final concentration of 0.4 mM. Cultures were grown with shaking for a further 3 h (37°C, 200 rpm). Un-induced, and hourly induction samples were collected to monitor protein expression. Cell were harvested by centrifugation (3200 × g, 15 min, 4°C) and lysed by sonication (Soniprep 150 MSE, Sanyo). His-tagged proteins were purified using Ni-NTA Spin Columns (QIAGEN). Columns were equilibrated with NPI-10 (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole), and washed with NPI-20 (NPI + 20 mM imidazole). Protein was eluted in NPI-500 (NPI + 500 mM imidazole). For SDS-PAGE analysis, samples were mixed with 4x LDS buffer (Invitrogen, Life Technologies) with or without β-mercaptoethanol (± reducing conditions) and resolved on 4–20% precast 10 × 10 cm gels (Expedeon, UK) in 1 × MOPS at 120 V. To visualize proteins, gels were rinsed with water and stained in InstantBlue (Expedeon) overnight. Gels were imaged using a GS800 Calibrated Densitometer (BioRad) at 63.5 μm resolution.
Two-dimensional Protein Gel Electrophoresis
Two-dimensional protein gel electrophoresis was conducted as described previously (Shaw et al., 2012). C. jejuni cells were harvested from broth culture (50 ml) by centrifugation at 3220 × g for 15 min at room temperature. Cell pellets were resuspended in 500 μl lysis buffer (50 mM Tris (pH 7.5), 0.3% sodium dodecyl sulfate (SDS), 0.2 M dithiothreitol, 3.3 mM MgCl2, 16.7 μg of RNase ml−1, and 1.67 U of DNase ml−1) and lysed (Soniprep 150 MSE, Sanyo) on ice until clear. The samples were then centrifuged (20,000 × g, 20 min, 4°C) to remove any unlysed cells. Total cell protein was quantified using a 2D Quant kit (GE Healthcare, UK) as per the manufacturer's instructions.
Protein Identification
Protein spots of interest were picked from 2D gels using the ProPick (Genomic Solution) and subject to in-gel trypsin digestion and LC-MS-MS as previously described (Shaw et al., 2012) except using the Proteome Discoverer program (Thermo) to convert the RAW file to Mascot Generic format for Mascot Analysis (Matrix Science).
For the protein overexpression SDS-PAGE gels, the protein bands were excised and subject to in-gel digestion as previously described (Ash et al., 2014) modified for an overnight Chymotrypsin digestion under both non-reducing, and reducing and alkylating conditions. The resulting peptides were then analyzed by LC-MS-MS on an Orbitrap Fusion (Thermo). MS Convert (Chambers et al., 2012) was used to convert the RAW file to Mascot Generic format for Mascot Analysis prior to either Mascot analysis for protein identification or Mass Matrix analysis (http://www.massmatrix.net/mm-cgi/home.py) for identification of potential disulfide bonds in the non-reduced protein bands.
Bioinformatics Analysis
Pfam [http://pfam.sanger.ac.uk/ (Finn et al., 2014)] and InterProScan [http://www.ebi.ac.uk/Tools/pfa/iprscan/ (Jones et al., 2014)] were used to search for protein domains. Domain architecture information was retrieved from Pfam. Protein sequences from either complete genome sequences or whole genome shotgun assemblies were downloaded from the Patric database (http://patricbrc.org/portal/portal/patric/Home) (Wattam et al., 2014) and a BLAST database was made using BioEdit (http://www.mbio.ncsu.edu/bioedit/bioedit.html). All BLAST searches were conducted within BioEdit. EMBL String (http://string-db.org/) (Franceschini et al., 2013) was used to find homologs of Cj1387c and investigate cj1387c-cj1388 genetic structure. The RNA-seq data of the C. jejuni NCTC 11168 wild-type have been deposited in the Gene Expression Omnibus (GEO) and Short Read Archive (SRA) databases, and are available via GEO accession number GSE49687. Transcript levels of individual genes were expressed as Reads Per Kilobase per Million mapped reads (RPKM) values, calculated after mapping of reads using CLC Genomics Workbench v5 (CLC Bio).
Results
C. jejuni Contains a PAS6 Domain-containing Protein that Exhibits Limited Architectural Variability
PAS domains are wide-spread signal-sensing domains involved in sensing a variety of signals including light, redox potential, oxygen, and small ligands (Taylor and Zhulin, 1999; Moglich et al., 2009). Searches of the C. jejuni NCTC 11168 genome sequence allowed the identification of five proteins containing predicted PAS domains. Of these, the CetB and CetC proteins, consisting of single PAS domains, and CetZ, consisting of two tandem PAS domains linked to a methyl-accepting chemotaxis domain are involved in energy and redox taxis (Hendrixson et al., 2001; Reuter and van Vliet, 2013). The Cj1491-92c two-component system has a PAS domain-containing histidine kinase, and controls expression of a gluconate dehydrogenase, and other proteins involved in heme or iron acquisition, and respiration (Luethy et al., 2015). Based on the Pfam classification of domains, all these PAS domains fall into the PAS9 family. These particular domains are found linked to a wide variety of output domains and of all the PAS families, exhibit the greatest number of architectures (Supplementary Figure 1). Further analysis of the C. jejuni NCTC 11168 genome revealed a protein that contained a PAS6 (YheO-like) domain linked to a helix-turn-helix domain, Cj1387c (Figure 1A). In contrast to the PAS domains found in the other C. jejuni signaling proteins, this member of the PAS clan is only found in prokaryotes and is represented by only five different architectures (Supplementary Figure 1) with the PAS6-Helix-turn-Helix configuration being the most abundant configuration (1816 sequences). Thus, this domain may have a specialized role distinct from signal sensing.
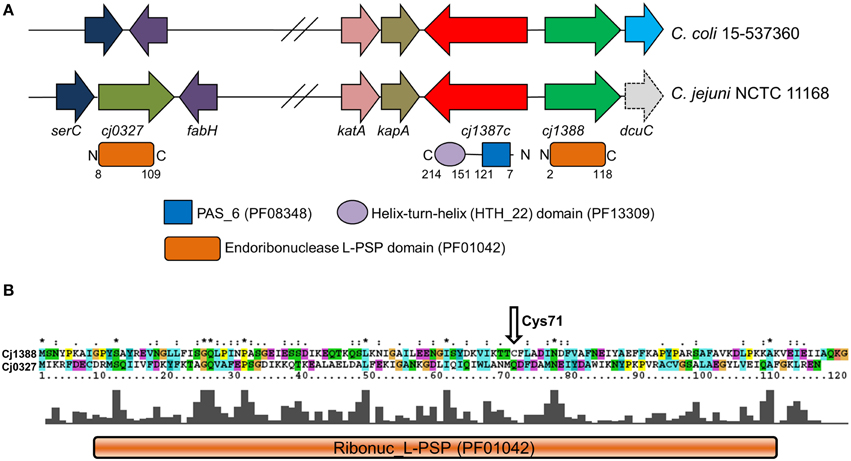
Figure 1. Locus and primary sequence analysis of the cj1387c-cj1388-cj0327 genes and encoded products. (A) Diagram showing the genetic organization of the cj0327 and cj1387c-cj1388 genes in the C. jejuni and C. coli genomes. The gene serC encodes phosphoserine aminotransferase, fabH encodes 3-oxoacyl-[acyl-carrier-protein] synthase, and dcuC encodes a C4-dicarboxylate transporter protein (dcuC in C. jejuni NCTC 11168 is a pseudogene, indicated by dashed lines). (B) Sequence alignment of Cj1388 and Cj0327. The position of the unique cysteine residue in Cj1388 is shown. “*” indicates positions which have a single, fully conserved residue. “:” indicates conservation amongst similar groups of amino acids. A full explanation of the ClustalX output can be found at http://www.clustal.org/download/clustalx_help.html.
