- 1Christian Doppler Laboratory for Monitoring of Microbial Contaminants, Institute for Milk Hygiene, Milk Technology and Food Science, Department for Farm Animals and Public Veterinary Health, University of Veterinary Medicine, Vienna, Austria
- 2Institute for Milk Hygiene, Milk Technology and Food Science, Department for Farm Animals and Public Veterinary Health, University of Veterinary Medicine, Vienna, Austria
When using bacteriophages to control food-borne bacteria in food production plants and processed food, it is crucial to consider that environmental conditions influence their stability. These conditions can also affect the physiological state of bacteria and consequently host–virus interaction and the effectiveness of the phage ability to reduce bacteria numbers. In this study we investigated the stability, binding, and replication capability of phage P100 and its efficacy to control Listeria monocytogenes under conditions typically encountered in dairy plants. The influences of SDS, Lutensol AO 7, salt, smear water, and different temperatures were investigated. Results indicate that phage P100 is stable and able to bind to the host under most conditions tested. Replication was dependent upon the growth of L. monocytogenes and efficacy was higher when bacterial growth was reduced by certain environmental conditions. In long-term experiments at different temperatures phages were initially able to reduce bacteria up to seven log10 units after 2 weeks at 4°C. However, thereafter, re-growth and development of phage-resistant L. monocytogenes isolates were encountered.
Introduction
Although bacteriophages have been known for about 100 years, commercial use of lytic bacteriophages to detect and control pathogenic bacteria has increased in recent years (Mann, 2005). In particular, the use of phages as an alternate class of antibacterial agents against food-borne pathogens is of growing interest due to advantages that they offer. These include high host specificity and the fact that phages do not change the quality and sensory perceptions of food (Hagens and Loessner, 2007, 2010; Mahony et al., 2011).
Phages and phage products are now commercially available against nearly all important food-borne bacteria. A phage that is commonly used to combat Listeria monocytogenes is P100. This phage was originally isolated from the waste water of a dairy plant and is commercially available as ListexTM P100. It has now been confirmed Generally Regarded As Safe (GRAS) by the US Food and Drug Administration (FDA; EFSA, 2009).
Listeria monocytogenes is one of the most important food-borne pathogens. Due to its ability to grow or persist at low pH values, high salt concentrations, low temperatures and in environments with low water activity, it is frequently found in adverse environments, such as food production plants (Koutsoumanis and Sofos, 2005; Swaminathan and Gerner-Smidt, 2007). When phages are used to combat bacteria in food production plants it is crucial to consider that factors such as heat, cold, dryness, nutrient deficit, curing, and exposure to chemical detergents or disinfectants influence phage stability, the physiological state of bacteria and consequently host–virus interactions (for instance attachment and replication of the phage) and the effectiveness of phages at reducing bacteria numbers (Ganegama Arachchi et al., 2013a,b; Chaturongakul and Ounjai, 2014; Denes and Wiedmann, 2014). In food production plants external factors such as temperature, pH, and water activity particularly affect the success of phage treatments (Garcia et al., 2008; Jończyk et al., 2011; Ly-Chatain, 2014).
The first requirement for the successful application of phages for biocontrol of bacteria is phage stability. Some authors assume a relationship between a phage’s morphology and its occurrence and infectivity in adverse environments, but evidence supportive of that hypothesis and predictions that can be made is lacking (Lasobras et al., 1997). In general, bacteriophage stability is highly variable and sensitivity of individual phage classes is highly diversified (Jończyk et al., 2011). It is known that the stability of a virus can be affected by changes of its virion or viral nucleic acid structure by various factors such as pH, ionic strength of the immediate environment, UV-light or heat (Maura and Debarbieux, 2011; Ly-Chatain, 2014). However, bacteriophages are the most abundant biological entities on earth, play a major role on nutrition, energy and global biogeochemical cycles (Fuhrman, 1999; Wommack and Colwell, 2000; Breitbart et al., 2002; Chibani-Chennoufi et al., 2004; Breitbart and Rohwer, 2005; Mann, 2005) and consequently are found in a range of adverse environments which theoretically could affect phage stability (Jończyk et al., 2011). For instance phages were found in environments with high UV irradiation and heat as the Sahara (Prigent et al., 2005) or hot springs (Breitbart et al., 2004) or in environments with various pH values and ionic strenghts like in food (Nel et al., 1987; Lu et al., 2003; Pringsulaka et al., 2011), cheese factories (Bruttin et al., 1997), humans (Bachrach et al., 2003), soil (Williamson et al., 2003), and sewage (Havelaar and Hogeboom, 1984). Moreover, maintenance of phage populations usually requires the presence of the bacterial host, which is also influenced by environmental factors (Maura and Debarbieux, 2011). As mentioned, bacterial fitness and physiological states can change the host–virus interaction.
The first step in host–virus interaction is attachment. On one hand attachment of the phages to bacteria and the susceptibility of the host can be reduced when bacterial fitness is compromised. A change in the physiological status of bacteria can lead to transcriptional responses that influence cell wall structures, which serve as receptors for phages (Denes and Wiedmann, 2014). Modified phage receptors could complicate or even prevent binding of the phage to the host. On the other hand, external factors, such as the presence of whey proteins, can result in non-specific binding or trapping of the phages thereby reducing host adsorption rates (Gill et al., 2006a). Another factor worth keeping in mind when bacteriophages are used to control food-borne bacteria, is accessibility to the target bacteria. Accessibility to the host can be limited when phages are applied in solid matrices or when the phage concentration and time of application are not optimal (Ly-Chatain, 2014).
