- Centre for Research in Infectious Diseases, School of Chemical and Biotechnology, Shanmugha Arts, Science, Technology & Research Academy University, Thanjavur, India
Cryptococcal meningitis caused by Cryptococcus neoformans, is a common opportunistic neural infection in immunocompromised individuals. Cryptococcus meningitis is associated with fungal burden with larger capsule size in cerebrospinal fluid (CSF). To understand the role of CSF constituents in capsule enlargement, we have evaluated the effect of artificial CSF on capsule induction in comparison with various other capsule inducing media. Two different strains of C. neoformans, an environmental and a clinical isolates were used in the present study. While comparing the various capsule inducing media for the two different strains of C. neoformans, it was observed that the capsule growth was significantly increased when grown in artificial CSF at pH 5.5, temperature 34°C for ATCC C. neoformans and 37°C for Clinical C. neoformans and with an incubation period of 72 h. In addition, artificial CSF supports biofilm formation in C. neoformans. While investigating the individual components of artificial CSF, we found that Mg2+ ions influence the capsule growth in both environmental and clinical strains of C. neoformans. To confirm our results we studied the expression of four major CAP genes namely, CAP10, CAP59, CAP60, and CAP64 in various capsule inducing media and in different concentrations of Mg2+ and Ca2+. Our results on gene expression suggest that, Mg2+ does have an effect on CAP gene expression, which are important for capsule biosynthesis and virulence. Our findings on the role of Mg2+ ion as a signal for capsule induction will promote a way to elucidate the control mechanisms for capsule biosynthesis in C. neoformans.
Introduction
Cryptococcal meningitis is a common, opportunistic neural infection in immunocompromised individuals (Satishchandra et al., 2007). It has emerged as a leading cause of morbidity and mortality in HIV positive and negative patients (Antinori, 2013). Recently, Center for Disease Control, USA, estimated the occurrence of approximately one million new cases of cryptococcal meningitis each year resulting in the death of 625,000 people worldwide (Park et al., 2009; CDC, 2015). The infection is acquired by inhalation of basidiospores, which resides in lungs and causes primary pulmonary infection, frequently asymptomatic. In immunocompromised host, the organism may disseminate either acutely or after a period of latency to extrapulmonary sites, with a particular predilection for the central nervous system (Bicanic and Harrison, 2004).
The etiologic agent, Cryptococcus neoformans has multiple virulence and predilection factors for invasion of central nervous system including capsule, melanin, phospholipase, mannitol, urease and proteinases (Buchanan and Murphy, 1998). The antiphagocytic polysaccharidic capsule is the prominent virulence factor of C. neoformans, which interferes with immunity and also serves as an antioxidant. The C. neoformans polysaccharide capsule structure are highly dynamic during budding or interaction with the host. The capsule undergoes changes in size and immunogenic properties as an adaptation in order to survive in the host (Vecchiarelli and Monari, 2012). GXM is the major component comprising 90% of capsule polysaccharide. C. neoformans shed substantial amount of exo-polysachharide during chronic infections resulting in profound consequences including resistance to host immune mechanisms, antimicrobial therapy and biofilm formation (Martinez and Casadevall, 2007). Recent studies reported the development of elevated intracranial pressure during excess release of exo-polysaccharide in CSF (Robertson et al., 2014).
During infection, the C. neoformans enlarges its size to form titan or giant cells. The increase in capsule size has profound consequences including, inhibition of phagocytosis, T-cell proliferation, and cytokine production. The giant cells have been reported in lung and CSF (García-Rodas et al., 2014). Cryptococcus meningitis is associated with fungal burden with larger capsule size in CSF which results in elevated intracranial pressure, less CSF inflammatory response and slower rate of fungal clearance (Robertson et al., 2014). The titan cells and capsule synthesis are regulated by cAMP dependent PKA. Significantly leading to PKA activation is also considered to be crucial for the generation of other virulence factors (Okagaki et al., 2011).
The activation of cAMP/PKA pathway increases the capsule biosynthesis and excess shedding of capsule favors the natural flocculation of C. neoformans cells to grow as a biofilm on various solid surfaces. The C. neoformans infections are becoming common due to the increased use of brain valve and other medical devices, such as ventriculoatrial shunt catheters, peritoneal dialysis fistula, cardiac valve, and prosthetic (Martinez and Fries, 2010; Liao et al., 2015). In fact, the increasing use of ventriculoperitoneal shunts to manage intracranial hypertension associated with cryptococcal meningoencephalitis highlights the importance of investigating the biofilm forming properties of this organism (Martinez and Casadevall, 2015). The biofilm formation is related to persistent infection as they resist the host defense mechanism and antifungal therapy. In addition to capsule, melanin production is another critical factor for pathogenicity, and melanized cells are more resistant to host cell response and antifungal therapy (Martinez and Casadevall, 2006).
Thus it is clear that increase in capsule size is vital for C. neoformans pathogenicity and virulence. Unfortunately it is difficult to achieve capsule growth under laboratory conditions as the mechanism for capsule enlargement remains unclear.
In the present study, analyses were performed for two different strains of C. neoformans, an environmental and a clinical isolate, to verify the responsiveness of the strains under different growth conditions for the expression of capsular polysaccharide.
Materials and Methods
Strains and Storage
Two C. neoformans strains were used in this study. The clinical C. neoformans was collected from Department of Microbiology, Government Medical College, Trichy, Tamil Nadu, India. The environmental strain was purchased from Microbial Culture Collection Centre at Chandigarh. (ATCC 14116). The cultures were maintained on potato dextrose agar slants and 20% glycerol stocks at –80°C.
Capsule Induction: (Medium and Conditions)
To study the effect of various capsule inducing media, C. neoformans were cultured in PDB at 37°C for 24 h. Cells were washed with sterile PBS three times and resuspended in PBS. Cells were adjusted to 0.1 OD600 which is equivalent to ∼107 CFU/ml and were inoculated in the following media: PDB, DMEM, MOPS [3-(N-morpholino)propanesulfonic acid], ACSF, ACSF+ 10% heat inactivated FCS. The media were maintained at different pH (5.5, 6.5, and 7.5) and temperatures (30, 34, 37°C). The capsule sizes were measured at three time intervals of 48, 72, and 96 h. Duplicates were maintained for the study (Frases et al., 2009b; Pettit et al., 2010; García-Rodas et al., 2014).
The preparation of ACSF media was as follows; [Stock I: 0.146 g NaCl (25 mM), 0.0186 g KCl (2.5 mM) in 10 ml double distilled water; Stock II: 0.015 g Na2HPO4 (1.25 mM) in 10 ml ddH2O; Stock III: 0.022 g CaCl2 (2 mM) in 10 ml ddH2O; Stock IV: 0.0120 g MgSO4 (1 mM) in 10 ml ddH2O; Stock V: 0.218 g NaHCO3 (26 mM) in 10 ml ddH2O]. All the 10 ml stocks were added in flask and made up to 100 ml with ddH2O to avoid precipitation, finally 0.1% glucose was added (Martinez and Casadevall, 2006).
The influence of individual components of ACSF [KCl (2.5 mM), CaCl2 (2 mM), MgSO4 (1 mM), and phosphate buffer (1.25 mM)] were tested for the induction of capsule growth in the presence of 0.1% glucose.
India Ink Staining and Light Microscopy
A drop of India ink was mixed with an aliquot each of C. neoformans cultures on a glass slide. The samples were examined using Nikon (Eclipse Ci-L) and images were taken with a camera (SLR, Canon D5100, Camera). To calculate relative size of capsule, diameters of whole cell, including capsule (Dwc) and cell body weight limited by cell wall (Dcb), were measured using ImageJ 1.48v software (National Institutes of Health, Washington, DC, USA). The size of the capsule relative to that of the whole cell was defined, as a percentage as {[(Dwc – Dcb)/Dwc] × 100}. Ten cells were measured for each determination and average was calculated (Frases et al., 2009a; García-Rodas et al., 2014).
Biofilm Formation
Cryptococcus neoformans was grown in PDB for 24 h at 34°C (for ATCC C. neoformans) and 37°C (for clinical C. neoformans) in a rotary shaker at 150 rpm (to early stationary phase). The cells were collected by centrifugation and adjusted to 0.1 OD600 with each media (ACSF, ACSF+FCS, Ca2+ +Mg2+ +Glucose, Ca2+ +glucose, Mg2+ +glucose, DMEM, MOPS, and PDB). The cells were then inoculated on glass coverslip for 72 h at 34°C (ATCC C. neoformans) and 37°C (clinical C. neoformans). The biofilm was then washed thrice with PBS, to remove non-adherent cells and media from the cover slips and stained with India ink. The stained biofilms were then observed using Trinocular microscope (Nikon) at 40×. The experiments were done twice (Martinez and Casadevall, 2005).
Capsule Gene Expression Analysis
Total RNA Extraction
For transcriptional pattern analysis, the ATCC C. neoformans and clinical C. neoformans were cultivated under following conditions, (i) PDB; (ii) PDB + Mg2+; (iii) MOPS; (iv) MOPS + Mg2+; (v) DMEM; (vi) DMEM + Mg2+; (vii) ACSF; (viii) Ca2+ + 0.1% glucose; (ix) Mg2+ + 0.1% glucose; (x) Mg2++ Ca2+ + 0.1% glucose. Based on the dose dependent studies of Mg2+, 2 mM Mg2+, and 0.5 mM Mg2+ were selected for ATCC C. neoformans and clinical C. neoformans, respectively. After 48 h incubation time, C. neoformans were harvested and washed in chilled PBS. The RNA was extracted using RNAeasy mini kit (74104, Qiagen).
cDNA Synthesis
cDNA synthesis was performed in a total volume of 50 μl including 2–10 μg of RNA sample with one step RT-PCR Kit (210201, Qiagen). PCR amplifications were made from templates (1:20) with target primers (2:20), and (10:20) Taq DNA polymerase master mix (180301, Amplicon). Quantification of the transcript levels was performed using the ImageJ software, normalizing against β-actin.
PCR Analysis
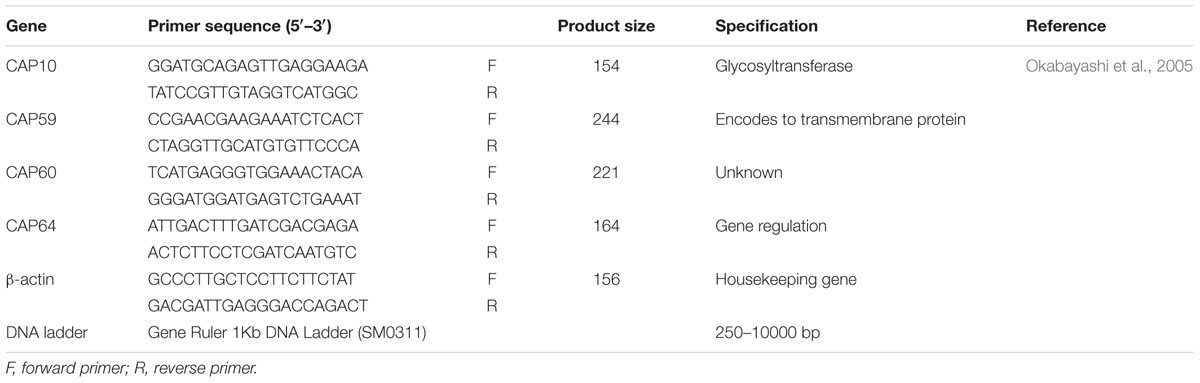
The PCR reactions were performed using a Taq DNA polymerase 2.0X Master mix with 1.5 mM MgCl2 (180301, Amplicon). The protocols of the PCR were as follows: initial denature at 95°C for 10 min, followed by 40 cycles at 95°C for 30 s, annealing at 57°C (β-actin and CAP59), 55°C (CAP10), 60°C (CAP60), or 54°C (CAP64) for 30 s, and extension at 72°C for 30 s. Primer pairs for PCR are listed in Table 1. These primer pairs were confirmed to amplify the genome DNA fragments of both ATCC and clinical strains (Bolano et al., 2001). The relative concentration of each gene was analyzed by ImageJ software. All experiments were performed in duplicates.
Statistical Analysis
All experiments were performed in duplicates and the data analysis was done using Minitab 16 software. One-way ANOVA and Tukey’s test were performed to test statistical significance for multiple comparisons. All graphs were prepared with GraphPad Prism 6, and were expressed as mean ± experiments done in duplicates.