Inactivation of cj1387c Results in a Flagella-mediated, Tightly Connected “Cell-train” Morphotype, Which Does Not Disrupt Motility
To investigate the role of Cj1387c in C. jejuni, the cj1387c gene was inactivated by insertion of an antibiotic resistance gene. The resulting strain was viable, suggesting that the Cj1387c protein does not encode an essential function, and had a wild-type growth phenotype (data not shown). When examined under light microscopy using the Ryu stain to visualize flagella, the Δcj1387c cells were observed to form cell chains (“trains”) of up to four cells (Figure 2), with cells linked by their flagella. These cell chains could not be disrupted by vortexing for 2 min (Figure 2D) or extrusion through a 21 G 0.8 mm needle.
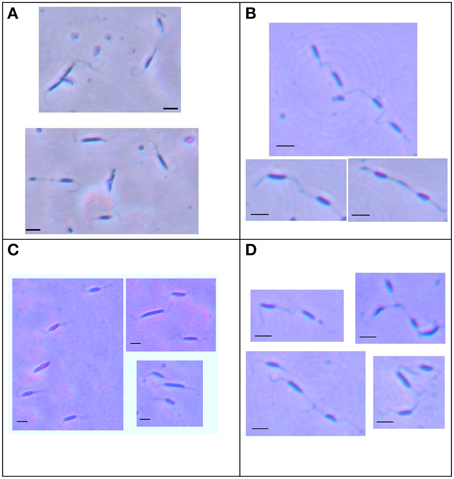
Figure 2. The cj1387c strain forms cell chains when grown in liquid broth. The wild-type (A), Δcj1387c strain (B), and cj1387c::cj1387* strain (C) were grown overnight in Brucella broth under microaerobic conditions at 37°C, and mounted directly on a twin-frost microscope slide. The Δcj1387c strain was also subject to vortexing for 2 min before mounting (D). Fresh Ryu stain was applied to the coverslip and cells photographed at × 100 magnification. Scale bar = 2 microns.
The role of flagella and the effect of the cell-train morphotype on motility were investigated by inactivation of cj1387c in an aflagellate ΔflaAB strain (Reuter and van Vliet, 2013), and the effect of flagellar rotation was investigated by inactivation of the pflA gene (cj1565c) in the Δcj1387c background, which is known to result in non-functional “paralysed” flagella (Yao et al., 1994). The Δcj1387c mutant displayed no defects in swimming motility when using light microscopy, and cell “trains,” containing two or three cells, were also motile (see Supplementary Video 1). Interestingly, these cell “trains” appeared to contain a dominant cell, leading the swimming cells, as gross directional changes were not observed. Swarming motility, as demonstrated using agar swarm plates, was also not significantly different from the wild-type (Figure 3). In contrast, the Δcj1387c-flaAB double mutant was not motile, and halo size was less than 10% of the wild-type halo on swarm plates. The Δcj1387c-flaAB cells showed no flagella or cell-cell contacts (Supplementary Figure 2), suggesting it is the flagella that are required for the cell-cell contacts observed in the Δcj1387c mutant. The ΔpflA-cj1387c double mutant possessed flagella but swarming motility was similar to the non-motile Δcj1387c-flaAB strain (Figure 3). The ΔpflA-cj1387c double mutant exhibited “cell-trains” observed in the single Δcj1387c mutant (Supplementary Figure 3). Taken together, this suggests that the “cell-train” phenotype, caused by inactivation of the cj1387c gene, is a result of interactions between flagella, but independent of a functional rotating flagellum.
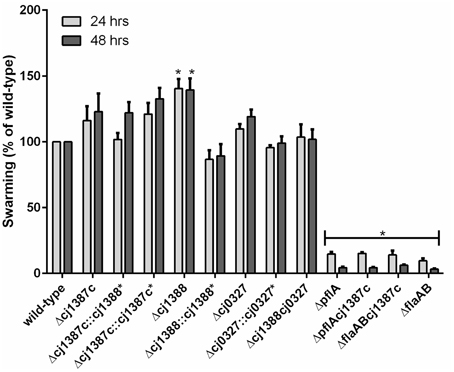
Figure 3. Inactivation of the cj1387c, cj1388, and cj0327 genes does not decrease swarming motility. Brucella broth supplemented with 0.4% agar and 0.01% TTC were inoculated with 5 μl of overnight culture and incubated in microaerobic conditions at 37°C. Halo formation was measured after 24 and 48 h and expressed as a percentage of the wild-type. Results are the mean from at least three biological replicates. An asterisk denotes statistically significant results, based on a one-sample t-test.
Cj1387c is a Repressor of the Downstream cj1388 Gene
The Cj1387c protein contains a helix-turn-helix domain (Figure 1A), suggesting that this protein may bind DNA, and therefore have some regulatory function. Hence the Δcj1387c mutant was compared to the wild-type strain using proteomics on two-dimensional gels. Only a single protein, identified as Cj1388, consistently showed differential expression when comparing the wild-type and Δcj1387c mutant (Figure 4). This protein was observed in both the 6 and 12 h samples of the Δcj1387c mutant, suggesting that Cj1387c represses cj1388 transcription.
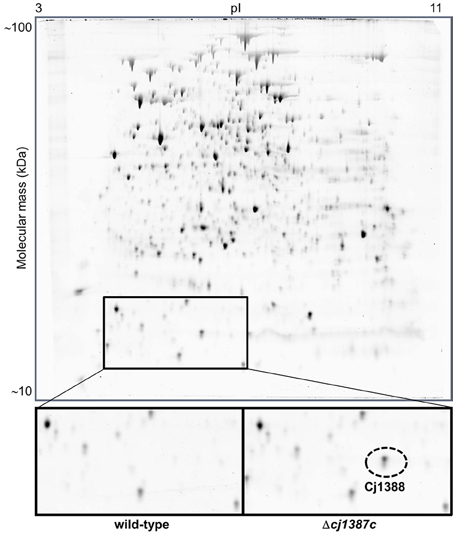
Figure 4. Inactivation of cj1387c results in increased expressed of Cj1388. The wild-type strain and the isogenic cj1387c mutant were grown for 12 h in Brucella medium, and total protein was separated by two-dimensional (2D) protein gel electrophoresis. A single protein difference was detected, which was identified as Cj1388. A representative 2D gel is shown, with the region containing Cj1388 magnified. The identity of Cj1388 was confirmed by excision of the protein fragment, trypsin digestion and mass spectroscopy. Cj1388 was identified using Mascot.