Following attachment of phages to the host, the phage genome is injected into the host cell and replication of the phage particle can commence. However, inadequate nutrition, poor environments and a switch to the stationary growth phase lead to decreased productivity of phage infection and to small burst size as phage replication is dependent on host cell growth (Chibani-Chennoufi et al., 2004; Denes and Wiedmann, 2014).
Even though attachment and replication have major influences on the effectiveness of phage treatments, external factors can directly affect the outcome of phage infections. For instance, the presence of inhibitory compounds such as antibodies, whey proteins or bacteriocins can reduce the effectiveness of phage treatments and can even lead to resistance against the phages used (Maura and Debarbieux, 2011; Tessema et al., 2011; Abedon, 2012; Meaden and Koskella, 2013; Vongkamjan et al., 2013; Chaturongakul and Ounjai, 2014; Ly-Chatain, 2014). Suboptimal application of phages can also lead to adaptation of the bacteria to the phage, the development of phage resistance and consequently ineffective treatment (Hagens and Loessner, 2010).
Although it is known that several factors influence the efficacy of phage treatments against food-borne pathogens, relevant studies detailing interactions are limited and current research is still at an early stage (Garcia et al., 2008; Ganegama Arachchi et al., 2013b; Vongkamjan et al., 2013). Until now it has not been clear as to what extent environmental factors influence bacterial susceptibility to phage infection (Denes and Wiedmann, 2014). Moreover, past failures in phage therapy were mostly caused by limited knowledge of phage biology (O’Flaherty et al., 2009). Therefore, some investigators recommend testing of phage persistence in the absence of target bacteria (Chan et al., 2013). Efficacy testing of phage treatments has been suggested for each type of application and under different environmental conditions on a case-by-case basis (Skurnik et al., 2007; Garcia et al., 2008; Ganegama Arachchi et al., 2013a).
Therefore, the main focus of this study was to test the effectiveness of phage P100 against L. monocytogenes under conditions that can be found in food-processing plants. The influence of various chemical and physical factors on the infectivity and persistence of phage P100 was tested. These included the effect of different temperatures, pH values, salt and detergent concentrations, smear water and Fraser broth on the stability preinfection, the attachment on the host, and replication after injection of viral nucleic acid. Moreover, the effectiveness of phage P100 at eliminating L. monocytogenes under the above conditions was investigated. Long-term infection experiments were also conducted to examine the development of resistant L. monocytogenes isolates at different temperatures.
Materials and Methods
Bacterial Strain, Phage, and Growth Conditions
The phage P100 susceptible Listeria strain used in this study was L. monocytogenes reference strain ATCC BAA-679 (EGDe). To establish standardized conditions, bacteria were grown in tryptone soya broth (TSB) with 0.6% (w/v) yeast extract (OxoidLdt., Hampshire) at 37°C. Overnight cultures were 10-fold diluted in fresh medium and incubated at 37°C for 3–4 h to obtain a maximum number of viable cells in the logarithmic growth phase (log phase). Phage P100 was supplied as the commercial preparation ListexTM P100 (EBI Food Safety Wageningen, Netherlands). PFU (plaque forming units) determination resulted in a phage titre of approximately 6 × 1010 PFU/ml. The ListexTM P100 preparation was diluted in SM buffer (5.8 g NaCl, 2.4 g Tris HCl, 1.0 g gelatine add. 1,000 ml, pH 7.0) to yield phages at other concentrations.
Influence of Environmental Factors on the Infectivity of P100
To investigate the persistence and infectivity of P100 in the dairy environment over a longer time period, phages were added to different smear water samples (5 × 1010 PFU/ml final concentration) and stored at 4 and 10°C. Two types of smear water were obtained from an Austrian dairy plant. Each smear water type was then split into four samples and each sample was pre-treated as follows: the first sample was the original untreated smear water obtained from the dairy plant. The second sample was autoclaved smear water. The third sample was smear water inoculated with L. monocytogenes EGDe (5 × 108 CFU). The fourth sample was smear water supernatant after centrifuging for 5 min at 8,000 × g in order to remove bacterial or eukaryotic (e.g., yeast) cells. Phage infectivity was determined every 10 days over a total of 117 days using the double agar overlay plaque assay (Kropinski et al., 2009) as previously described (Fister et al., 2016). As a non-treatment control, phage infectivity following incubation in SM buffer was monitored. Each experiment with each type of smear water was performed in duplicate.
To test the short-time influence of chemicals on P100 stability in terms of infectivity, phages were incubated in TSB containing either 2 M NaCl (Fisher Scientific, Leics, UK), the detergents Lutensol AO 7 (BASF, Ludwigshafen, Germany) and sodium dodecyl sulfate (SDS; SIGMA-ALDRICH, Steinheim, Germany; 5% each) and TSB adjusted to the pH values of 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 using 0.5 M HCl or NaOH (Merck, Darmstadt, Germany). After 1, 6, and 24 h the number of phages was determined using the double agar overlay plaque assay. For comparison and as a non-treatment control, phages were incubated in TSB. Each experiment was carried out at least in triplicate on different days.
Adsorption Tests
To determine binding and replication characteristics of P100, adsorption tests were performed under different environmental conditions. The variables were salt concentration, pH, concentration of detergents and temperature.
Log phase bacteria cultures were adjusted to an OD600 of 0.6. Thereafter bacteria were centrifuged at 8,000 × g for 5 min. The pellet was resuspended in TSB medium representing the chemical conditions described above (see Influence of Environmental Factors on the Infectivity of P100; pH 2 and 12 were not further tested as phage infectivity was reduced more than seven log10 units within 1 h). Phage P100 was added to a MOI (multiplicity of infection, ratio of phages to bacteria) of 0.1. In order to determine adsorption of the phages on the host under these conditions, the infection was stopped by the addition of ice-cold TSB followed by centrifugation for 2 min at 8,000 rpm after 0, 10, 20, 30, 40, 50, and 60 min as described by Wendlinger et al. (1996). Afterward, phages remaining in the supernatant were serially diluted in SM buffer and quantified using the double agar overlay plaque assay. To monitor the replication ability of the phage at the conditions described, the number of phages was measured after stopping the infection after 0, 1, 3, and 6 h (and after 12 and 24 h at pH 4). All experiments were carried out at least twice in duplicate.