Results
Comparison of Capsule Induction in Various Medium
We analyzed the effect of various nutrient based culture media for capsule growth and virulence trait expression for two different C. neoformans strains of clinical and environmental origin. While comparing the capsule growth in different media, it was found that ACSF significantly increased capsule growth in both ATCC C. neoformans and clinical C. neoformans compared to other media. The order of capsule growth for the strains are as follows: ACSF (69%) > ACSF+FCS (63%) > MOPS (62%) > DMEM (61%) > PDB (55%) and ACSF (68%) > ACSF+FCS (51%) > DMEM (49%) > MOPS (40%) > PDB (37%) for ATCC C. neoformans and Clinical C. neoformans, respectively. In both strains the capsule was induced by ∼32% when compared with the control media (Figures 1 and 2). Statistical analysis confirmed that ACSF was the best among the tested media (p < 0.0001; Supplementary Table 1).
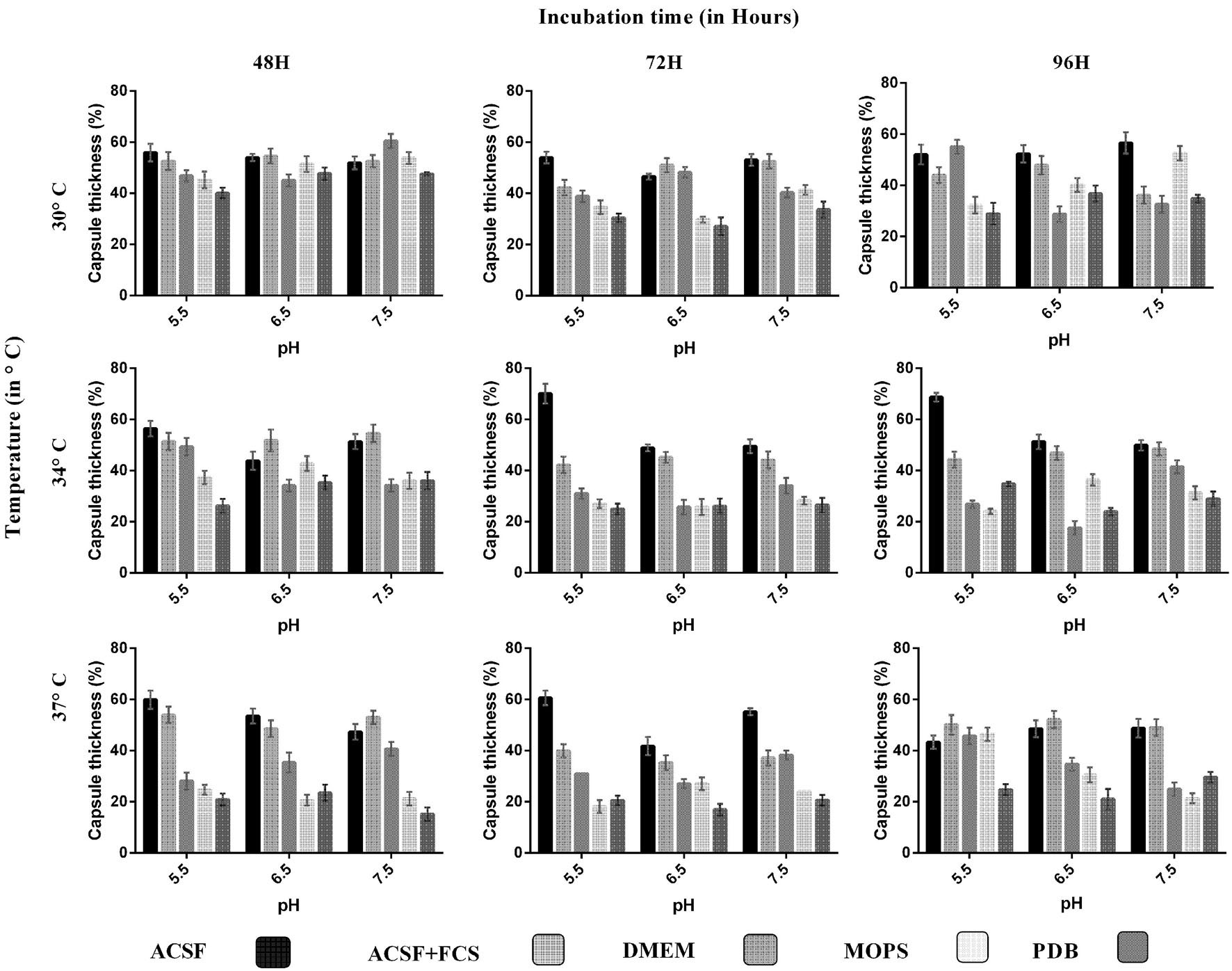
FIGURE 1. Comparison of capsule induction in ATCC C. neoformans in different media (ACSF, ACSF+FCS, DMEM, MOPS, and PDB) at various pH, temperature, and incubation period. The percentage of the capsule thickness [(total size∗ – cell size)/(total size∗) × 100] for ≥8 cells per group is shown. Bars represent standard errors (p < 0.0001). The greater capsule growth was observed in ACSF media (70%) at pH 5.5, 37°C, 72 h, followed by DMEM 60%, ACSF+FCS 55%, MOPS 53%, and PDB 47%. ∗Total size = capsule size + cell size.
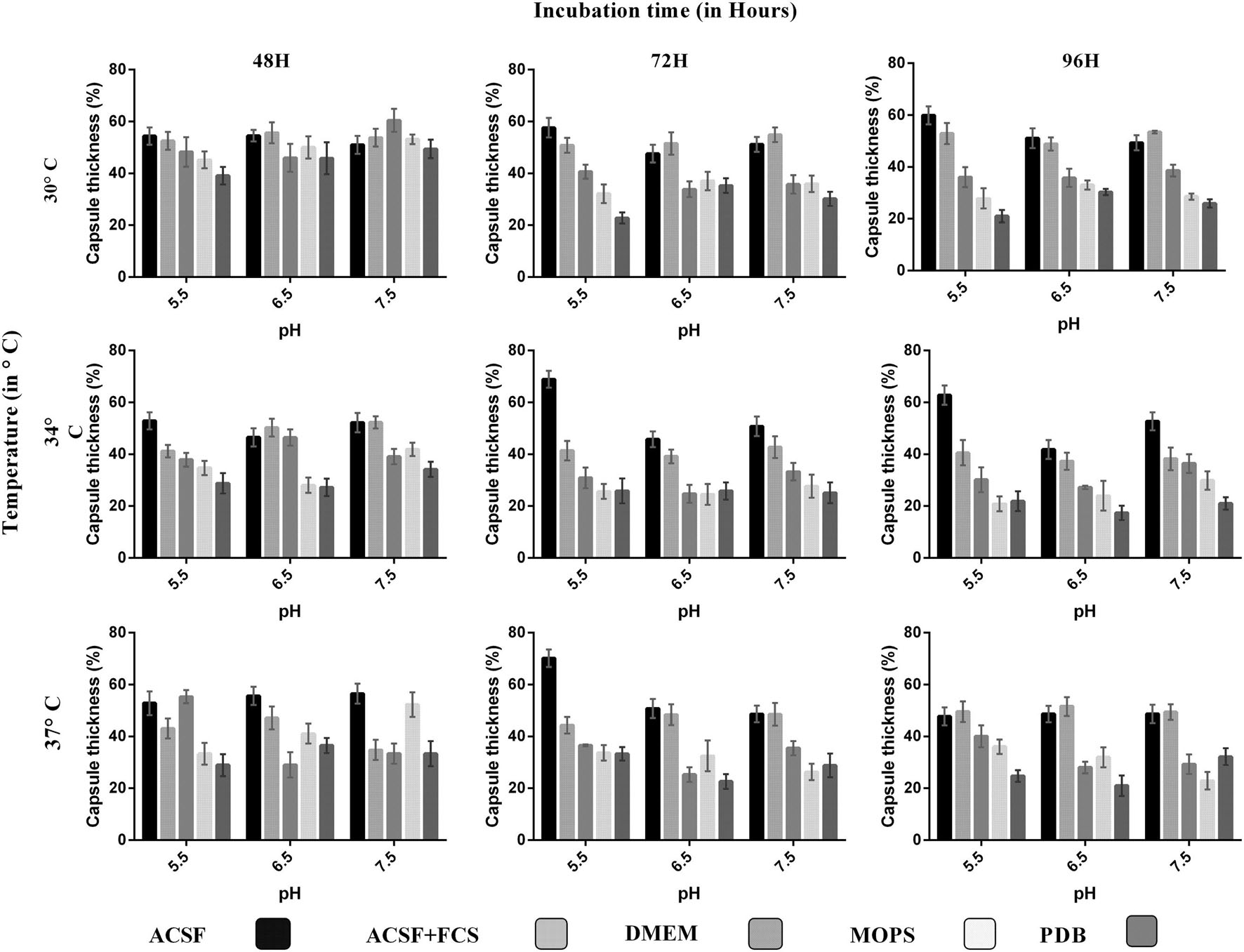
FIGURE 2. Comparison of capsule growth in clinical C. neoformans in different media (ACSF, ACSF+FCS, DMEM, MOPS, and PDB) at various pH, temperature, and incubation period. The higher capsule induction was observed in ACSF media at pH 5.5, 37°C, 72 h, which is followed by ACSF 70%, ACSF+FCS 56%, DMEM 55%, MOPS 53%, and PDB 49%. The cell size, capsule size, and percentage of the capsule thickness for ≥8 cells per group are shown. Bars represent standard errors (p < 0.0001).
The order of decrease in capsule growth in different medium shows that the capsule size decreases with increase in glucose concentration. Low glucose induces the production of cAMP and activates cAMP–PKA pathway (Pukkila-Worley and Alspaugh, 2004). However, single inputs are not sufficient for capsule induction, other inducing since signals are also needed (Zaragoza and Casadevall, 2004). Thus the effect of pH, temperature were also studied. All our test media contains considerable amount of salts and ions except PDB, which is observed as weakest medium for capsule induction. Serum induced capsule growth; however, the inducing effect was dependent on composition of the medium. Zaragoza and Casadevall (2004) observed capsule induction when grown in PBS with serum whereas not in sabouraud medium. Similarly in our study, 10% FCS induced capsule in the presence of ACSF. In summary, ACSF induced greater capsule growth in both the strains (Zaragoza and Casadevall, 2004).
Effect of pH, Temperature, and Incubation Period
We investigated the effect of pH, temperature and incubation period for the capsule induction in C. neoformans. To study the effect of pH, each media with different pH at 5.5, 6.5, or 7.5, based on PDB as control was selected for the study. From the results obtained, we observed a diverse range of capsule induction in different media. We achieved significant capsule induction (70%) for both the strains in ACSF with pH 5.5. A prominent increase in capsule size than the cell size for ATCC C. neoformans was noticed. Whereas, for other media, the capsule and cell size were relatively equal with capsule induction in the range of 34–54%. The ambient pH for capsule induction was observed to vary with the media types which are shown in Figures 3 and 4. As suggested by previous reports the effect of pH on capsule growth might be due to the alterations in cell wall stability, protein stability, morphogenesis, nutrient uptake (O’Meara et al., 2010) and changes in capsule structure (García-Rodas et al., 2011; Park et al., 2014). The exact mechanism by which pH regulates capsule growth is not clearly known. O’Meara and Alspaugh (2012) observed that there was no significant change in the expression of capsule biosynthesis genes at physiological pH. Effect of pH on the expression of capsule biosynthesis genes are required to elucidate the mechanism of capsule induction at different pH, which could be an important criteria for establishment and spread of infection (O’Meara and Alspaugh, 2012).