The Cj1388 protein comprises a single Endoribonuclease L-PSP (liver perchloric acid-soluble protein) domain (PF01042), see Figure 1, a domain that is predicted to inhibit protein synthesis via degradation of messenger RNA (Morishita et al., 1999). A search of the C. jejuni NCTC 11168 genome revealed the presence of a further Endoribonuclease L-PSP domain protein, Cj0327, which has 34% identity to Cj1388 (Figure 1B). The cj0327 gene is not located adjacent to a putative regulatory protein, but is downstream of the SerC protein (phosphoserine aminotransferase) involved in serine biosynthesis (Figure 1A). A BLAST search of 75 C. jejuni and 45 C. coli genomes from the PATRIC database (either complete or WGS) revealed that Cj1387c, Cj1388 and Cj0327 were highly conserved in C. jejuni (74/75, 75/75, and 71/75 respectively), but that in C. coli, homologs of Cj0327 were completely absent, while the Cj1387c-1388 system is well conserved. A comprehensive search using the String database, using cj1387c as a query, showed that in organisms containing a cj1387c ortholog, while the genetic arrangement is varied, the cj1388 ortholog is always either directly adjacent to the cj1387c ortholog, or separated by a single gene (Supplementary Figure 4).
Cj1388 and Cj0327 are Non-essential and are not Required for Motility
Insertional inactivation was used to determine the role of Cj1388 and Cj0327 proteins in C. jejuni. Mutants were created in the wild-type background. To make a double Δcj1388cj0327 mutant, the cj0327 disruption construct (Cmr) was transformed into the Δcj1388 (Kmr) background. Although cj0327 has been proposed to be an essential gene (Stintzi et al., 2005), the cj0327 mutants were viable and showed wild-type growth phenotypes. Ryu staining showed the presence of bi-polar flagella, as in the wild-type strain, and all mutants were motile (Figure 3). The Δcj1388 mutant had a significantly larger halo than the wild-type strain after both 24 and 48 h, whereas swarming motility of the Δcj0327 and Δcj1388cj0327 strains was not different from wild-type.
Cj1387c, Cj1388, and Cj0327 Contribute to Autoagglutination
Autoagglutination (AAG) was used to quantify flagella-mediated cell-cell contacts as observed in the Δcj1387c strain, and assess the possible roles of Cj1388 and Cj0327 in this phenotype. C. jejuni is known to autoagglutinate, and this activity is known to be dependent on the flagella (Golden and Acheson, 2002). Overnight AAG (i.e., 24 h incubation, hereafter referred to as the final AAG measurement) was significantly lower in the Δcj1387c strain compared to the wild-type: 10% of the starting A600 compared to 20% in the wild-type (Figure 5A). The AAG phenotype could be restored by expression of cj1387c from the constitutive fdxA promoter in pseudogene cj0046 (Supplementary Figure 5). AAG was significantly reduced in a strain containing two copies of Cj1387c (WT::cj1387c*) compared to the wild-type. This may be explained by the low level of cj1388 transcript that was detected by RNA-seq analysis (Porcelli et al., 2013; Handley et al., 2015), with a RPKM value of 1.97. We hypothesize that overexpression of cj1387c from two gene copies might therefore result in full repression of cj1388 transcript, resulting in decreased AAG compared to the wild-type. The T1∕2, describing the steepest part of an 8 h AAG assay, was 60% of the wild-type in the Δcj1387c strain (Figure 5B). The increased AAG phenotype of the Δcj1387c strain is consistent with the microscopy observations showing flagella-mediated cell-cell contacts.
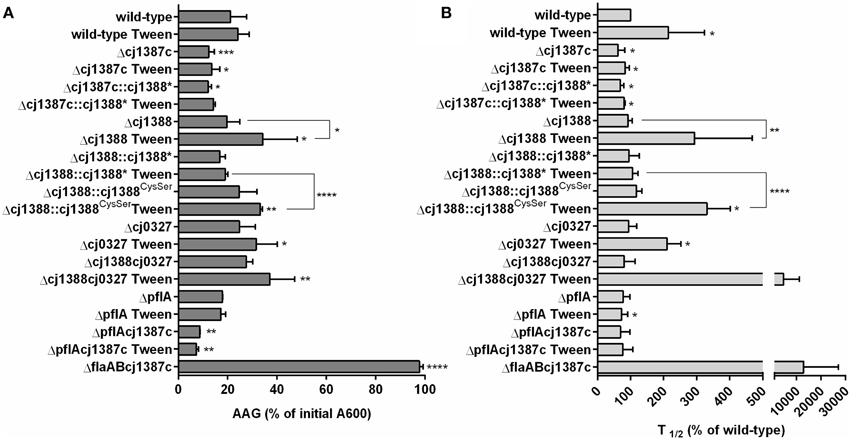
Figure 5. Inactivation of cj1387c increases autoagglutination, while inactivation of cj1388 and cj0327 decreases autoagglutination in media supplemented with Tween-20. AAG of cultures grown overnight in Brucella broth was measured over an 8 h time period and after 24 h at room temperature in air. The percentage of the initial A600 following 24 h (A) and t1∕2 of the steepest part of the AAG curve over the initial 8 h was calculated and expressed as a percentage of the wild-type (B). Results show the mean of at least three biological replicates. Significantly different results, determined using either an unpaired t-test (A) or one-sample t-test (B) are indicated by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
To assess the strength and nature of the flagella-flagella interactions, AAG assays were repeated in media supplemented with sub-inhibitory concentrations of various detergents (Svensson et al., 2009). Assays in media supplemented with either 0.00025% SDS or 0.05% Triton X-100 had no effect on either AAG rate or final AAG levels (data not shown). However, AAG in media supplemented with 0.002% Tween-20 did affect AAG in a strain-dependent manner (Figure 5). Tween-20 marginally slowed the AAG rate of the wild-type, and this effect could be increased by increasing the level of Tween-20 (data not shown). The Δcj1387c strain showed no response to Tween-20, either in rate or final AAG. In Tween 20-supplemented media, the rate of AAG of the Δcj1388 strain was significantly slower than the wild-type (almost 300% of that observed in the wild-type) and final AAG was around 34% of the initial A600. Complementation of the cj1388 mutant with constitutively expressed cj1388 restored the wild-type phenotype. The final AAG measurements of the Δcj0327 and Δcj1388-cj0327 strains were significantly different from the wild-type in Tween 20-supplemented media. Combining both the cj1388 and cj0327 mutations had a dramatic effect on AAG rate: the Δcj1388-cj0327 double mutant behaved essentially as a Δcj1387c-flaAB double mutant over the course of an 8 h AAG assay. AAG in the Δcj1387c-flaAB strain is totally abolished due to absence of flagella. AAG of the ΔpflA strain was similar to the wild-type, suggesting that flagella rotation is not required for AAG. Like in the Δcj1387c strain, AAG in the ΔpflA-cj1387c double mutant was increased, relative to the wild-type. The ΔpflA-cj1387c double mutant actually showed a significantly lower final AAG value compared to the single Δcj1387c strain.