Chemical Influence on the Efficacy of Phage Treatment (Short-Term)
To analyze the influence of different chemical conditions on the efficacy of phage treatments, L. monocytogenes EGDe was infected with phage P100 and grown in Fraser Listeria Selective Enrichment Broth base (Merck, Darmstadt, Germany), TSB and TSB medium with different salt concentrations, detergent (Lutensol AO 7 and SDS) concentrations and in TSB adjusted to different pH values (see Influence of Environmental Factors on the Infectivity of P100). Additionally, the efficacy of phage treatment in smear water, obtained from an Austrian dairy plant, was investigated. The influence of these factors on the growth of uninfected L. monocytogenes EGDe was monitored as a control.
In detail, two concentrations of log-phase L. monocytogenes EGDe cultures (approximately 2.5 × 107 and 2.5 × 106 CFU/ml, 1:10 and 1:100 dilutions of a OD610 = 0.6 log phase culture, respectively) were infected with P100, with a MOI of 10, and were incubated at 37°C in TSB media representing the chemical conditions described above. The OD610 of these infections was measured for 24 h every hour in a TECAN F100 microplate reader (Tecan Austria GmbH., Groeding, Austria). The experiments were performed at least twice on different days and in duplicate.
Influence of Temperature on the Efficacy of P100 Treatments (Long-Term)
To examine the influence of temperature on the efficacy of phage treatments, 500 μl of a log phase L. monocytogenes EGDe culture was incubated with an equal volume of ListexTM P100 dilutions (2 × 1010 and 2 × 109 PFU/ml, resulting in MOIs of 10 and 100) for 30 min at room temperature. Thereafter, 5 ml of TSB was added and the infected Listeria stored at 4, 10, and 20°C for 17 weeks. The number of surviving bacteria was determined weekly using the plate count method and compared to a non-infected control of the same isolate (treated with SM buffer instead of ListexTM P100). All plating was performed in duplicate on TSA with at least two different dilutions and plates were incubated overnight at 37°C. The experiment was performed twice.
Screening for Newly Formed Resistances and Confirmation of Insensitive Isolates
When re-growth of L. monocytogenes in the long-term temperature experiments (Influence of Temperature on the Efficacy of P100 Treatments (Long-Term)) was observed, single colonies growing on the TSA plates of the experiment were selected and used for preparation of overnight cultures. These cultures were then used to screen for insensitive L. monocytogenes isolates using cross streak tests (Miller, 1998) as follows: 50 μl P100 (6 × 1010 PFU/ml) were allowed to run from the top to the bottom of TSA+Y plates. After drying, the streak was crossed with the cultures of re-growing L. monocytogenes isolates. L. monocytogenes EGDe was used as sensitive control. Plates were incubated overnight at 37°C. Suspicious isolates, which grew in the phage-zone of the plates, were confirmed as L. monocytogenes by plating on selective ALOA and PALCAM agar. Additionally, suspicious isolates were confirmed as L. monocytogenes by PCR as described by Rossmanith et al. (2006). Resistance and reduced susceptibility were confirmed by small drop plaque assays (Mazzocco et al., 2009). Stability of resistance was tested by passaging the isolates for five passages.
Results
Phage P100 Is Stable under Most Tested Chemical Conditions
For the investigation of P100 stability in SM buffer and different types of smear water over a period of 4 months, comparison revealed similar results for samples that were stored at 4 and 10°C (Figures 1A,B). At both incubation temperatures the decrease in the number of infective phages was lowest when P100 was stored in SM buffer, followed by phages stored in autoclaved smear water. In both experimental conditions, the number of phages decreased within the first month by about 1–2 log10 units and did not change distinctly until the end of the investigation. Phages stored in untreated smear water were reduced by about 2.5–3 log10 units. The highest phage reduction was observed in smear water containing L. monocytogenes EGDe. In this case P100 was reduced by four log10 units.
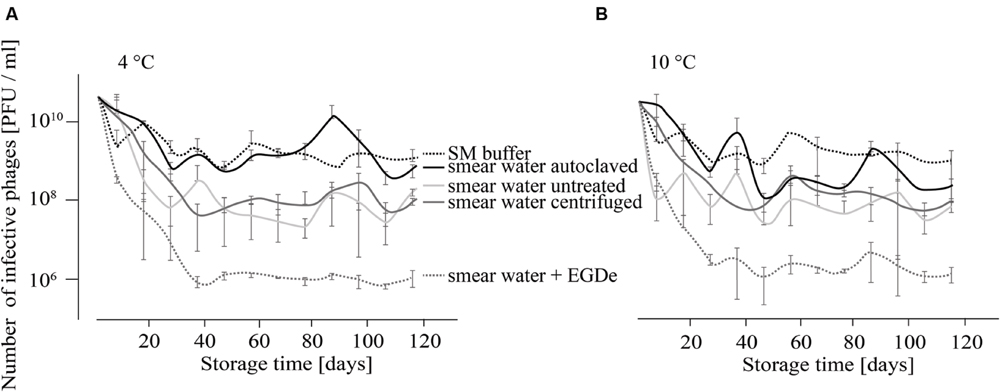
FIGURE 1. Infectivity of P100 stored in smear water and SM buffer at 4°C (A) and 10°C (B) for 117 days. Smear water was either untreated (as obtained from the dairy plant), autoclaved or centrifuged in order to remove bacterial or eukaryotic (e.g., yeast) cells. Further, L. monocytogenes EGDe was added to one smear water sample. Experiments were done twice in duplicates and mean values and standard deviations were shown.