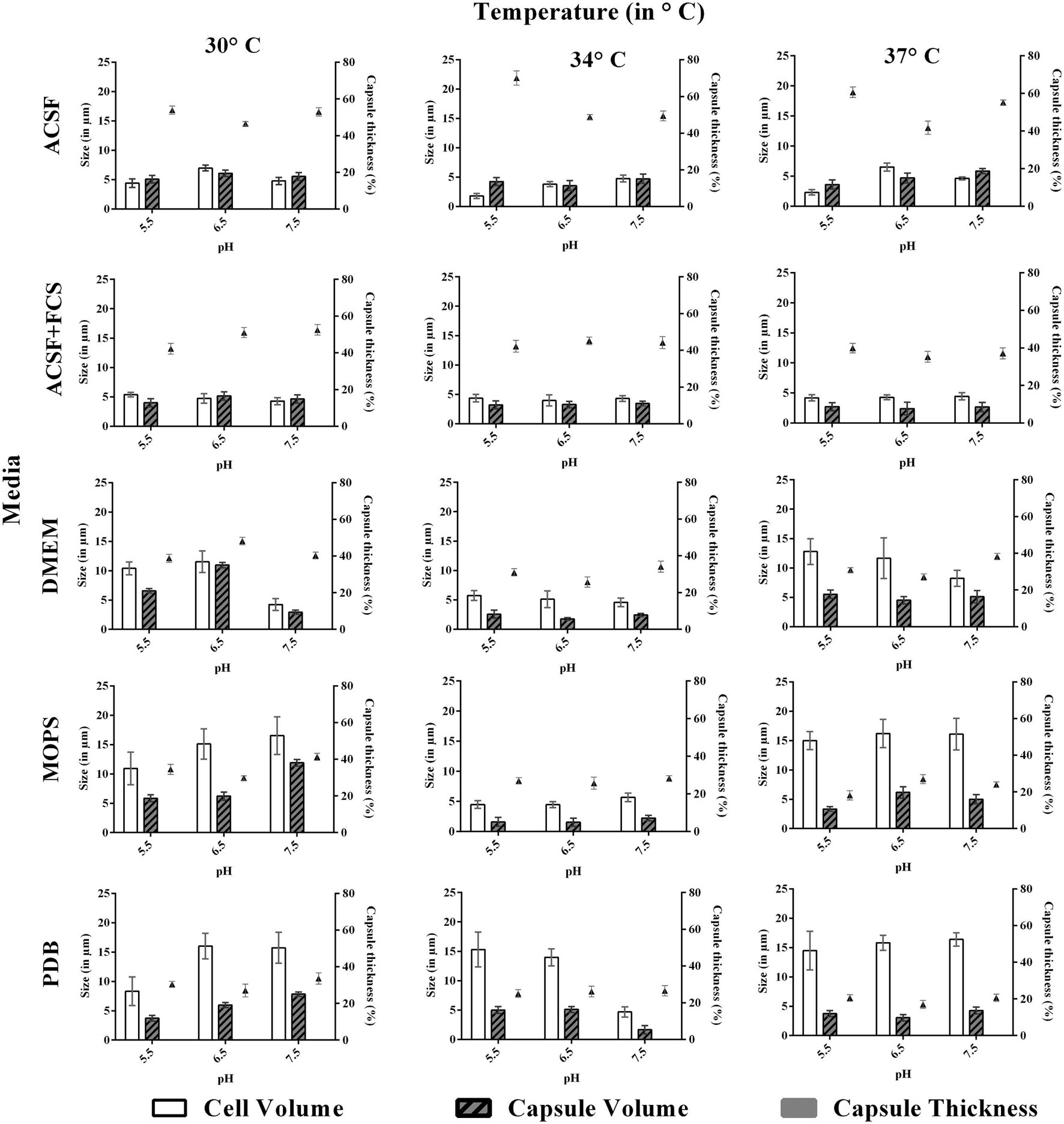
FIGURE 3. Effect of pH and temperature on capsule induction in ATCC C. neoformans in different media at 72 h. Left Y-axis represents size (in μm) for cell and capsule, compared with capsule thickness (in %) through right Y-axis. The cell size, capsule size and percentage of the capsule thickness for ≥8 cells per group are shown. Bars represent standard errors (p < 0.0001).
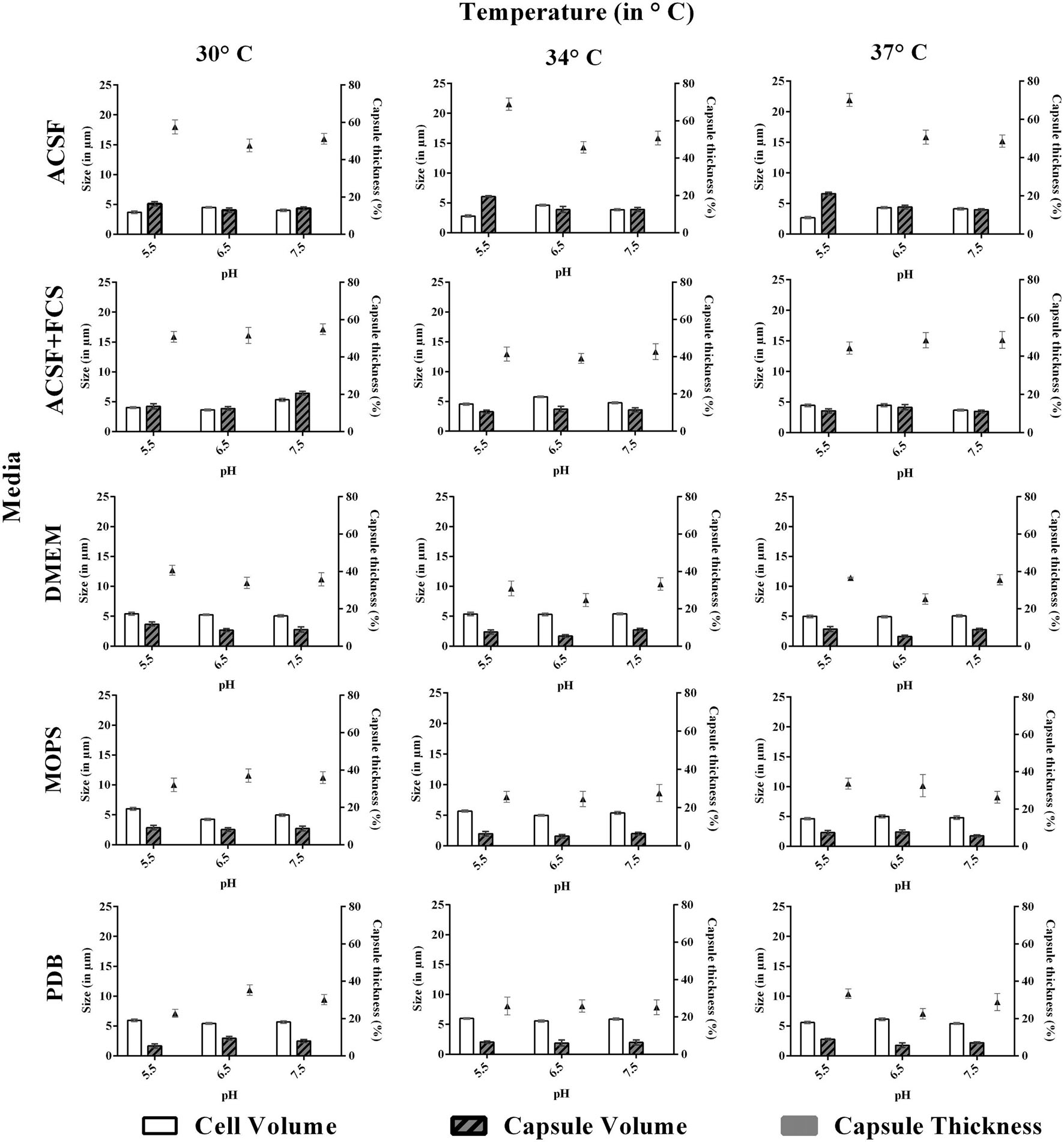
FIGURE 4. Effect of pH and temperature on capsule induction in clinical C. neoformans in different media at 72 h. Left Y-axis represents size (in μm) for cell and capsule, compared with capsule thickness (in %) through right Y-axis. The cell size, capsule size and percentage of the capsule thickness for ≥8 cells per group are shown. Bars represent standard errors (p < 0.0001).
Similar to pH, the effect of temperature required for capsule induction was studied (Figures 3 and 4). From the results obtained it is clear that temperature does have an effect on capsule growth. However, we could not conclude whether an increase or decrease in temperature is influencing the capsule formation. ACSF induced capsule growth at 30, 34, 37°C but significant induction was at 34°C for ATCC C. neoformans and 37°C for clinical C. neoformans. However in our study, temperature has an effect on cell and capsule volume, postulating that the effect of physiological factors varies with type of strains used for the study.
We then investigated the effect of incubation period for capsule induction in C. neoformans. The study was conducted for a period of 96 h, and the capsule size was measured at regular intervals of 24 h. For both the strains, the optimum capsule induction period was 72 h when grown in ACSF at their optimum temperature (34 and 37°C) and pH (5.5). Thus it is clear that capsule induction appears to vary with respect to changes in media, temperature and pH. However, longer incubations did not significantly change the capsule size in both strains.
In summary, comparison of various capsule inducing media for the two different strains of C. neoformans shows that capsule growth was significantly increased when grown in ACSF at pH 5.5, temperature 34°C for ATCC C. neoformans and 37°C for Clinical C. neoformans and with an incubation period of 72 h. Using this information, we have made an attempt to understand the effect of individual components of ACSF on capsule induction.
Evaluation of ACSF Components
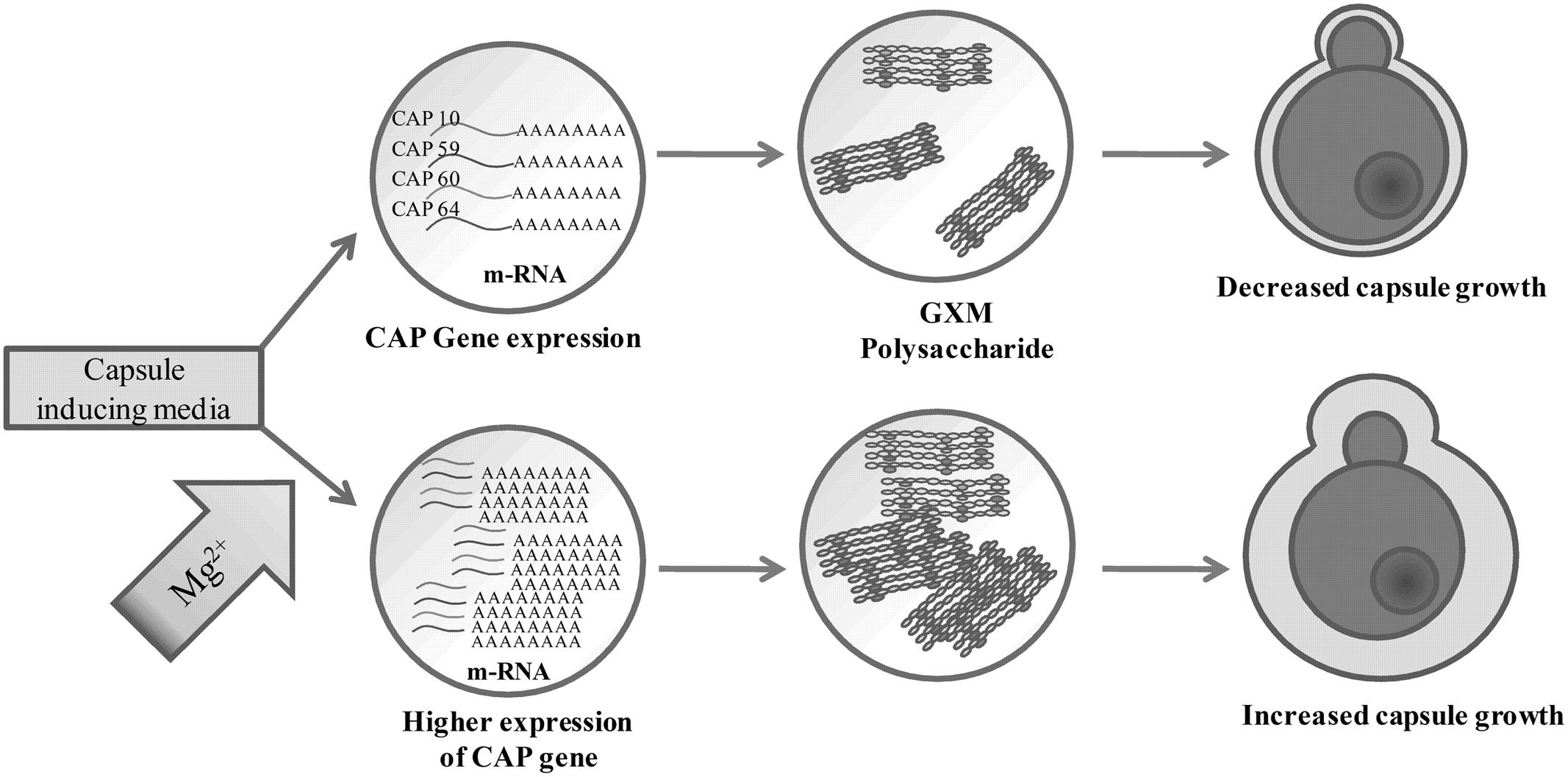
The individual components of ACSF were analyzed to find out the most important capsule inducing factor. The defined medium has interesting components like KCl (2.5 mM), CaCl2 (2 mM), MgSO4 (1 mM), and phosphate buffer (1.25 mM). When each individual component of ACSF was supplemented with 0.1% glucose, we noticed Mg2+ as the prominent capsule inducing factor in both strains. The order of capsule induction for different salts for clinical C. neoformans is Mg2+ (58%) > Mg2++ Ca2+ (52%) > Ca2+ (48%) > PO43-(46%) > glucose (46%). For ATCC C. neoformans, Mg2+ (59%) > Mg2++ Ca2+ (57%) > Ca2+ (54%) > PO43-(52%) > glucose (50%) and no capsule induction was observed in KCl for both the strains (Figure 5). So we increased the pH of the KCl containing medium for capsule synthesis. Surprisingly capsule induction was observed at higher pH (pH 7.5). The effect of ACSF ions on capsule formation were studied by comparing the cell size and capsule thickness. From the results we obtained, it is very clear that C. neoformans when grown in the medium containing Mg2+ ion, show increased capsule production even though the cell size was relatively small. Whereas in case of effect of ions like K+, PO43-, the cell size was greater than capsule. In the presence of Ca2+, the cell and capsule thickness were equal, whereas a significant induction was observed when Ca2+ medium was supplemented with Mg2+. The effect of Mg2+ ion in capsule induction is clearly evident in Figure 5. A similar kind of results was observed with clinical C. neoformans. However, in both the strains, Mg2+ significantly induced to capsule growth under any of the conditions tested, like different pH (pH 5.5, 6.5, and 7.5), temperature (30, 34, and 37°C), induction period (48, 72, 96 h). Statistically Mg2+ was found to enhance capsule compared with other salts. Through One-way ANOVA analysis Mg2+ was found to be the best salt compared to other ACSF salts (p < 0.0001; Supplementary Table 2). These results show the importance of Mg2+ in C. neoformans capsule growth.