Cj1388 Forms a Disulfide Linked Dimer
Examination of the Cj1388 primary sequence revealed a single cysteine residue at position 71 (Figure 1B). Reasoning that this residue may be involved in disulfide bridge formation, two over-expression constructs were made. Firstly, cj1388 was cloned into pET28a to make an N-terminal translationally-fused his-tagged protein. The cysteine codon was then substituted for a serine codon (TGC to AGC) using site-directed mutagenesis. Both constructs were transformed into E. coli strain BL21 (DE3) and protein expression was induced with IPTG. SDS-PAGE analysis showed that both expression strains resulted in the clear over-expression of a soluble protein of the expected size. Repeating SDS-PAGE analysis of the Cj1388H6 fractions in non-reducing conditions showed the appearance of a 44 kDa protein band, calculated to be 2.4 times the size of the purified protein observed in reducing conditions (Figure 6). The identity of both the higher and lower molecular protein bands was confirmed to be Cj1388 using LC-MS-MS ion search by Mascot with full coverage of the predicted protein sequence when using a chymotrypsin digest for the reduced and alkylated protein. Searching the upper band digest for the presence of the disulfide-linked peptide using the Mass Matrix program disappointingly failed to identify the crosslinked peptide species. SDS-PAGE analysis of the Cj1388Cys71Ser fractions in non-reducing conditions did not show the 44 kDa fragment. Thus, Cj1388 can form a dimer where subunits are connected via a disulfide bridge. To test if dimer formation is required for function, the cj1388 complementation construct was subjected to site-directed mutagenesis to substitute Cys71 for serine, as in the expression construct. This construct was then transformed into the Δcj1388 strain to make strain cj1388::cj1388Cys71Ser. While the cj1388 complement strain can rescue the Tween20 phenotype of the Δcj1388 mutant, the cj1388::cj1388Cys71Ser cannot, suggesting that dimer formation may be required for function of Cj1388 in modulating flagella interactions in media containing Tween 20 (Figure 5).
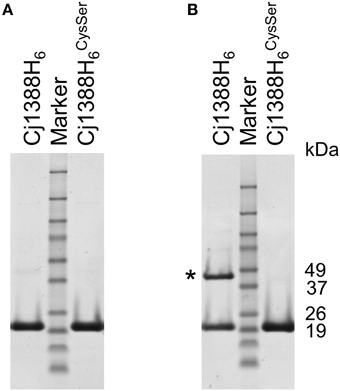
Figure 6. Cj1388 forms a dimer via a disulfide bridge. Cj1388H6 or Cj1388 HCysSer6 were cloned into pET28a and induced with IPTG. SDS-PAGE in non-reducing conditions (B) shows the appearance of a protein fragment twice the size of the purified protein (marked with an asterisk) not visible in reducing conditions (A) or in samples containing Cj1388HCysSer6.
Given that Cj1388 is predicted to consist of a single endoribonuclease domain, its ability to degrade nucleic acid was tested. Purified Cj1388 did not show any nuclease activity on C. jejuni ribosomal RNA or DNA and lysates from IPTG-induced cultures expressing Cj1388 were negative on DNase indicator agar plates (Gaasbeek et al., 2010), data not shown.
Cj1388 and Cj0327 have a Role in Virulence in the Galleria mellonella Model
Bacterial flagella are known to be highly antigenic (Hayashi et al., 2001; Smith et al., 2003), and motility in C. jejuni is a known virulence factor (Lee et al., 1986; Szymanski et al., 1995; Carrillo et al., 2004). Given that the Cj1387c-1388 system affects AAG, which is dependent on functioning flagella, we sought to assess the role of Cj1388 and Cj0327 on virulence using the Galleria mellonella virulence model (Champion et al., 2010). The Δcj1388, Δcj0327, and Δcj1388-cj0327 strains showed attenuation in Galleria larvae, with the Δcj1388 strain showing the greatest level of attenuation (Figure 7). The virulence phenotype of the Δcj1388 and Δcj0327 strains could be restored by complementation with their cognate gene.
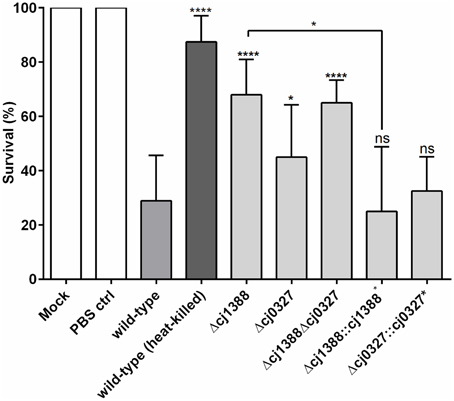
Figure 7. Strains lacking Cj1387c, Cj1388, and/or Cj0327 are attenuated in the Galleria wax moth larvae model. Galleria larvae were injected with 10 μl of cells (1 × 106 cells) harvested from Skirrow plates and washed in PBS. Mock injection, injection with PBS, and injection with heat-killed C. jejuni were included as negative controls. Results show the mean of at least four biological replicates, each containing 10 larvae. Significantly different results, determined using unpaired t-tests, are shown using asterisks (*p < 0.05, ****p < 0.0001).
Discussion
The regulatory cascade controlling expression of flagellar genes in Campylobacter is increasingly well understood; however, there are fewer insights into the regulatory control of post-translational modification of flagella. The paradigm of sensing the environment followed by modulating behavior, enzyme activity, or gene expression to adapt to those changes is well established. Thus, it is not surprising that post-translational control of flagella should also be subject to environmental control. In this study we have identified a new PAS-domain-containing repressor protein, which affects flagella-flagella interactions resulting in higher levels of autoagglutination. Proteomics analysis suggests that this regulatory protein represses expression of the adjacent downstream gene encoding a dimeric effector protein Cj1388. This protein, in concert with a homolog encoded in a different region of the chromosome, affects autoagglutination and may play a role in virulence. Homologs of Cj1387c and Cj1388 in other organisms are always found linked suggesting that this system always functions in concert.
PAS domains are known to sense diverse environmental signals and participate in dimerization (Ma et al., 2008; Slavny et al., 2010). Unlike the PAS domain found in other Campylobacter proteins, the (YheO-like) PAS domain found in Cj1387c is only found in bacteria and exhibits a very limited architectural diversity. Very little is known about this member of the PAS family; the sensed stimuli or signal, if any, for Cj1387c is not known. The Cj1388 effector protein appears to be a dimer, formed via a disulfide bridge between two monomers. This suggests that there could be a link to redox control; indeed, redox sensing in PAS domain sensors is well established (Hill et al., 1996; Xie et al., 2010; Sousa et al., 2013).