As a next step in the short-term experiments, phage P100 was incubated in TSB containing different detergents or adjusted to different pH values since food can have a wide range of pH values. Infectivity of P100 was then determined after 1, 6, and 24 h of incubation (Table 1). At pH 2, P100 numbers were rapidly reduced below the detection limit, indicating a reduction of at least 7–8 log10 units within 1 h. At pH 3, P100 concentrations decreased within 1 h by at least 5.4 log10 units and P100 numbers were reduced below the detection limit (7–8 log10) at 6 h. At pH 4–10, P100 numbers were not distinctly reduced and even after 24 h the decrease in phage number was ≤0.5 log10 units. At pH 11 phage numbers were reduced by nearly 1 log10 level within 1 h. After 24 h, infectivity of phages was also reduced by about 1 log10 unit compared to the control. Five percent Lutensol AO 7 did not change the phage number within 1 day. The second tested detergent, SDS, reduced P100 numbers by about 0.3 log10 units, when the SDS concentration in TSB was 5%. After 6 and 24 h of incubation, the phage concentration was decreased by 0.6 and 1.2 log10 units.
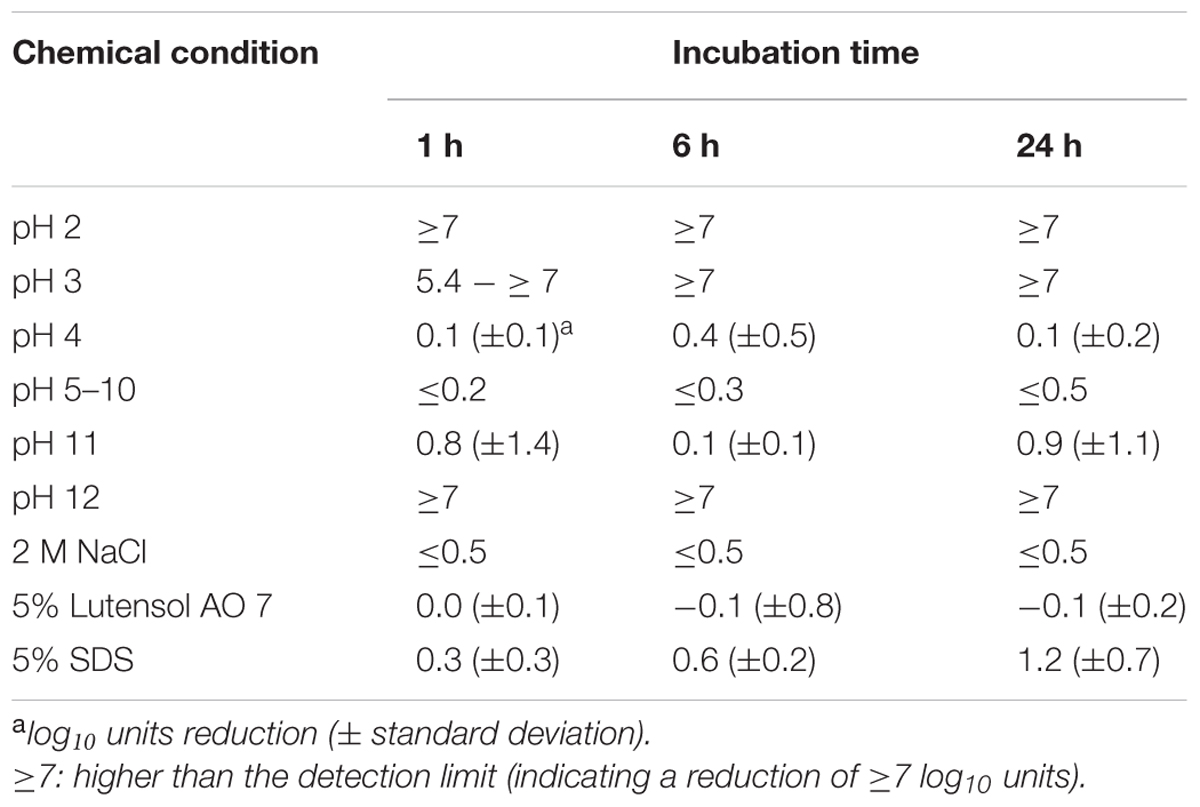
TABLE 1. Reduction of infectivity of P100 after incubation in TSB adjusted to different pH values, containing NaCl or detergents.
Binding of Phage P100 Was Possible, but Its Replication Was Not Observed at all Tested Chemical Conditions
In order to test the influence of different chemical conditions on the attachment of P100 to L. monocytogenes EGDe, adsorption tests in different smear water samples, in Fraser medium, in TSB media containing different NaCl or detergent concentrations and in TSB media with different pH values were performed and the respective results are shown in Figure 2. For all tested incubation conditions distinct binding of phage P100 to L. monocytogenes, indicated by an initial drop in free phage counts in the supernatant, was found. At NaCl concentrations up to 0.1 M an increase of free phages in the supernatant was observed after 30 min (Figure 2A), indicating replication of P100. At 0.5 M NaCl the number of unattached phages increased after 40 min and at 1 and 2 M NaCl no increase in free phage numbers was observed within 60 min. In Lutensol and SDS-containing TSB medium, in all tested smear water samples and in the selective Fraser medium, there was also no detected increase in unattached phages within 1 h, except in the presence of 0.1% SDS (Figures 2B,D,E). At all tested pH values an increase of free phages was observed 40 min after infection (Figure 2C). The number of unattached phages 60 min after infection was lowest at pH values 3 and between 8 and 11.