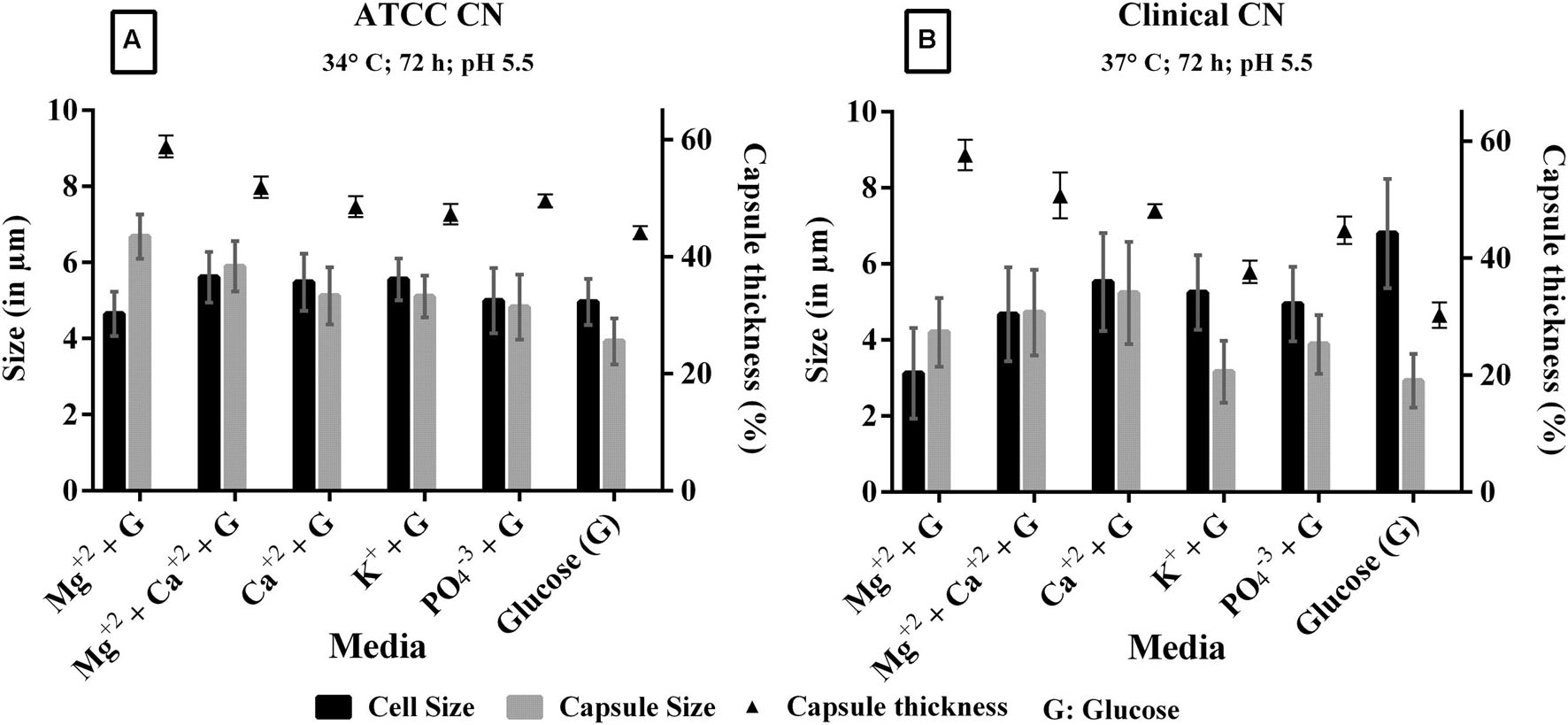
FIGURE 5. Effect of ACSF salts [Magnesium (Mg2+), Calcium (Ca2+), Potassium (K+), Phosphate (PO43-) salts were supplemented with 0.1% glucose] on capsule induction in (A) ATCC C. neoformans at 34°C, 72 h, pH 5.5; (B) clinical C. neoformans at 37°C, 72 h, pH 5.5. 0.1% glucose used as a control. Left Y-axis represents size (in μm) for cell and capsule, compared with capsule thickness (%) through right Y-axis. The cell size, capsule size, and percentage of the capsule thickness for ≥8 cells per group are shown. Bars represent standard errors (p < 0.0001); G, Glucose.
There are earlier studies on C. neoformans capsule growth with some salts like NaCl, MgCl2, CaCl2, phosphate buffer, MOPS, FeS04, (Lian et al., 2005; Nimrichter et al., 2007; Robertson et al., 2012; Kretschmer et al., 2014). High concentrations of NaCl inhibited the capsule growth suggesting that Na neutralizes the negatively charged GlcA residues (Nimrichter et al., 2007). A recent study by Kretschmer et al. (2014) reported that phosphate ions are helpful for the nutrition acquisition and influence capsule synthesis. The author observed that phosphate uptake is critical for capsule synthesis as it is integrated with the key signaling pathways for nutrient sensing. The effect of iron on capsule induction is varied with the induction being greater in both iron limited and iron rich condition, like brain tissue. The role of iron, however, in capsule induction is not clearly understood (Kretschmer et al., 2014).
The three major events in C. neoformans capsule formation includes, capsule synthesis, assembly and degradation (Bose et al., 2003). Once the capsule components are synthesized they are assembled to form a thick outer covering to form mature C. neoformans, which requires the involvement of multiple regulatory pathways. The dynamics of capsule size is affected by the varied environmental cues. There are reports suggesting the role of divalent ions (Mg2+, Ca2+) in capsule assembly and capsule growth (Nimrichter et al., 2007; Robertson et al., 2012) and they are dose dependent. Increasing the concentration of divalent ions decreases the capsule assembly. In our study the effect of Mg2+ alone is significantly greater than Mg2+ + Ca2+, suggesting its vital role in capsule synthesis.
Magnesium Acts as Signal for Increased Capsule Production and Dose-Dependency
Since Mg2+ in ACSF was shown to increase capsule size, we sought to compare its concentration with human CSF. Mg2+ is found at a concentration of 0.97 mM in human CSF and might be a capsule inducing factor that triggers larger capsule size in patients with cryptococcus meningitis. Therefore, we also performed Mg2+ dose-dependency on C. neoformans capsule induction. The media whichever was not having magnesium was selected for the study to confirm that magnesium is one of the important capsule inducing factor. Hence, various concentrations of Mg2+ (0–4 mM Mg2+) were supplemented in MOPS, PDB, and phosphate buffer. As expected, the medium supplemented with Mg2+ produced larger capsule sizes than the control (media without Mg2+; Figure 6). The dose-dependent studies clearly demonstrate that capsule induction by Mg2+ is dose responsive. The optimum concentration required, however, varied between the strains. Dose-dependent analysis of Mg2+ on capsule thickness showed that 2.0 mM Mg2+ gave the best result for ATCC C. neoformans. With increase in Mg2+ concentration, there was a small decrease in capsule thickness, and a similar kind of result was observed for clinical C. neoformans at 0.5 mM Mg2+. This concentration is very similar to the concentration of human CSF (Figure 6). C. neoformans capsule induction was observed in microscopic analysis for both ATCC and clinical strains, microscopic images showed capsule induction in different media and ions (Figures 7 and 8). The One-way ANOVA confirmed that 2.0 mM and 0.5 mM Mg2+ concentration is required for significant capsule induction in ATCC C. neoformans and clinical C. neoformans, respectively (p < 0.0001; Supplementary Table 3).