Deleting Δcj1387c resulted in a clear “cell-train” morphology. Ryu flagella staining suggested that the cells were connected via flagella and accordingly, combining the cj1387c disruption with a flaAB mutant abolished cell chain formation. Flagella rotation, however, is dispensable for cell chain formation as the cj1387c mutant in a paralyzed flagella background showed the “cell-train” morphology and rapid AAG. The paralyzed flagella mutant in strain 81–176 is known to show normal AAG (Guerry et al., 2006) and we confirm this observation for the NCTC 11168 background. Interaction between flagella during AAG is therefore most likely to occur via a physical interaction between flagella filaments. Interestingly, cell chains are still motile, and chains of up to three cells could be observed with rapid darting motility. Given that flagella rotation is governed by CheY and subject to chemotactic control, this raises the intriguing question as to which cells in a cell-chain determine direction and how does directional dominance arise? The role of these cell chains in the normal physiology and survival of C. jejuni is not known. The flagella-flagella interactions may merely be a consequence of disrupting the normal flagella glycan decoration pathways, which are known to be essential for flagella assembly and function (Goon et al., 2003; Asakura et al., 2013). Clearly, disrupting cj1387c-88 does not abolish glycosylation, as the cells still possess flagella and are motile and no gross differences in glycoprotein could be detected by Alcian Blue staining. Changes in glycosylation decoration of the flagella are however the most likely explanation for the changes in AAG observed in this study. AAG is known to be important for microcolony formation at the initiation of biofilm formation (Cole et al., 2004; Boddey et al., 2006; Moreira et al., 2006), and in C. jejuni, flagella are required for robust biofilm formation (Joshua et al., 2006; Reuter et al., 2010). The biofilm phenotype of the Δcj1387c and Δcj1388 strains was not different from the wild-type as assessed by microscopy and crystal violet staining.
Both Cj1388 and Cj0327 are small (13 kDa) proteins consisting of single endoribonuclease L-PSP domain. This domain was first characterized as liver perchloric acid-soluble protein (hence L-PSP) from Rat liver and shown to inhibit protein synthesis (cell free system using rabbit reticulocyte lysate) and degrade in vitro transcribed mRNA (Oka et al., 1995; Morishita et al., 1999). Non-reducing SDS-PAGE and substitution of the single cysteine residue in Cj1388 demonstrated that this protein likely forms a disulfide stabilized dimer. A complement construct carrying the Cys71Ser substitution also failed to complement the AAG-Tween phenotype, suggesting that dimerization is required for function. If indeed these proteins do function to degrade mRNA, this suggests that post-transcriptional regulation may play a role in regulating flagella function. While both Cj1388 and Cj0327 score as significant for a Ribonuclease L-PSP domain, they are only 36% identical. Cj0327 has two cysteine residues, and it is unknown if these contribute to inter- or intra-domain disulfide bridge formation. Based on the levels of AAG in Tween-supplemented media, disrupting either gene has the same consequence (reduced AAG) and this phenotype is most extreme when both mutations are combined. Degenerate function has been seen previously in C. jejuni: the Cet energy taxis system can function with either CetB and CetC (Reuter and van Vliet, 2013) and both FlaA and FlaB flagella proteins are incorporated into the flagella filament (Logan et al., 1987; Guerry et al., 1991). Moreover, C. jejuni strain RM1221 contains three extra-cellular DNases (Gaasbeek et al., 2010). The presence of two Ribonuclease L-PSP proteins in C. jejuni is an example of functional redundancy, although C. coli has only the single cj1387c-linked gene. Also, based on the String analysis, Cj0327 homologs are much less common than the linked cj1387c-88 system.
Disrupting both cj1388 and cj0327 genes resulted in significant attenuation in the Galleria wax moth larvae model, and virulence in this model was restored by constitutive in trans expression of Cj1388. It is well established that changes in glycosylation and AAG affect adhesion to and invasion of human intestinal cells (Guerry et al., 2006). Although flagella from Campylobacter and Helicobacter lack TLR5-recognition sites (Galkin et al., 2008), the sheer size of the flagella suggests that it will be highly antigenic. As part of the insect immune system, N-acetylglucosamine-specific lectins are known to facilitate phagocytosis by the haemocyte cells (insect phagocytes) (Kavanagh and Reeves, 2004) and this process is further augmented by the action of lysozyme, which degrades bacterial peptidoglycan exposing lectin-specific molecules such as teichoic acid and lipomannans (Wilson and Ratcliffe, 2000). Therefore, changes in surface polysaccharides, on either the flagella or cell might be expected to influence virulence in an insect model. The Cj1388-Cj0327 proteins may therefore represent hitherto unknown virulence determinants.
In summary, we have identified a novel system that affects flagella-flagella interactions. We propose to designate the Cj1387c repressor as CfiR (Campylobacter flagella Interaction Regulator) and name the effectors as CfiP (Cj1388) and CfiQ (Cj0327). Proteomics analysis shows that CfiP is repressed by CfiR. We present a model whereby CfiPQ control flagella glycan decoration, which influences cell aggregation and virulence (Figure 8). Deletion of CfiPQ results in attenuation in the Galleria model and a disruption of flagella-mediated autoagglutination by the surfactant Tween-20; derepressed expression of disulfide-linked CfiP dimers results in tightly connected “cell-trains” mediated by the flagella, and increased aggregation. CfiRP homologs are found in other bacterial species, and always linked on the chromosome. Therefore, this system, acting in concert, may control glycan modification in other bacteria, which may influence virulence and immuno-modulation in pathogens such as S. pyogenes, S. enterica, V. parahaemolyticus, and Y. pestis.