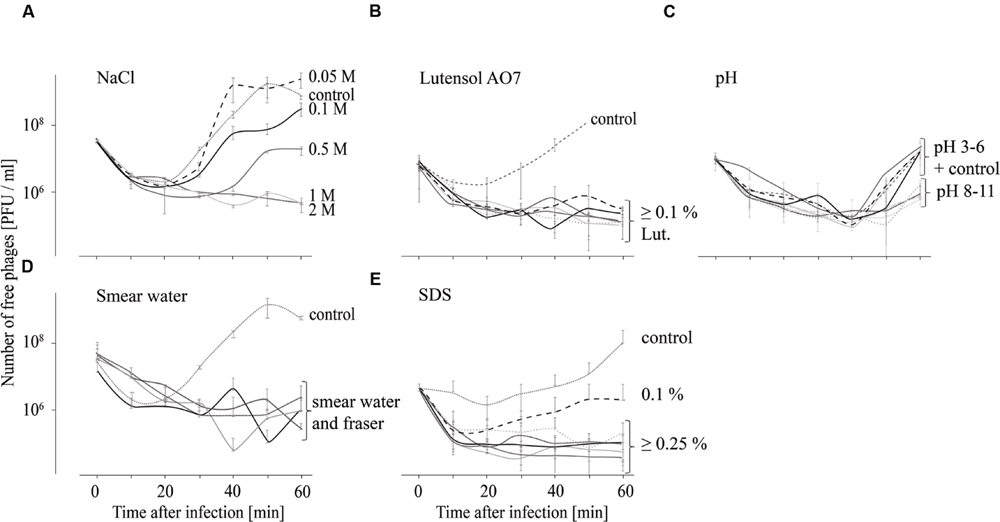
FIGURE 2. Adsorption tests performed over 60 min indicating attachment (decreasing number of free phages) of phage P100 to L. monocytogenes in TSB containing NaCl (A), Lutensol AO 7 (B), TSB adjusted to pH values 3–11 (C), smear water and Fraser (D), and TSB containing SDS (E). All experiments were done twice and in duplicates and mean values and standard deviations were shown.
As replication of phages was not observed at all tested conditions within 1 h after infection, adsorption tests were also performed over 6 h in order to obtain an indication if phage replication at different chemical conditions (described above) is possible. Results indicate that replication of phages is not possible at all tested conditions (Figure 3) although phage stability was not strongly impaired at all tested conditions within the first 6 h (Table 1). When the influence of salt was tested, the number of free phages was very similar in TSB media containing up to 0.1 M NaCl compared with the TSB control (Figure 3A). The number of free phages in TSB containing 0.5 or 1 M NaCl distinctly increased later compared with the TSB control. In TSB media containing 2 M NaCl an increase in free phages was observed after 1 h. However, after 6 h phage concentration was similar to that at the beginning of the experiment. These results indicate that replication of P100 is influenced by increasing concentrations of NaCl. Adsorption tests in TSB media with pH values ranging from 5 to 11 indicate that replication of phage P100 in L. monocytogenes is possible (Figure 3B). At pH value 4 the number of free phages decreased for up to 3 h after infection. Thereafter, there was a slight increase in phages up to 6 h after infection. The concentration of phages measured at the beginning of the experiment was not reached indicating no replication of phage P100 at pH 4. Even 12 or 24 h after infection the number of free phages at pH 4 did not increase indicating that no replication was possible (Supplementary Figure S1). The effect of detergents on replication of phage P100 was tested by performing adsorption tests in TSB media containing 0.1–5% Lutensol AO 7 or SDS (Figures 3C,D). At all Lutensol AO 7 concentrations (≤0.25%) the number of free phages increased between 1 and 3 h, indicating that P100 replication is possible. In TSB media containing 0.5–5% Lutensol AO 7, the concentration of free phages also increased between 1 and 3 h, but after 6 h the phage concentrations had still not reached initial values. In the presence of the second detergent, SDS, no increase of free phages was observed when concentrations ≥0.25% are present (Figure 3D). These data indicate that phage replication did not take place. In contrast, in TSB containing 0.1% SDS phage numbers were observed to increase after 1 h, indicating replication of P100.