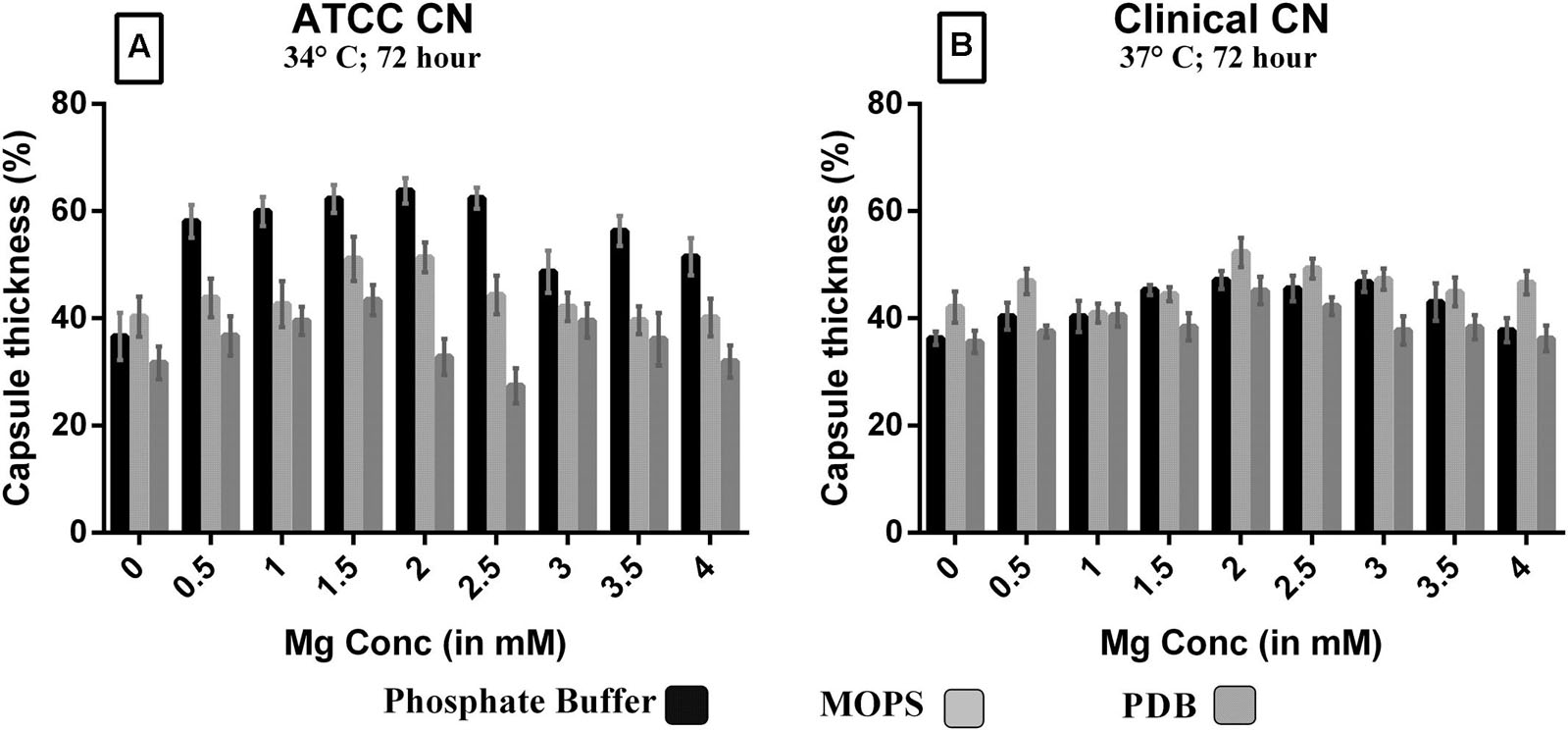
FIGURE 6. Effect of Mg2+ with phosphate buffer, MOPS and PDB on capsule induction with increasing Mg2+ concentration (0.0 – 4.0 mM) in ATCC C. neoformans at 34°C, 72 h, pH 5.5 (A); clinical C. neoformans at 37°C, 72 h, pH 5.5 (B); 0.1% glucose used as a control. Y-axis represents capsule thickness (in %). The higher capsule size was observed with phosphate buffer + 2.0 mM Mg2+ in ATCC C. neoformans (67%) whereas in clinical C. neoformans (58%) sample, PDB + 0.5 mM Mg2+. The cell size, capsule size and percentage of the capsule thickness for ≥8 cells per group is shown. Bars represent standard errors (p < 0.0001).
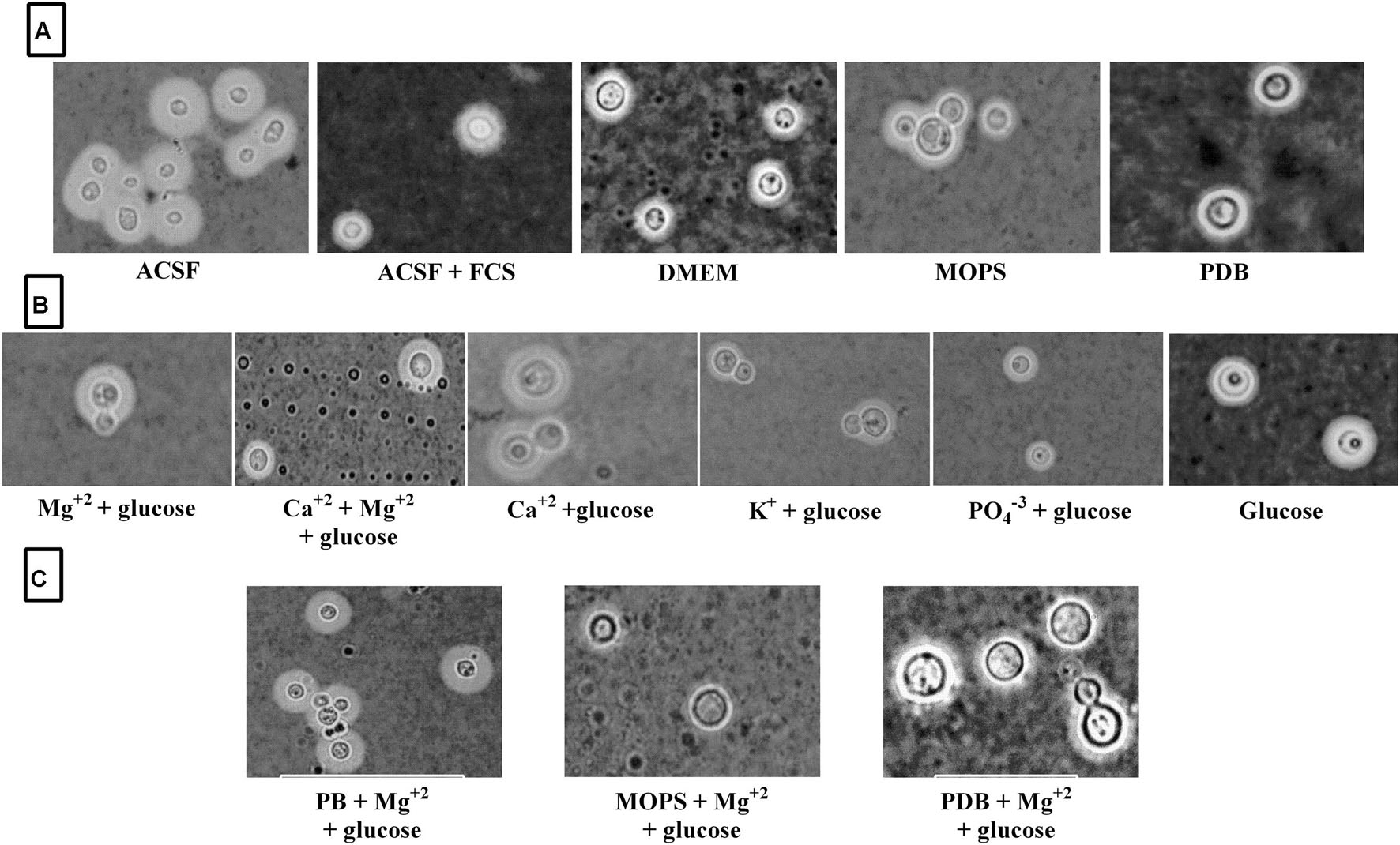
FIGURE 7. ATCC C. neoformans capsule induction in different media. (A) Capsule induction in different media; (B) Capsule induction in individual ACSF salts; (C) Capsule induction in different media supplemented with Mg2+ ion.
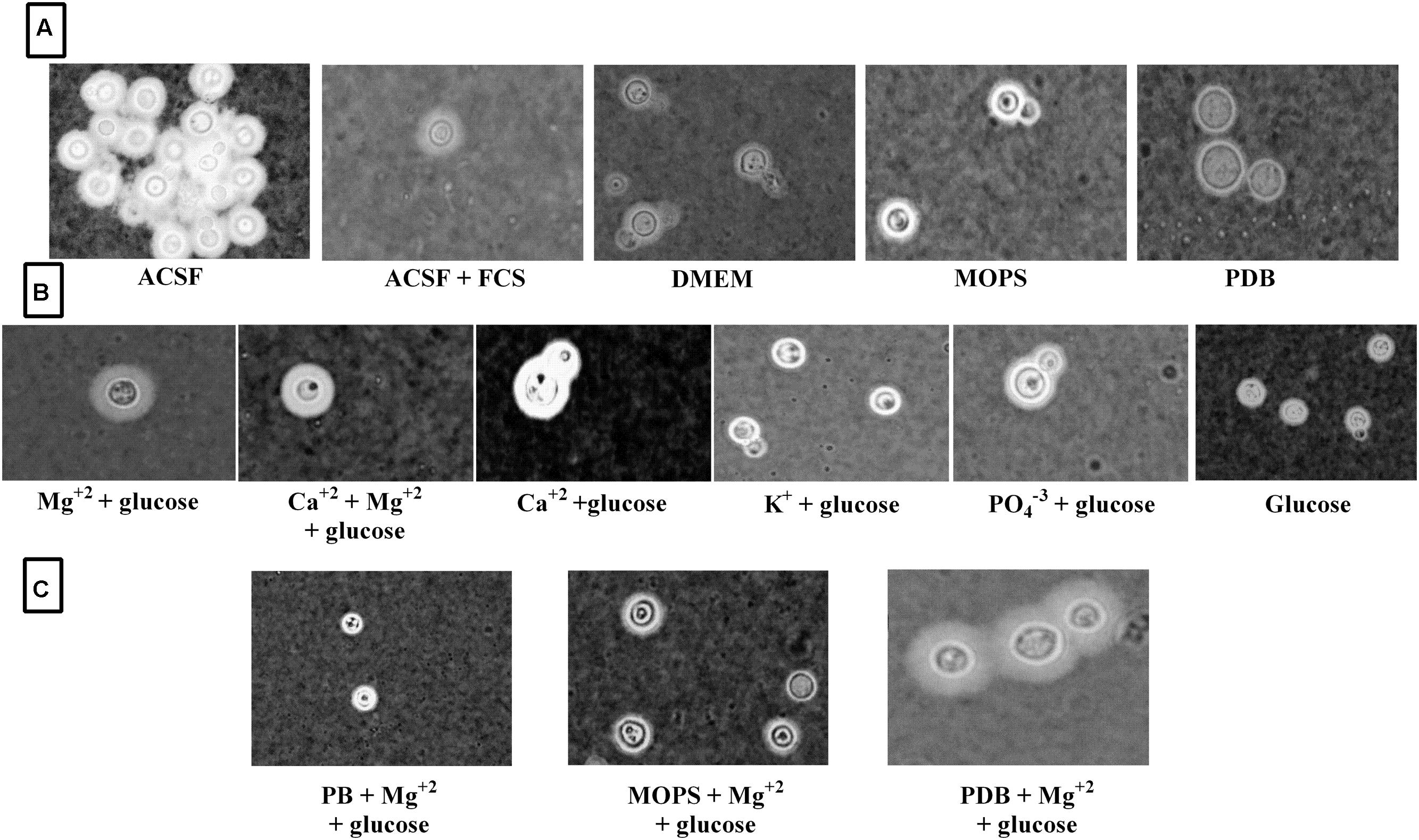
FIGURE 8. Clinical C. neoformans capsule induction in different media. (A) Capsule induction in different media; (B) Capsule induction in individual ACSF salts; (C) Capsule induction in different media supplemented with Mg2+ ion.
Capsule growth are regulated by multiple pathways and are specifically associated with stress response. cAMP is one such pathway, which is regulated during stress conditions like low glucose, low amino acids, increased CO2 (Miwa et al., 2004; Maidan et al., 2005). During stress conditions cAMP levels are elevated and this activates the kinase Pka1, which in turn activates transcriptional factors like Nrg1, Rim101 that are involved in capsule assembly and enlargement, respectively. Mg2+ is one of major divalent cation that has important role in cell division, enzyme activation and stress suppression (Nimrichter et al., 2007). It is reported that Mg2+ is the most important divalent ion absolutely required for the synthesis of adenylate cyclase which in turn is required for the production of cAMP (Bahn et al., 2004). The cAMP–PKA pathway regulates virulence in C. neoformans, including capsule production, melanin formation. From our results we assume that C. neoformans when grown in ACSF which has abundant salts and less glucose, activates the cAMP–PKA pathway which are regulated well in the presence of Mg2+ ions.
Biofilm Formation
Cryptococcus neoformans biofilms are becoming common due to the increased use of brain valves and other medical devices. The knowledge on mechanism of biofilm formation by Cryptococcus sp. is still in its infancy. The exopolysaccharide of C. neoformans renders it to grow as biofilms on various medical devices. We intended to investigate the efficiency of each media to enhance capsule formation which in turn helps C. neoformans to grow as biofilm (Martinez and Casadevall, 2007; Robertson et al., 2012). The biofilm formation is shown in Figure 9. The result supports our finding that ACSF is the efficient capsule inducing medium for both the strains of C. neoformans. Our further studies are intended to form biofilms on different solid supports including medical devices for a better understanding of the role of Mg2+ ions in biofilm formation using advanced imaging techniques.