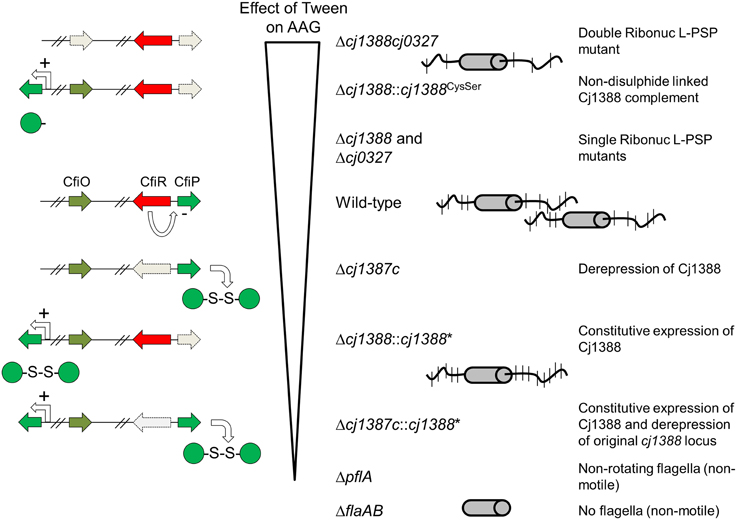
Figure 8. Model showing the effect of disrupting the cj1387c-cj1388-cj0327 genes on autoagglutination (AAG). Strains are shown ranked in the order in which AAG is decreased in media supplemented with Tween 20 compared to un-supplemented media. Cj1388 expression is proposed to be de-repressed when cj1387c is inactivated resulting in greater AAG, which cannot be disrupted by Tween. Deleting both cj1388 and/or cj0327 results in a Tween-dependent decrease in AAG. Complementation by Cj1388 rescues this phenotype, and this activity is dependent on a single cysteine residue in Cj1388, which forms a dis- sulfide-linked dimer in vitro. AAG is dependent on flagella glycan decoration, suggesting that Cj1388 and Cj0327 have a role in mediating glycan modification of flagella.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Bruce Pearson for the gift of plasmids pCj1387::KanC1 and pCj1387::CatC1, Duncan Gaskin for the gift of plasmid pUCΔpflA, and members of the IFR Campylobacter research group for helpful discussions. We would also like to thank Maddy Houchen for microbiology media support. We gratefully acknowledge the support of the Biotechnology and Biological Sciences Research Council (BBSRC) via the BBSRC Institute Strategic Programme (BB/J004529/1). Paula M. Periago thanks the Spanish Ministerio de Ciencia e Innovación for the grant of the Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de I-D+i 2008–2011. Helen Brown was funded from BBSRC CASE studentship (BB/I15321/1) with CASE funding from Campden BRI.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2015.00770
References
Asakura, H., Hashii, N., Uema, M., Kawasaki, N., Sugita-Konishi, Y., Igimi, S., et al. (2013). Campylobacter jejuni pdxA affects flagellum-mediated motility to alter host colonization. PLoS ONE 8:e70418. doi: 10.1371/journal.pone.0070418
Ash, A., Mulholland, F., Burnett, G. R., and Wilde, P. J. (2014). Structural and compositional changes in the salivary pellicle induced upon exposure to SDS and STP. Biofouling 30, 1183–1197. doi: 10.1080/08927014.2014.977268
Barrero-Tobon, A. M., and Hendrixson, D. R. (2012). Identification and analysis of flagellar coexpressed determinants (Feds) of Campylobacter jejuni involved in colonization. Mol. Microbiol. 84, 352–369. doi: 10.1111/j.1365-2958.2012.08027.x
Barrero-Tobon, A. M., and Hendrixson, D. R. (2014). Flagellar biosynthesis exerts temporal regulation of secretion of specific Campylobacter jejuni colonization and virulence determinants. Mol. Microbiol. 93, 957–974. doi: 10.1111/mmi.12711
Bereswill, S., Fischer, A., Plickert, R., Haag, L. M., Otto, B., Kuhl, A. A., et al. (2011). Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS ONE 6:e20953. doi: 10.1371/journal.pone.0020953
Boddey, J. A., Flegg, C. P., Day, C. J., Beacham, I. R., and Peak, I. R. (2006). Temperature-regulated microcolony formation by Burkholderia pseudomallei requires pilA and enhances association with cultured human cells. Infect. Immun. 74, 5374–5381. doi: 10.1128/IAI.00569-06
Boll, J. M., and Hendrixson, D. R. (2013). A regulatory checkpoint during flagellar biogenesis in Campylobacter jejuni initiates signal transduction to activate transcription of flagellar genes. MBio 4, e00432–e00413. doi: 10.1128/mBio.00432-13
Carrillo, C. D., Taboada, E., Nash, J. H., Lanthier, P., Kelly, J., Lau, P. C., et al. (2004). Genome-wide expression analyses of Campylobacter jejuni NCTC11168 reveals coordinate regulation of motility and virulence by flhA. J. Biol. Chem. 279, 20327–20338. doi: 10.1074/jbc.M401134200
Chambers, M. C., Maclean, B., Burke, R., Amodei, D., Ruderman, D. L., Neumann, S., et al. (2012). A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920. doi: 10.1038/nbt.2377
Champion, O. L., Karlyshev, A. V., Senior, N. J., Woodward, M., La Ragione, R., Howard, S. L., et al. (2010). Insect infection model for Campylobacter jejuni reveals that O-methyl phosphoramidate has insecticidal activity. J. Infect. Dis. 201, 776–782. doi: 10.1086/650494
Chen, S., Beeby, M., Murphy, G. E., Leadbetter, J. R., Hendrixson, D. R., Briegel, A., et al. (2011). Structural diversity of bacterial flagellar motors. EMBO J. 30, 2972–2981. doi: 10.1038/emboj.2011.186
Cole, S. P., Harwood, J., Lee, R., She, R., and Guiney, D. G. (2004). Characterization of monospecies biofilm formation by Helicobacter pylori. J. Bacteriol. 186, 3124–3132. doi: 10.1128/JB.186.10.3124-3132.2004
Elliott, K. T., and Dirita, V. J. (2008). Characterization of CetA and CetB, a bipartite energy taxis system in Campylobacter jejuni. Mol. Microbiol. 69, 1091–1103. doi: 10.1111/j.1365-2958.2008.06357.x
Finn, R. D., Bateman, A., Clements, J., Coggill, P., Eberhardt, R. Y., Eddy, S. R., et al. (2014). Pfam: the protein families database. Nucleic Acids Res. 42, D222–D230. doi: 10.1093/nar/gkt1223
Franceschini, A., Szklarczyk, D., Frankild, S., Kuhn, M., Simonovic, M., Roth, A., et al. (2013). STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41, D808–D815. doi: 10.1093/nar/gks1094
Gaasbeek, E. J., Wagenaar, J. A., Guilhabert, M. R., van Putten, J. P., Parker, C. T., and van der Wal, F. J. (2010). Nucleases encoded by the integrated elements CJIE2 and CJIE4 inhibit natural transformation of Campylobacter jejuni. J. Bacteriol. 192, 936–941. doi: 10.1128/jb.00867-09
Galkin, V. E., Yu, X., Bielnicki, J., Heuser, J., Ewing, C. P., Guerry, P., et al. (2008). Divergence of quaternary structures among bacterial flagellar filaments. Science 320, 382–385. doi: 10.1126/science.1155307
Golden, N. J., and Acheson, D. W. (2002). Identification of motility and autoagglutination Campylobacter jejuni mutants by random transposon mutagenesis. Infect. Immun. 70, 1761–1771. doi: 10.1128/IAI.70.4.1761-1771.2002
Goon, S., Kelly, J. F., Logan, S. M., Ewing, C. P., and Guerry, P. (2003). Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol. Microbiol. 50, 659–671. doi: 10.1046/j.1365-2958.2003.03725.x
Guerry, P., Alm, R. A., Power, M. E., Logan, S. M., and Trust, T. J. (1991). Role of two flagellin genes in Campylobacter motility. J. Bacteriol. 173, 4757–4764.
Guerry, P., Ewing, C. P., Schirm, M., Lorenzo, M., Kelly, J., Pattarini, D., et al. (2006). Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. 60, 299–311. doi: 10.1111/j.1365-2958.2006.05100.x
Handley, R. A., Mulholland, F., Reuter, M., Ramachandran, V. K., Musk, H., Clissold, L., et al. (2015). PerR controls oxidative stress defence and aerotolerance, but not motility-associated phenotypes of Campylobacter jejuni. Microbiology. doi: 10.1099/mic.0.000109. [Epub ahead of print].
Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., et al. (2001). The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103. doi: 10.1038/35074106
Heimbrook, M. E., Wang, W. L., and Campbell, G. (1989). Staining bacterial flagella easily. J. Clin. Microbiol. 27, 2612–2615.
Hendrixson, D. R. (2006). A phase-variable mechanism controlling the Campylobacter jejuni FlgR response regulator influences commensalism. Mol. Microbiol. 61, 1646–1659. doi: 10.1111/j.1365-2958.2006.05336.x
Hendrixson, D. R., Akerley, B. J., and DiRita, V. J. (2001). Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol. Microbiol. 40, 214–224. doi: 10.1046/j.1365-2958.2001.02376.x
Hendrixson, D. R., and DiRita, V. J. (2003). Transcription of sigma54-dependent but not sigma28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol. Microbiol. 50, 687–702. doi: 10.1046/j.1365-2958.2003.03731.x
Hill, S., Austin, S., Eydmann, T., Jones, T., and Dixon, R. (1996). Azotobacter vinelandii NIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-sensitive switch. Proc. Natl. Acad. Sci. USA. 93, 2143–2148.
Jones, P., Binns, D., Chang, H. Y., Fraser, M., Li, W., McAnulla, C., et al. (2014). InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240. doi: 10.1093/bioinformatics/btu031
Joshua, G. W., Guthrie-Irons, C., Karlyshev, A. V., and Wren, B. W. (2006). Biofilm formation in Campylobacter jejuni. Microbiology 152, 387–396. doi: 10.1099/mic.0.28358-0
Kavanagh, K., and Reeves, E. P. (2004). Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 28, 101–112. doi: 10.1016/j.femsre.2003.09.002
Konkel, M. E., Klena, J. D., Rivera-Amill, V., Monteville, M. R., Biswas, D., Raphael, B., et al. (2004). Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 186, 3296–3303. doi: 10.1128/JB.186.11.3296-3303.2004
Le, M. T., Porcelli, I., Weight, C. M., Gaskin, D. J., Carding, S. R., and van Vliet, A. H. (2012). Acid-shock of Campylobacter jejuni induces flagellar gene expression and host cell invasion. Eur. J. Microbiol. Immunol. (Bp). 2, 12–19. doi: 10.1556/EuJMI.2.2012.1.3
Lee, A., O'Rourke, J. L., Barrington, P. J., and Trust, T. J. (1986). Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect. Immun. 51, 536–546.
Lertsethtakarn, P., Ottemann, K. M., and Hendrixson, D. R. (2011). Motility and chemotaxis in Campylobacter and Helicobacter. Annu. Rev. Microbiol. 65, 389–410. doi: 10.1146/annurev-micro-090110-102908
Logan, S. M., Harris, L. A., and Trust, T. J. (1987). Isolation and characterization of Campylobacter flagellins. J. Bacteriol. 169, 5072–5077.
Logan, S. M., Kelly, J. F., Thibault, P., Ewing, C. P., and Guerry, P. (2002). Structural heterogeneity of carbohydrate modifications affects serospecificity of Campylobacter flagellins. Mol. Microbiol. 46, 587–597. doi: 10.1046/j.1365-2958.2002.03185.x
Luethy, P. M., Huynh, S., Parker, C. T., and Hendrixson, D. R. (2015). Analysis of the Activity and Regulon of the Two-component Regulatory System Composed by Cjj1484 and Cjj1483 of Campylobacter jejuni. J. Bacteriol. 197, 1592–1605. doi: 10.1128/JB.02564-14
Ma, X., Sayed, N., Baskaran, P., Beuve, A., and van den Akker, F. (2008). PAS-mediated dimerization of soluble guanylyl cyclase revealed by signal transduction histidine kinase domain crystal structure. J. Biol. Chem. 283, 1167–1178. doi: 10.1074/jbc.M706218200
Marchant, J., Wren, B., and Ketley, J. (2002). Exploiting genome sequence: predictions for mechanisms of Campylobacter chemotaxis. Trends Microbiol. 10, 155–159. doi: 10.1016/S0966-842X(02)02323-5
McNally, D. J., Lamoureux, M. P., Karlyshev, A. V., Fiori, L. M., Li, J., Thacker, G., et al. (2007). Commonality and biosynthesis of the O-methyl phosphoramidate capsule modification in Campylobacter jejuni. J. Biol. Chem. 282, 28566–28576. doi: 10.1074/jbc.M704413200
Misawa, N., and Blaser, M. J. (2000). Detection and characterization of autoagglutination activity by Campylobacter jejuni. Infect. Immun. 68, 6168–6175. doi: 10.1128/IAI.68.11.6168-6175.2000
Moglich, A., Ayers, R. A., and Moffat, K. (2009). Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 17, 1282–1294. doi: 10.1016/j.str.2009.08.011
Moreira, C. G., Palmer, K., Whiteley, M., Sircili, M. P., Trabulsi, L. R., Castro, A. F., et al. (2006). Bundle-forming pili and EspA are involved in biofilm formation by enteropathogenic Escherichia coli. J. Bacteriol. 188, 3952–3961. doi: 10.1128/JB.00177-06
Morishita, R., Kawagoshi, A., Sawasaki, T., Madin, K., Ogasawara, T., Oka, T., et al. (1999). Ribonuclease activity of rat liver perchloric acid-soluble protein, a potent inhibitor of protein synthesis. J. Biol. Chem. 274, 20688–20692.
Neal-McKinney, J. M., and Konkel, M. E. (2012). The Campylobacter jejuni CiaC virulence protein is secreted from the flagellum and delivered to the cytosol of host cells. Front. Cell. Infect. Microbiol. 2:31. doi: 10.3389/fcimb.2012.00031
Nuijten, P. J., van Asten, F. J., Gaastra, W., and van der Zeijst, B. A. (1990). Structural and functional analysis of two Campylobacter jejuni flagellin genes. J. Biol. Chem. 265, 17798–17804.
Oka, T., Tsuji, H., Noda, C., Sakai, K., Hong, Y. M., Suzuki, I., et al. (1995). Isolation and characterization of a novel perchloric acid-soluble protein inhibiting cell-free protein synthesis. J. Biol. Chem. 270, 30060–30067.