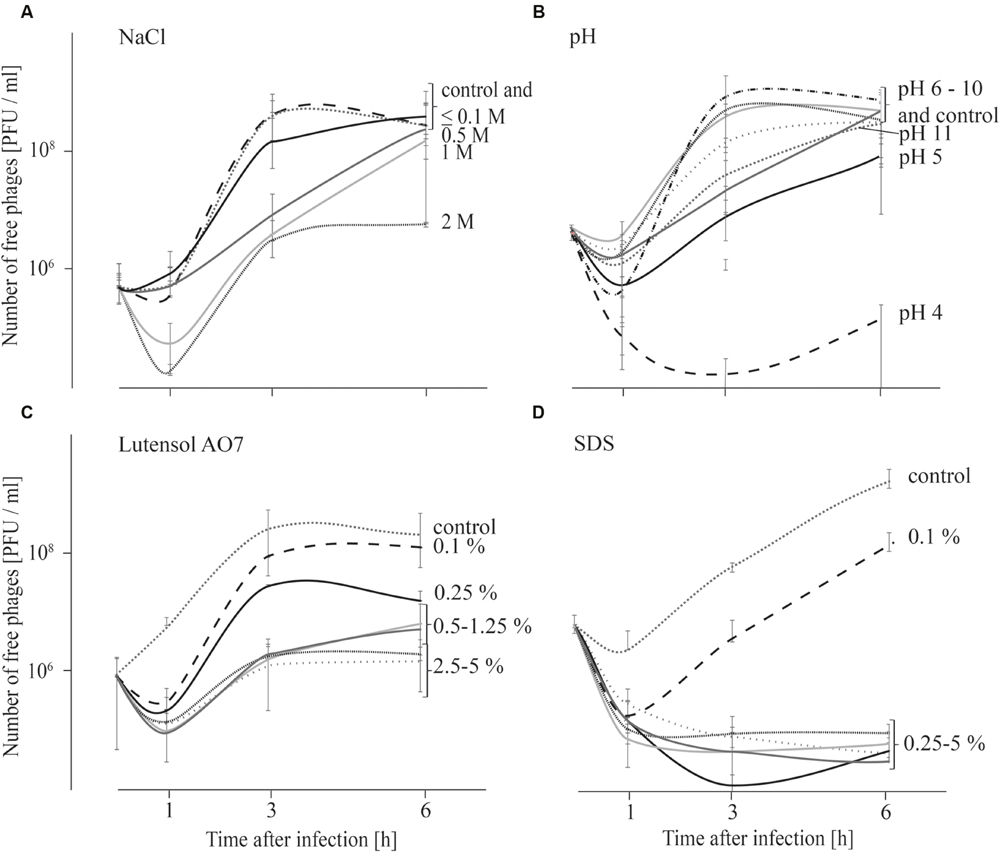
FIGURE 3. Adsorption tests performed over 6 h in TSB media containing NaCl (A), adjusted to different pH values (B) and containing the detergents Lutensol AO 7 (C) or SDS (D). Graphs show the number of free (unattached extracellular) phages. All experiments were carried out twice and in duplicates and mean values and standard deviations are shown.
Chemical Conditions that Reduce the Growth of L. monocytogenes Lead to Higher Efficacy of Phage Treatments
In order to test the effect of different chemical conditions on the efficacy of P100 to reduce L. monocytogenes EGDe, bacteria (2.5 × 107 and 2.5 × 106 CFU/ml) were infected with P100 with a MOI of 10 and the growth of bacteria in TSB media adjusted to different pH values or containing different NaCl or detergent concentrations was monitored by measurement of OD610 over 24 h. All experiments were carried out twice and in duplicate. In some cases, growth of L. monocytogenes was observed at shifted time points or there was growth in one experiment, but not when repeated. In Supplementary Figures S2–S4 each single growth curve that was measured is shown. For the sake of simplicity Figures 4–6 show representative growth curves for one measurement of each tested condition.
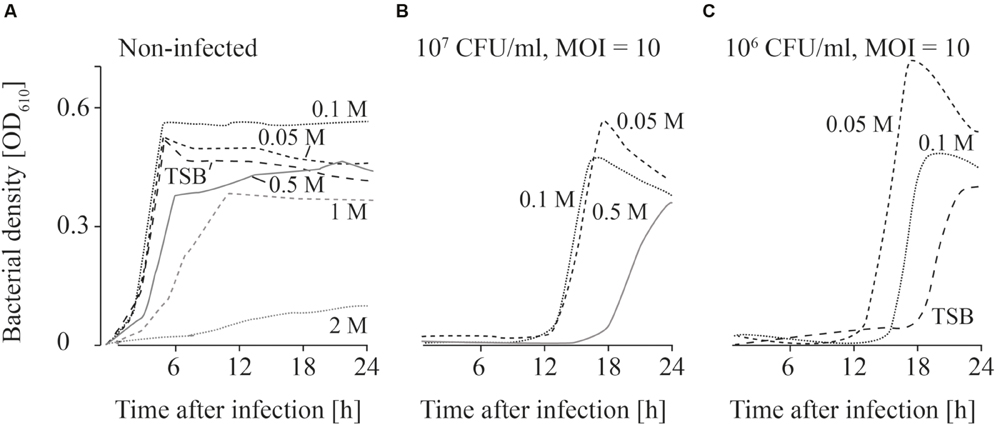
FIGURE 4. Growth curves of uninfected (A) and infected (B,C; MOI = 10) L. monocytogenes in TSB and NaCl containing TSB media. Bacteria concentrations at the beginning of the infections were 107 CFU/ml (A,B) and 106 CFU/ml (C). All NaCl concentrations (0–2 M) were tested. For the sake of clarity only the curves of growing bacteria were demonstrated while the others on base line level were not depicted. Moreover, one of four independent experiments is representatively shown.
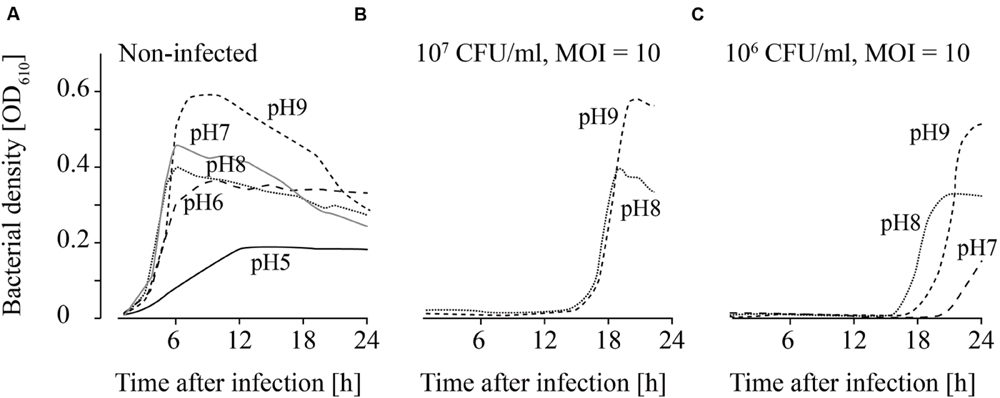
FIGURE 5. Growth of uninfected (A) and infected (B,C; MOI = 10) L. monocytogenes in TSB adjusted to different pH values. The bacteria concentrations at the beginning of the infections were 107 CFU/ml (A) and (B) and 106 CFU/ml (C). All pH values (4–11) were tested. For the sake of clarity only the curves of growing bacteria were demonstrated while the others on base line level were not depicted. Moreover, one of four independent experiments is representatively shown.