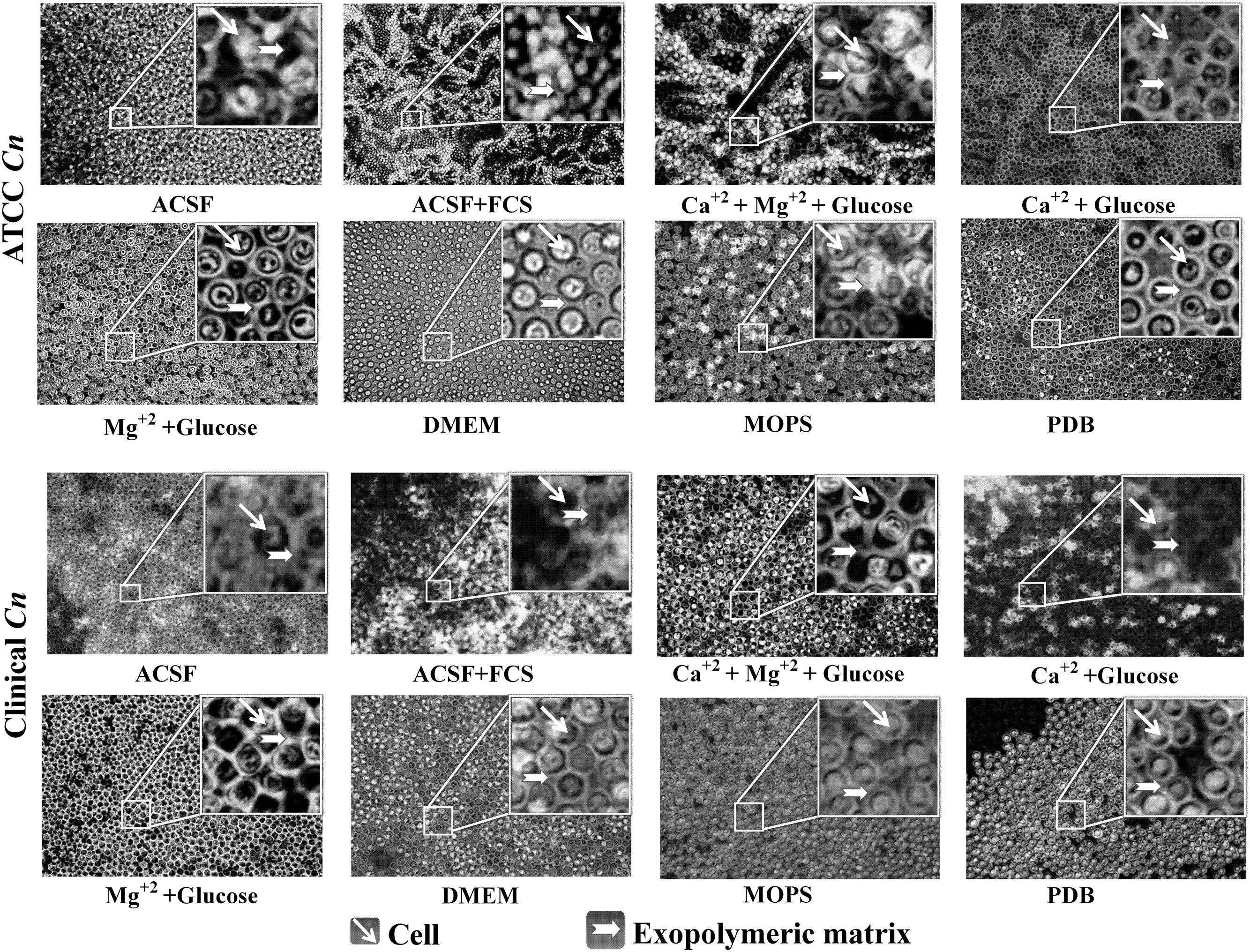
FIGURE 9. Biofilm formation on coverslip with different media. Biofilm observed at 40× light microscope by India ink stain. Images of a mature biofilm show yeast cells and capsular polysaccharide.
Capsule Gene Expression Analysis
To look specifically at capsule biosynthesis genes induced by Mg2+, we tested the effect of different capsule induction media on the expression of genes important for capsule biosynthesis. We analyzed four major CAP genes namely, CAP10, CAP59, CAP60, and CAP64. Our result (Figure 10) on gene expression shows that different media were able to stimulate the expression of CAP10, CAP59, and CAP60, whereas none of the media were able to stimulate the expression of CAP64. These results were similar for both environmental and clinical isolates of C. neoformans.
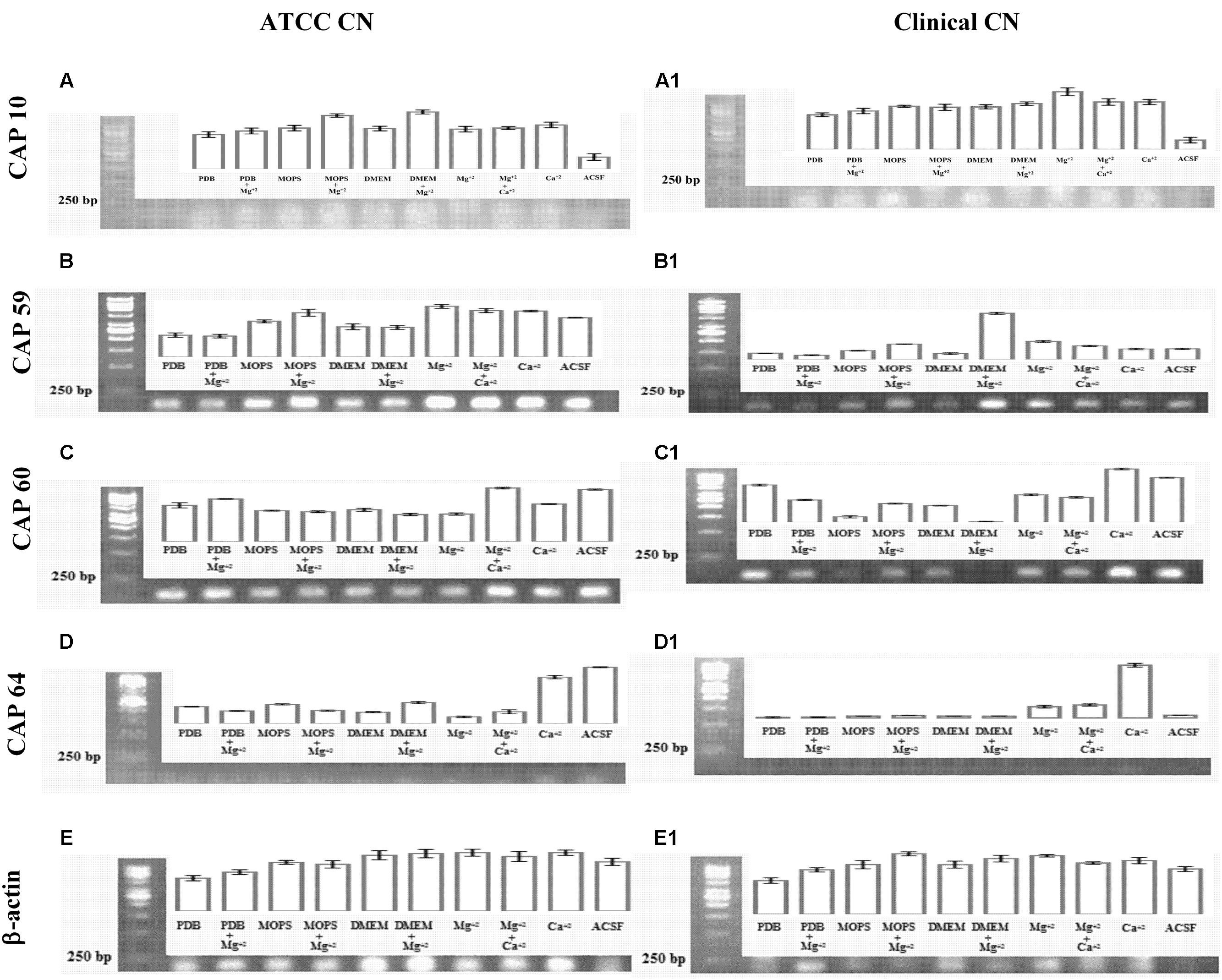
FIGURE 10. Capsule associated gene expression. CAP gene expression of ATCC C. neoformans: (A) CAP10 gene; (B) CAP59 gene; (C) CAP60 gene; (D) CAP64 gene; (E) β-actin gene. CAP gene expression clinical C. neoformans: (A1) CAP10 gene; (B1) CAP59 gene; (C1) CAP60 gene; (D1) CAP64 gene; (E1) β-actin gene.
With regard to CAP10, gene, presence of Mg2+ in the media lead to enhanced expression of the gene when compared to Mg2+-free media, with best expression seen with MOPS + Mg2+ in the case of environmental isolate. However, with clinical isolate, presence of Mg2+ resulted in lower expression of CAP10 gene when compared to Mg2+-free media. Presence of Ca2+ did not have any significant effect on CAP10 expression in both isolates. For both environmental and clinical isolates of C. neoformans, ACSF did not promote significant CAP10 expression.
For CAP59 expression, again presence of Mg2+ in the media leads to increase in expression of the gene in MOPS and DMEM for both environmental and clinical isolates. Presence of Mg2+ in PDB did not induce significantly higher CAP59 gene expression in both isolates. Interestingly, CAP59 gene in environmental isolate showed higher expression in ACSF media when compared to clinical isolate.
Unlike CAP59, CAP60 gene expression in clinical isolate was enhanced in the presence of Mg2+ in the case of MOPS alone. For this isolate, presence of Mg2+ in PDB, and DMEM produced significantly lower levels of CAP60 gene expression. In the case of environmental isolate, presence of Mg2+ had a stimulating effect only in the case of PDB, while with MOPS and DMEM, the expression looked almost similar in both Mg2+-free and Mg2+-containing media. But for both isolates, ACSF induced a strong expression of CAP60 gene.
Interestingly, for both isolates, CAP64 gene expression was not detected in any of the media, including with Mg2+, and the reasons for this are unclear. The environmental and clinical isolates displayed differences in expression of capsular polysaccharide under different growth conditions. Interestingly, our study demonstrates that the virulent genes (CAP genes) are highly expressed in the presence of Mg2+ ions in both clinical and environmental isolates. Together these results suggest that, Mg2+ does have an effect on CAP gene expression, which are important for capsule biosynthesis and virulence.
Discussion
Meningitis is caused by a variety of microbes, however, those caused by fungus especially Cryptococcus is becoming quite common (Karkowska-Kuleta et al., 2009). Cryptococcosis of the CNS is life threatening and presents as meningitis or meningoencephalitis. Initial infection, latency or dissemination of the pathogen is determined by specific morphological features of the microbe. Since infections are rapid in immunocompromised individuals, it strongly suggests the importance of both innate and adaptive immune components (Johnston and May, 2013). Phagocytic cells, especially macrophages of the innate immunity and regulatory arm of the adaptive immune system also appear to be important for cryptococcal clearance (Feldmesser et al., 2001; Taborda and Casadevall, 2002). Capsule is the best-studied virulence factor in C. neoformans, wherein it has been shown to inhibit phagocytosis by macrophages, through masking opsonin function (Levitz and DiBenedetto, 1989). Capsule is also important for protecting cryptococci from reactive oxygen species within the lysosome and is also related to proliferative ability of the pathogen inside macrophages (Feldmesser et al., 2001; Zaragoza et al., 2008). Hence studies related to capsule biosynthesis has been a promising topic of interest for C. neoformans researchers enabling them to understand capsule synthesis and in turn its pathobiology. Reports have evidently shown that C. neoformans cells express antigenic variation in the capsule during murine infection and transmigration through the blood–brain barrier (Jain et al., 2006; Vecchiarelli and Monari, 2012). Thus changes in capsule structure and size are observed during mammalian infection. Since capsule size is highly dynamic based on various environment cues, studies have been carried out under various in vitro conditions mimic in vivo capsule size changes during infection. Previous studies with mammalian serum, DMEM, MOPS have suggested the capsule production varies with media and growth conditions of C. neoformans strains (Haynes et al., 2011; O’Meara and Alspaugh, 2012). Also, a recent study on various nitrogen sources suggested that the capsule growth is induced by urea, which is a prevalent nitrogen source in human CSF (Frazzitta et al., 2013). CSF is widely used as the best diagnostic tool for the identification of Cryptococcus sp., as the fungal burden with large capsule size are easily visualized with an India ink stain (Fridlington et al., 2006). A recent interesting study has correlated capsular size in CSF as representative of that found in the brain (Robertson et al., 2014). The predominance of C. neoformans in CSF, made us to evaluate the suitability of the CSF as the favorable environment for C. neoformans growth. However, we have performed the study for both environmental and clinical isolate. In general, environmental and clinical isolates are different based on their place of origin and habitat. For instance, environmental strains are isolated from soil, pigeon and chicken excreta, trunk hollows of Tamarindus indica, and decaying wood. Similarly clinical strains are frequently isolated from CSF of immunocompromised individuals (Emmons, 1951, 1955; Lazéra et al., 1996; Souza et al., 2005). The differences in the genotype, serotype, mating type, and antifungal susceptibility between the environmental and clinical C. neoformans has been analyzed by various investigators. Majority of these studies have demonstrated the existence of similarity in genotype, serotype, and mating type in environmental and clinical isolates (Halliday et al., 1999; Campbell et al., 2005; Escandon et al., 2006). However the virulence trait expressions are found to be different between them. For example, phospholipase, urease activity, capsule synthesis, and melanin production are higher in case of clinical isolates, suggesting the significant role of host factors in virulence trait expression. In addition, antifungal susceptibilities are also found to be varied between the clinical and environmental C. neoformans (Chowdhary et al., 2011). Thus we studied the responsiveness of environmental and clinical isolates in different growth conditions. The media comprised of various salts like Mg2+, Ca2+, PO43-, K+, and with 0.1% of glucose. Our comparative statistical studies with other media like DMEM, MOPS, PDB, and ACSF+FCS showed that capsule induction was significantly higher in ACSF for both the strains. However, one limitation of the present study is the influence of ambient CO2 on capsule induction, which could be an effective component under in vivo conditions.