Parkhill, J., Wren, B. W., Mungall, K., Ketley, J. M., Churcher, C., Basham, D., et al. (2000). The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403, 665–668. doi: 10.1038/35001088
Poly, F., Ewing, C., Goon, S., Hickey, T. E., Rockabrand, D., Majam, G., et al. (2007). Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagellar filament. Infect. Immun. 75, 3859–3867. doi: 10.1128/IAI.00159-07
Porcelli, I., Reuter, M., Pearson, B. M., Wilhelm, T., and van Vliet, A. H. (2013). Parallel evolution of genome structure and transcriptional landscape in the Epsilonproteobacteria. BMC Genomics 14:616. doi: 10.1186/1471-2164-14-616
Raphael, B. H., Pereira, S., Flom, G. A., Zhang, Q., Ketley, J. M., and Konkel, M. E. (2005). The Campylobacter jejuni response regulator, CbrR, modulates sodium deoxycholate resistance and chicken colonization. J. Bacteriol. 187, 3662–3670. doi: 10.1128/JB.187.11.3662-3670.2005
Reuter, M., Mallett, A., Pearson, B. M., and van Vliet, A. H. (2010). Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl. Environ. Microbiol. 76, 2122–2128. doi: 10.1128/AEM.01878-09
Reuter, M., and van Vliet, A. H. (2013). Signal balancing by the CetABC and CetZ chemoreceptors controls energy taxis in Campylobacter jejuni. PLoS ONE 8:e54390. doi: 10.1371/journal.pone.0054390
Shaw, F. L., Mulholland, F., Le Gall, G., Porcelli, I., Hart, D. J., Pearson, B. M., et al. (2012). Selenium-dependent biogenesis of formate dehydrogenase in Campylobacter jejuni is controlled by the fdhTU accessory genes. J. Bacteriol. 194, 3814–3823. doi: 10.1128/JB.06586-11
Slavny, P., Little, R., Salinas, P., Clarke, T. A., and Dixon, R. (2010). Quaternary structure changes in a second Per-Arnt-Sim domain mediate intramolecular redox signal relay in the NifL regulatory protein. Mol. Microbiol. 75, 61–75. doi: 10.1111/j.1365-2958.2009.06956.x
Smith, K. D., Andersen-Nissen, E., Hayashi, F., Strobe, K., Bergman, M. A., Barrett, S. L., et al. (2003). Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4, 1247–1253. doi: 10.1038/ni1011
Song, Y. C., Jin, S., Louie, H., Ng, D., Lau, R., Zhang, Y., et al. (2004). FlaC, a protein of Campylobacter jejuni TGH9011 (ATCC43431) secreted through the flagellar apparatus, binds epithelial cells and influences cell invasion. Mol. Microbiol. 53, 541–553. doi: 10.1111/j.1365-2958.2004.04175.x
Sousa, E. H., Tuckerman, J. R., Gondim, A. C., Gonzalez, G., and Gilles-Gonzalez, M. A. (2013). Signal transduction and phosphoryl transfer by a FixL hybrid kinase with low oxygen affinity: importance of the vicinal PAS domain and receiver aspartate. Biochemistry 52, 456–465. doi: 10.1021/bi300991r
Stintzi, A., Marlow, D., Palyada, K., Naikare, H., Panciera, R., Whitworth, L., et al. (2005). Use of genome-wide expression profiling and mutagenesis to study the intestinal lifestyle of Campylobacter jejuni. Infect. Immun. 73, 1797–1810. doi: 10.1128/IAI.73.3.1797-1810.2005
Svensson, S. L., Davis, L. M., MacKichan, J. K., Allan, B. J., Pajaniappan, M., Thompson, S. A., et al. (2009). The CprS sensor kinase of the zoonotic pathogen Campylobacter jejuni influences biofilm formation and is required for optimal chick colonization. Mol. Microbiol. 71, 253–272. doi: 10.1111/j.1365-2958.2008.06534.x
Szymanski, C. M., King, M., Haardt, M., and Armstrong, G. D. (1995). Campylobacter jejuni motility and invasion of Caco-2 cells. Infect. Immun. 63, 4295–4300.
Takata, T., Fujimoto, S., and Amako, K. (1992). Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect. Immun. 60, 3596–3600.
Tam, C. C., Rodrigues, L. C., Viviani, L., Dodds, J. P., Evans, M. R., Hunter, P. R., et al. (2012). Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 61, 69–77. doi: 10.1136/gut.2011.238386
Taylor, B. L., and Zhulin, I. B. (1999). PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63, 479–506.
Thibault, P., Logan, S. M., Kelly, J. F., Brisson, J. R., Ewing, C. P., Trust, T. J., et al. (2001). Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J. Biol. Chem. 276, 34862–34870. doi: 10.1074/jbc.M104529200
van Vliet, A. H., Wooldridge, K. G., and Ketley, J. M. (1998). Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J. Bacteriol. 180, 5291–5298.
Wattam, A. R., Abraham, D., Dalay, O., Disz, T. L., Driscoll, T., Gabbard, J. L., et al. (2014). PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 42, D581–D591. doi: 10.1093/nar/gkt1099
Wilson, R., and Ratcliffe, N. A. (2000). Effect of lysozyme on the lectin-mediated phagocytosis of Bacillus cereus by haemocytes of the cockroach, Blaberus discoidalis. J. Insect Physiol. 46, 663–670. doi: 10.1016/S0022-1910(99)00154-7
Wosten, M. M., van Dijk, L., Veenendaal, A. K., de Zoete, M. R., Bleumink-Pluijm, N. M., and van Putten, J. P. (2010). Temperature-dependent FlgM/FliA complex formation regulates Campylobacter jejuni flagella length. Mol. Microbiol. 75, 1577–1591. doi: 10.1111/j.1365-2958.2010.07079.x
Wosten, M. M., Wagenaar, J. A., and van Putten, J. P. (2004). The FlgS/FlgR two-component signal transduction system regulates the fla regulon in Campylobacter jejuni. J. Biol. Chem. 279, 16214–16222. doi: 10.1074/jbc.M400357200
Xie, Z., Ulrich, L. E., Zhulin, I. B., and Alexandre, G. (2010). PAS domain containing chemoreceptor couples dynamic changes in metabolism with chemotaxis. Proc. Natl. Acad. Sci. U.S.A. 107, 2235–2240. doi: 10.1073/pnas.0910055107
Yao, R., Burr, D. H., Doig, P., Trust, T. J., Niu, H., and Guerry, P. (1994). Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol. Microbiol. 14, 883–893.
Keywords: Campylobacter, flagella, transcriptional repression, PAS domains
Citation: Reuter M, Periago PM, Mulholland F, Brown HL and van Vliet AHM (2015) A PAS domain-containing regulator controls flagella-flagella interactions in Campylobacter jejuni. Front. Microbiol. 6:770. doi: 10.3389/fmicb.2015.00770
Received: 11 March 2015; Accepted: 14 July 2015;
Published: 30 July 2015.
Edited by:
Dongsheng Zhou, Beijing Institute of Microbiology and Epidemiology, ChinaReviewed by:
Karl Graham Wooldridge, University of Nottingham, UKDennis Linton, University of Manchester, UK
Copyright © 2015 Reuter, Periago, Mulholland, Brown and van Vliet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark Reuter, Institute of Food Research, Norwich Research Park, Colney Lane, Norwich NR4 7UA, UK, mark.reuter@ifr.ac.uk
 Mark Reuter
Mark Reuter Paula M. Periago
Paula M. Periago Francis Mulholland
Francis Mulholland Helen L. Brown
Helen L. Brown Arnoud H. M. van Vliet
Arnoud H. M. van Vliet