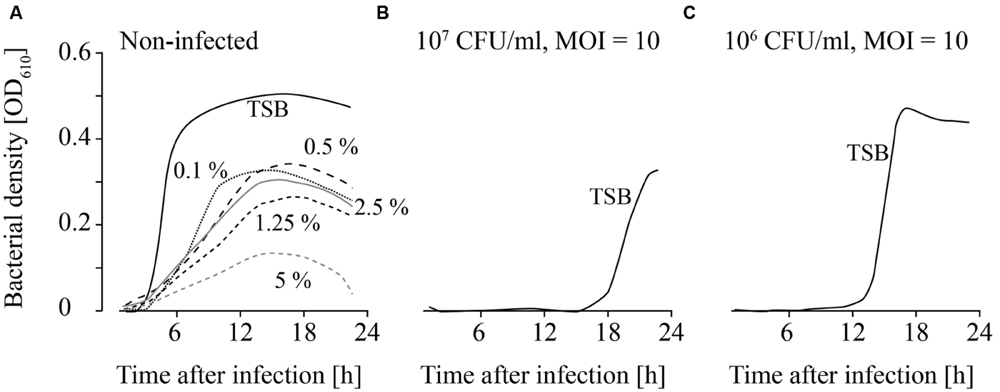
FIGURE 6. Growth of uninfected (A) and infected (B,C; MOI = 10) L. monocytogenes in TSB containing different Lutensol AO 7 concentrations. Bacteria concentrations at the beginning of the infections were 107 CFU/ml (A,B) and 106 CFU/ml (C). All concentrations (0–5%) were tested. For the sake of clarity only the curves of growing bacteria were demonstrated while the others on base line level were not depicted. Moreover, one of four independent experiments is representatively shown.
When the effects of Fraser medium (Supplementary Figures S2A–D) and NaCl were tested on the growth of uninfected L. monocytogenes, a distinct reduction in growth was only observed in TSB medium containing 2 M NaCl (Figure 4A; Supplementary Figure S2). When 107 CFU/ml were infected with phage P100 (MOI = 10), growth of L. monocytogenes was suppressed in TSB and TSB containing 1 and 2 M NaCl (Figure 4B). Growth of L. monocytogenes in TSB containing between 0.05 and 0.5 M NaCl and in Fraser broth (Supplementary Figures S2E–H) was observed in all experiments. When lower bacteria concentrations (106 CFU/ml) were infected, in three out of four experiments growth of L. monocytogenes was observed in Fraser medium (Supplementary Figures S2I–L), in 0.05 and 0.1 M NaCl containing TSB medium and in half of the experiments in TSB media (Figure 4C).
Uninfected L. monocytogenes were able to grow in TSB adjusted to pH values ranging from 6 to 9 (Figure 5A). Bacterial growth was also observed at pH 5 in three of four experiments, although lower OD values were obtained. When higher L. monocytogenes concentrations (107 CFU/ml) were infected, phage P100 was not able to suppress growth of L. monocytogenes at pH values 8 and 9 longer than 12–20 h (Figure 5B). In one of four repetitions at pH 7, growth of L. monocytogenes was observed. When lower bacteria concentrations were infected (106 CFU/ml), growth of L. monocytogenes was possible at pH 8 and 9 (Figure 5C). At pH 7 bacteria growth was monitored in two of four repetitions and at pH 6 in three of four repetitions.
Detergents showed the strongest impact on the growth of both, infected and non-infected, L. monocytogenes. L. monocytogenes was able to grow at all Lutensol AO 7 concentrations (0.1–5%) tested (Figure 6A). However, even at low Lutensol concentrations, growth of L. monocytogenes was distinctly reduced compared with growth in TSB media without Lutensol. When phages were added, growth was suppressed for at least 24 h at all concentrations tested (Figures 6B,C). The only exception was 0.1% Lutensol in TSB. Here, L. monocytogenes started to grow 15 h post-infection in one out of four replicates (Supplementary Figures S4I–L).
L. monocytogenes was not able to grow in TSB containing 0.1–5% SDS for the first 24 h (data not shown). Consequently, no infection experiments were performed.
Development of Phage Insensitive L. monocytogenes Isolates Was Observed at all Tested Temperatures
As growth of L. monocytogenes at low temperatures is slow, the influence of temperature and host–phage ratio on the efficacy of P100 treatments was tested in longer-term experiments. L. monocytogenes EGDe was infected with MOIs of 10 and 100, incubated at 4, 10, and 20°C and the number of surviving bacteria determined weekly for 17 weeks.
At 4°C L. monocytogenes was reduced by six and seven log10 units (MOI = 10 and MOI = 100) within the first 2 weeks (Figure 7A). Thereafter re-growth was observed. At 10 and 20°C the reduction in L. monocytogenes counts was maximal at 4–5 log10 units and re-growth of L. monocytogenes was also observed (Figures 7B,C).