The results of capsule induction were also supported by the biofilm formation studies. The higher capsule production enables C. neoformans to grow as biofilm and the acapsular mutants are unable to adhere to plastic materials (Martinez and Casadevall, 2006). Though a simple coverslip method was used for the biofilm formation, the biofilm of C. neoformans was clearly observed, when grown in ACSF, ACSF salts and other capsule inducing media (Figure 9). In a similar kind of study, C. neoformans strain B3501 was observed to form a strong biofilm in minimal medium containing glucose (Martinez and Casadevall, 2006).
We have extended our study to find the effect of pH and temperature on capsule induction. Though there are reports on the effect of pH and temperature on capsule induction, we also tried similar kind of experiments as the strains selected for the present study are different from those used by other authors. As also suggested by previous reports we could not conclude the role of pH in capsule induction (Gupta and Fries, 2010; O’Meara et al., 2010). The influence of pH varies with the type of media used. Thus to find the significance of pH in capsule induction, expression of capsule biosynthesis genes are required to elucidate the mechanism of capsule induction at different pH. Similarly, the effect of temperature for significant capsule induction varied with the two strains. The capsule production was higher at 37 and 34°C for clinical Cn and ATCC Cn, respectively. The results show that the temperature required for cell and capsule growth is different for different strains of C. neoformans. Zaragoza et al. (2003) reported that temperature does have an effect on cell size but not on capsule size. The authors observed that even a temperature shift from 24 to 37°C, did not affect the proportion of capsule present in the cells. However, from our results, we suggest that temperature does have an effect on capsule growth. Following these results, we used optimized conditions for capsule formation (ACSF, pH 5.5, 72 h, 34°C for ATCC Cn and similar conditions at 37°C for clinical Cn) to assess the potential of ACSF salts in inducing capsule formation. Similar studies were done previously by some authors to find the significant role of Mg2+ + Ca2+, PO43-, FeSO4, and NaCl in capsule formation. PO43- was found to be an essential mineral for nutrient uptake by C. neoformans for capsule growth (Kretschmer et al., 2014), and Mg2+, and Ca2+ ions was found to be important for capsule assembly (Robertson et al., 2012). Whereas the results from our CAP gene expression analyses, suggests the essentiality of Mg2+ ion in C. neoformans capsule formation. Mg2+ ion is one of the most important divalent ion which has many functions including as an enzyme activator. From the previous reports, the significance of cAMP signaling in expression of virulence factors like capsule and melanin are well established. The regulation of cAMP pathway is mediated by adenylate cyclase which are activated in the presence of Mg2+ ion (Cramer et al., 2006). Even though Mg2+ is identified as the important structural component of biological molecules, the molecular identity of its regulation in yeast cell is poorly elucidated (Udeh et al., 2013). Pathogenicity of C. neoformans is in part due to capsule production which is primarily known to block phagocytic response of macrophages (Syme et al., 1999; Del Poeta, 2004; Oliveira et al., 2010). Mutation of four specific genes related to capsule biosynthesis and exocytosis, namely CAP10, CAP59, CAP60, and CAP64 has been shown to results in a acapsular phenotype (Okabayashi et al., 2005; Alarousy et al., 2011; Chun et al., 2011). However, the exact role of these four genes is still unclear, though CAP10 is a glycosyltransferase, CAP59 is important for capsule exocytosis, and CAP60 and CAP64 are probably involved in regulation of capsule biosynthesis (Chang and Kwon-Chung, 1998, 1999; García-Rivera et al., 2004; Chun et al., 2011). The increase in capsule size is associated with increase in virulence and resistance to host immune mediators and thus an understanding of the mechanism of capsule synthesis could be important to identify drug targets for effective therapy. Increase in capsule size has been previously shown to be induced by high CO2, low iron and glucose levels, while culture of C. neoformans in hypertonic medium containing 1 M NaCl was shown to decrease capsule thickness (Okabayashi et al., 2005; Guimarães et al., 2010). Apart from these reports there are no other studies to the best of our knowledge that have looked at the influence of buffer salts on capsule gene expression. In this study, we specifically looked at the effect of divalent cation Mg2+ on capsule gene expression and our results showed that Mg2+ had a differential effect on CAP gene expression and this effect was different for both the environmental and clinical isolates of C. neoformans. Mg2+ had an inducing effect on CAP10 gene of environmental isolate alone and similarly induced the expression of CAP59 in both isolates, while for CAP60, Mg2+ was able to induce its expression in clinical isolates. Under certain incubation conditions, Mg2+ appeared to have no inducing effect on the CAP genes and this was true in the case of CAP64 gene, wherein we failed to detect its expression in any of the media used, whereas the microscopic analysis revealed increased capsule size, suggesting the involvement of multiple CAP genes in capsule biosynthesis. ACSF on the other hand proved to be effective in inducing CAP59 in environmental isolate. Taken together our result on CAP gene expression (Figure 10) shows that the expression of CAP10, CAP59, and CAP60 was enhanced in the presence of Mg2+ for both the isolates, suggesting that Mg2+ concentration might be critical in regulating capsule synthesis and virulence of C. neoformans in vivo, and the exact concentrations of this important divalent cation in blood and CSF could influence the pathogenicity of C. neoformans during active infection. However, this remains a critical parameter to be assessed in meningitis patients to correlate with treatment outcome. Our findings on the role of Mg2+ ion as an inducing factor for capsule induction (Figures 11) could open up new avenues for targeting the capsule biosynthesis pathway in C. neoformans.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This study was financially supported by the Science and Engineering Research Board (SERB), Department of Science and Technology, New Delhi, under the Fast Track Young Scientist Scheme (No: SB/FT/LS-249/2012) to JR and (SERB/F/1266/2012-13) to TR.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Science and Engineering Research Board (SERB), Department of Science and Technology, New Delhi, for financial support. We sincerely thank the SASTRA University and its management for providing us the infrastructure needed to carry out our research work.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00325
Abbreviations
ACSF, artificial cerebrospinal fluid; C. neoformans, Cryptococcus neoformans; cAMP, cyclic adenosine monophosphate; CAP gene, capsule associated gene; Cn, Cryptococcus neoformans; CSF, cerebrospinal fluid; DMEM, Dulbecco’s modified eagle medium; FCS, fetal calf serum; GXM, glucuronoxylomannan; MOPS, 4-morpholinepropanesulfonic acid; PDB, potato dextrose broth; PKA, protein kinase.
References
Alarousy, R., Abo, H., Yazeed, E., Kotb, H., Abdalla, K., and Refai, M. (2011). Amplification of capsule-associated genes from Cryptococcus neoformans. N. Y. Sci. J. 4, 1–5.
Antinori, S. (2013). New insights into HIV/AIDS-associated cryptococcosis. ISRN AIDS 2013, 471363. doi: 10.1155/2013/471363
Bahn, Y. S., Hicks, J. K., Giles, S. S., Cox, G. M., and Heitman, J. (2004). Adenylyl cyclase-associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade. Eukaryot. Cell 3, 1476–1491. doi: 10.1128/EC.3.6.1476-1491.2004
Bicanic, T., and Harrison, T. S. (2004). Cryptococcal meningitis. Br. Med. Bull. 72, 99–118. doi: 10.1093/bmb/ldh043
Bolano, A., Stinchi, S., Preziosi, R., Bistoni, F., Allegrucci, M., Baldelli, F., et al. (2001). Rapid methods to extract DNA and RNA from Cryptococcus neoformans. FEMS Yeast Res. 1, 221–224. doi: 10.1016/S1567-1356(01)00030-7
Bose, I., Reese, A. J., Ory, J. J., Doering, T. L., and Janbon, G. (2003). A yeast under cover: the capsule of Cryptococcus neoformans. Eukaryot. Cell 2, 655–663. doi: 10.1128/EC.2.4.655
Buchanan, K. L., and Murphy, J. W. (1998). What makes Cryptococcus neoformans a pathogen? Emerg. Infect. Dis. 4, 71–83. doi: 10.3201/eid0401.980109
Campbell, L. T., Fraser, J. A., Nichols, C. B., Dietrich, F. S., Carter, D., and Heitman, J. (2005). Clinical and environmental isolates of Cryptococcus gattii from Australia that retain sexual fecundity. Eukaryot. Cell 4, 1410–1419. doi: 10.1128/EC.4.8.1410-1419.2005
CDC (2015). C. neoformans Infection Statistics|Fungal Diseases. Available at: http://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/statistics.html [Accessed February 4, 2016].
Chang, Y. C., and Kwon-Chung, K. J. (1998). Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun. 66, 2230–2236.
Chang, Y. C., and Kwon-Chung, K. J. (1999). Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J. Bacteriol. 181, 5636–5643.
Chang, Y. C., Penoyer, L. A., and Kwon-Chung, K. J. (1996). The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun. 64, 1977–1983.
Chowdhary, A., Randhawa, H. S., Sundar, G., Kathuria, S., Prakash, A., Khan, Z., et al. (2011). In vitro antifungal susceptibility profiles and genotypes of 308 clinical and environmental isolates of Cryptococcus neoformans var. grubii and Cryptococcus gattii serotype B from north-western India. J. Med. Microbiol. 60, 961–967. doi: 10.1099/jmm.0.029025-0
Chun, C. D., Brown, J. C. S., and Madhani, H. D. (2011). A major role for capsule-independent phagocytosis-inhibitory mechanisms in mammalian infection by Cryptococcus neoformans. Cell Host Microbe 9, 243–251. doi: 10.1016/j.chom.2011.02.003
Cramer, K. L., Gerrald, Q. D., Nichols, C. B., Price, M. S., and Alspaugh, J. A. (2006). Transcription factor Nrg1 mediates capsule formation, stress response, and pathogenesis in Cryptococcus neoformans. Eukaryot. Cell 5, 1147–1156. doi: 10.1128/EC.00145-06
Del Poeta, M. (2004). Role of phagocytosis in the virulence of Cryptococcus neoformans. Eukaryot. Cell 3, 1067–1075. doi: 10.1128/EC.3.5.1067-1075.2004
Emmons, C. W. (1955). Saprophytic Sources of Cryptococcus neoformans associated with the Pigeon (Columba livia). Am. J. Hyg. 62, 227–232.
Escandon, P., Sanchez, A., Martinez, M., Meyer, W., and Castaneda, E. (2006). Molecular epidemiology of clinical and environmental isolates of the Cryptococcus neoformans species complex reveals a high genetic diversity and the presence of the molecular type VGII mating type a in Colombia. FEMS Yeast Res. 6, 625–635. doi: 10.1111/j.1567-1364.2006.00055.x
Feldmesser, M., Tucker, S., and Casadevall, A. (2001). Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 9, 273–278. doi: 10.1016/S0966-842X(01)02035-2
Frases, S., Pontes, B., Nimrichter, L., Rodrigues, M. L., Viana, N. B., and Casadevall, A. (2009a). The elastic properties of the Cryptococcus neoformans capsule. Biophys. J. 97, 937–945. doi: 10.1016/j.bpj.2009.04.043
Frases, S., Pontes, B., Nimrichter, L., Viana, N. B., Rodrigues, M. L., and Casadevall, A. (2009b). Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proc. Natl. Acad. Sci. U.S.A. 106, 1228–1233. doi: 10.1073/pnas.0808995106
Frazzitta, A. E., Vora, H., Price, M. S., Tenor, J. L., Betancourt-Quiroz, M., Toffaletti, D. L., et al. (2013). Nitrogen source-dependent capsule induction in human-pathogenic Cryptococcus species. Eukaryot. Cell 12, 1439–1450. doi: 10.1128/EC.00169-13
Fridlington, E., Colome-Grimmer, M., Kelly, E., and Kelly, B. C. (2006). Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch. Dermatol. 142, 25–27. doi: 10.1001/archderm.142.1.25
García-Rivera, J., Chang, Y. C., Kwon-Chung, K. J., and Casadevall, A. (2004). Cryptococcus neoformans CAP59 (or Cap59p) is involved in the extracellular trafficking of capsular glucuronoxylomannan. Eukaryot. Cell 3, 385–392. doi: 10.1128/EC.3.2.385-392.2004
García-Rodas, R., Casadevall, A., Rodríguez-Tudela, J. L., Cuenca-Estrella, M., and Zaragoza, O. (2011). Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS ONE 6:e24485. doi: 10.1371/journal.pone.0024485
García-Rodas, R., Cordero, R. J. B., Trevijano-Contador, N., Janbon, G., Moyrand, F., Casadevall, A., et al. (2014). Capsule growth in Cryptococcus neoformans is coordinated with cell cycle progression. MBio 5, 1–13. doi: 10.1128/mBio.00945-14
Guimarães, A. J., Frases, S., Cordero, R. J. B., Nimrichter, L., Casadevall, A., and Nosanchuk, J. D. (2010). Cryptococcus neoformans responds to mannitol by increasing capsule size in vitro and in vivo. Cell. Microbiol. 12, 740–753. doi: 10.1111/j.1462-5822.2010.01430.x
Gupta, G., and Fries, B. C. (2010). Variability of phenotypic traits in Cryptococcus varieties and species and the resulting implications for pathogenesis. Future Microbiol. 5, 775–787. doi: 10.2217/fmb.10.44
Halliday, C. L., Bui, T., Krockenberger, M., Malik, R., Ellis, D. H., and Carter, D. A. (1999). Presence of?? and a mating types in environmental and clinical collections of Cryptococcus neoformans var. gattii strains from Australia. J. Clin. Microbiol. 37, 2920–2926.
Haynes, B. C., Skowyra, M. L., Spencer, S. J., Gish, S. R., Williams, M., Held, E. P., et al. (2011). Toward an integrated model of capsule regulation in Cryptococcus neoformans. PLoS Pathog. 7:e1002411. doi: 10.1371/journal.ppat.1002411
Jain, N., Li, L., McFadden, D. C., Banarjee, U., Wang, X., Cook, E., et al. (2006). Phenotypic switching in a Cryptococcus neoformans variety gattii strain is associated with changes in virulence and promotes dissemination to the central nervous system. Infect. Immun. 74, 896–903. doi: 10.1128/IAI.74.2.896-903.2006
Johnston, S. A., and May, R. C. (2013). Cryptococcus interactions with macrophages: evasion and manipulation of the phagosome by a fungal pathogen. Cell. Microbiol. 15, 403–411. doi: 10.1111/cmi.12067
Karkowska-Kuleta, J., Rapala-Kozik, M., and Kozik, A. (2009). Fungi pathogenic to humans: molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochim. Pol. 56, 211–224.
Kretschmer, M., Reiner, E., Hu, G., Tam, N., Oliveira, D. L., Caza, M., et al. (2014). Defects in phosphate acquisition and storage influence virulence of Cryptococcus neoformans. Infect. Immun. 82, 2697–2712. doi: 10.1128/IAI.01607-14
Lazéra, M. S., Pires, F. D. A., Camillo-Coura, L., Nishikawa, M. M., Bezerra, C. C. F., Trilles, L., et al. (1996). Natural habitat of Cryptococcus neoformans var. neoformans in decaying wood forming hollows in living trees. Med. Mycol. 34, 127–131. doi: 10.1080/02681219680000191
Levitz, S. M., and DiBenedetto, D. J. (1989). Paradoxical role of capsule in murine bronchoalveolar macrophage-mediated killing of Cryptococcus neoformans. J. Immunol. 142, 659–665.
Lian, T., Simmer, M. I., D’Souza, C. A., Steen, B. R., Zuyderduyn, S. D., Jones, S. J. M., et al. (2005). Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 55, 1452–1472. doi: 10.1111/j.1365-2958.2004.04474.x
Liao, S., Bitoun, J., and Nguyen, A. (2015). Deficiency of PdxR in Streptococcus mutans affects vitamin B6 metabolism, acid tolerance response and biofilm formation. Mol. Oral Microbiol. 30, 255–268. doi: 10.1111/omi.12090
Maidan, M. M., De Rop, L., Serneels, J., Exler, S., Rupp, S., Tournu, H., et al. (2005). The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol. Biol. Cell 16, 1971–1986. doi: 10.1091/mbc.E04-09-0780
Martinez, L. R., and Casadevall, A. (2005). Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect. Immun. 73, 6350–6362. doi: 10.1128/IAI.73.10.6350
Martinez, L. R., and Casadevall, A. (2006). Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 50, 1021–1033. doi: 10.1128/AAC.50.3.1021
Martinez, L. R., and Casadevall, A. (2007). Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 73, 4592–4601. doi: 10.1128/AEM.02506-06
Martinez, L. R., and Casadevall, A. (2015). Biofilm formation by Cryptococcus neoformans. Microbiol. Spectr. 3, 1–11. doi: 10.1128/microbiolspec.MB-0006-2014.Correspondence
Martinez, L. R., and Fries, B. C. (2010). Fungal biofilms: relevance in the setting of human disease. Curr. Fungal Infect. Rep. 4, 266–275. doi: 10.1007/s12281-010-0035-5
Miwa, T., Takagi, Y., Shinozaki, M., Yun, C., Schell, W. A., Perfect, J. R., et al. (2004). Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot. Cell 3, 919–931. doi: 10.1128/EC.3.4.919
Nimrichter, L., Frases, S., Cinelli, L. P., Viana, N. B., Nakouzi, A., Travassos, L. R., et al. (2007). Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot. Cell 6, 1400–1410. doi: 10.1128/EC.00122-07
Okabayashi, K., Kano, R., Watanabe, S., and Hasegawa, A. (2005). Expression of capsule-associated genes of Cryptococcus neoformans. Mycopathologia 160, 1–7. doi: 10.1007/s11046-005-0139-6
Okagaki, L. H., Wang, Y., Ballou, E. R., O’Meara, T. R., Bahn, Y. S., Alspaugh, J. A., et al. (2011). Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli Δ. Eukaryot. Cell 10, 1306–1316. doi: 10.1128/EC.05179-11
Oliveira, D. L., Freire-de-Lima, C. G., Nosanchuk, J. D., Casadevall, A., Rodrigues, M. L., and Nimrichter, L. (2010). Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 78, 1601–1609. doi: 10.1128/IAI.01171-09
O’Meara, T. R., and Alspaugh, A. J. (2012). The Cryptococcus neoformans capsule: a sword and a shield. Clin. Microbiol. Rev. 25, 387–408. doi: 10.1128/CMR.00001-12
O’Meara, T. R., Norton, D., Price, M. S., Hay, C., Clements, M. F., Nichols, C. B., et al. (2010). Interaction of Cryptococcus neoformans Rim101 and protein kinase a regulates capsule. PLoS Pathog. 6:e1000776. doi: 10.1371/journal.ppat.1000776
Park, B. J., Wannemuehler, K. A., Marston, B. J., Govender, N., Pappas, P. G., and Chiller, T. M. (2009). Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530. doi: 10.1097/QAD.0b013e328322ffac
Park, Y. D., Shin, S., Panepinto, J., Ramos, J., Qiu, J., Frases, S., et al. (2014). A role for LHC1 in higher order structure and complement binding of the Cryptococcus neoformans capsule. PLoS Pathog. 10:e1004037. doi: 10.1371/journal.ppat.1004037
Pettit, R. K., Repp, K. K., and Hazen, K. C. (2010). Biofilms to antifungal agents. Med. Mycol. 48, 421–426. doi: 10.3109/13693780903136879
Pukkila-Worley, R., and Alspaugh, J. A. (2004). Cyclic AMP signaling in Cryptococcus neoformans. FEMS Yeast Res. 4, 361–367. doi: 10.1016/S1567-1356(03)00241-1
Robertson, E. J., Najjuka, G., Rolfes, M. A., Akampurira, A., Jain, N., Anantharanjit, J., et al. (2014). Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in hiv-associated cryptococcal meningitis. J. Infect. Dis. 209, 74–82. doi: 10.1093/infdis/jit435
Robertson, E. J., Wolf, J. M., and Casadevall, A. (2012). EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans. Appl. Environ. Microbiol. 78, 7977–7984. doi: 10.1128/AEM.01953-12
Satishchandra, P., Mathew, T., Gadre, G., Nagarathna, S., Chandramukhi, A., Mahadevan, A., et al. (2007). Cryptococcal meningitis: clinical, diagnostic and therapeutic overviews. Neurol. India 55, 226–232. doi: 10.4103/0028-3886.35683
Souza, L. K., Fernandes Ode, F., Kobayashi, C. C., Passos, X. S., Costa, C. R., Lemos, J. A., et al. (2005). Antifungal susceptibilities of clinical and environmental isolates of Cryptococcus neoformans in Goiania city, Goias, Brazil. Rev. Inst. Med. Trop. Sao Paulo 47, 253–256. doi: 10.1590/S0036-46652005000500003
Syme, R. M., Bruno, T. F., Kozel, T. R., and Mody, C. H. (1999). The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect. Immun. 67, 4620–4627.
Taborda, C. P., and Casadevall, A. (2002). CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16, 791–802. doi: 10.1016/S1074-7613(02)00328-X
Udeh, O., Udeh, H. O., and Kgatla, T. E. (2013). Role of magnesium ions on yeast performance during very high gravity fermentation. J. Brew. Distill. 4, 19–45. doi: 10.5897/JBD2013.0041
Vecchiarelli, A., and Monari, C. (2012). Capsular material of Cryptococcus neoformans: virulence and much more. Mycopathologia 173, 375–386. doi: 10.1007/s11046-011-9513-8
Zaragoza, O., and Casadevall, A. (2004). Experimental modulation of capsule size in Cryptococcus neoformans. Biol. Proced. Online 6, 10–15. doi: 10.1251/bpo68
Zaragoza, O., Chrisman, C. J., Castelli, M. V., Frases, S., Cuenca-Estrella, M., Rodríguez-Tudela, J. L., et al. (2008). Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 10, 2043–2057. doi: 10.1111/j.1462-5822.2008.01186.x
Keywords: biofilm, Cryptococcus neoformans, capsule, CAP gene, magnesium ions
Citation: Rathore SS, Raman T and Ramakrishnan J (2016) Magnesium Ion Acts as a Signal for Capsule Induction in Cryptococcus neoformans. Front. Microbiol. 7:325. doi: 10.3389/fmicb.2016.00325
Received: 11 August 2015; Accepted: 29 February 2016;
Published: 15 March 2016.
Edited by:
Dominique Sanglard, University of Lausanne and University Hospital Center, SwitzerlandReviewed by:
John C. Panepinto, State University of New York at Buffalo, USAGeorge Tegos, Massachusetts General Hospital, USA
Copyright © 2016 Rathore, Raman and Ramakrishnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jayapradha Ramakrishnan, antibioticbiology@gmail.com, kavijayashal@gmail.com
 Sudarshan S. Rathore
Sudarshan S. Rathore Thiagarajan Raman
Thiagarajan Raman Jayapradha Ramakrishnan
Jayapradha Ramakrishnan