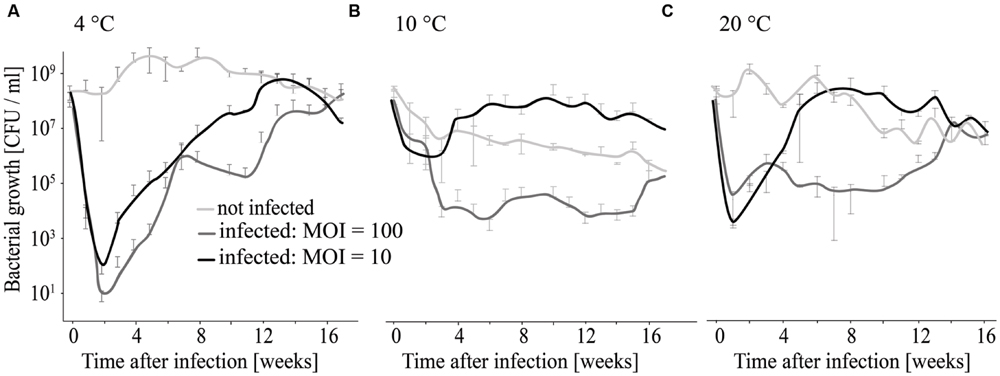
FIGURE 7. Long-term infection experiment using different MOIs and temperatures for incubation. Growth of infected and non-infected L. monocytogenes was monitored at 4°C (A), 10°C (B), and 20°C (C).
As described above, following an initial reduction of bacteria at the beginning of infection, re-growth of L. monocytogenes was observed at all tested temperatures and at both tested phage concentrations. In order to determine if survival and re-growing L. monocytogenes results from decreased efficacy of phage P100 or is caused by the resistance development, L. monocytogenes isolates were randomly selected at the end of the experiment. Afterward, they were tested for their susceptibility to P100. L. monocytogenes isolates that were obtained from uninfected samples were all susceptible to phage P100. In contrast, all L. monocytogenes isolates that were obtained from infected samples (independent of which MOI was used for infection and at which temperature the infected bacteria were incubated), had reduced susceptibility to P100 (at least four log10 units reduction compared to non-infected L. monocytogenes EGDe, see Supplementary Table S1). Moreover, susceptibility to the phage did not change over five passages, indicating that insensitivity to P100 was stable.
Discussion
In food production bacteriophages have become more and more attractive as tools to eliminate L. monocytogenes (Guenther et al., 2009; Soni et al., 2010; Guenther and Loessner, 2011; Soni et al., 2012; Oliveira et al., 2014). In a recent publication Fister et al. (2016) investigated the basic issues regarding the occurrence of P100 resistant L. monocytogenes associated with the application of ListexTM. A connection between use of ListexTM and the presence of resistant L. monocytogenes strains was demonstrated. Additionally, the host relationship was investigated in vitro.
However, in vivo especially in food production plants there are harsh environmental conditions. Therefore, this study took into account the influence of several of these chemical and physical factors on P100 that are typically encountered in the food-production environment. These conditions potentially influence the ability of P100 to reproduce by acting on the phage’s infection cycle. Relevant factors are: (i) the stability of the phage before infection, (ii) the attachment process after contact with the host, (iii) replication after attachment, injection of the phage genome, and finally (iv) the efficacy of the infection, according to reduction of bacterial cell numbers. These four parameters were used to analyze the efficacy of P100 against L. monocytogenes.
In following the sequence of events during the reproductive infection cycle of P100 we examined the influence of pH, salt concentration and the presence of two detergents as representative of chemical factors present in the production environment. The respective experiments were conducted short-term (up to 24 h). Moreover, long-term experiments were conducted for 120 days.
First of all the stability of the persisting phage particles was investigated, as loss of phage infectivity would result in a rapid decrease of overall efficacy of phage treatments (Gill et al., 2010). We tested the stability of phage P100 because during the cheese ripening process the pH increases and this consequently supports the growth of Listeria (Guenther and Loessner, 2011). In this study we did not observe a significant reduction in phage P100 numbers in TSB media over the pH range 4–10 within 24 h (Table 1).
These data indicate that P100 is stable over a wide pH range. In contrast, other phages were decreased on sliced apples due to the low pH (4.4) and were consequently not able to prevent microbial growth (Leverentz et al., 2003). Our results showed that P100 numbers rapidly decreased by >5 log10 units within 1 h, only when the pH values were ≤2 and ≥12. These results reflect the importance to test the stability of each phage individually and indicate that phage P100 can probably be inactivated by the use of disinfectants with very low or very high pH values.
As salt is one of the most frequent additions to food, and since it plays an important role in cheese production (brine wash contains 15–20% NaCl; Carlton et al., 2005), we tested the effect of various NaCl concentrations on phage P100. Salt is known to influence osmotic pressure, leading to breaks in phage heads and tails (Jończyk et al., 2011). However, in our study no reduction in phage infectivity was observed when P100 was stored for up to 24 h in TSB containing 2 M NaCl.
Cleaning and disinfection are also major ongoing activities in the food safety environment and in food production. The most commonly used detergents for cleaning are SDS and a group of detergents summarized as Lutensols. Lutensols are polymeric ethoxylated aliphatic alcohols that act as non-ionic tenside detergents, which means that they, like SDS, are surfactants. The particular advantage of Lutensols is that they are considered to be biodegradable and significantly less toxic than SDS (Smułek et al., 2015). In our study we found no reduction in phage P100 infectivity in the presence of up to 5% Lutensol AO 7 in TSB within 24 h, whereas 5% SDS reduced phage infectivity by about 1.2 log10 units within 24 h. This finding is in agreement with another study, in which it was speculated that SDS may be capable of denaturing the capsid proteins of non-enveloped viruses (Howett et al., 1999).
Smear water is a major application area for ListexTM. Therefore we tested the influence of smear water on the stability of P100 at different temperatures. As recommended by other authors, phage persistence with and without target bacteria was examined (Chan et al., 2013).
The infectivity of P100 was reduced faster in smear water than in SM buffer (Figure 1). The presence of L. monocytogenes increased this effect distinctly, which was expressed as 2 log10 units reduction in the PFU number. This could be due to different reasons. One reason could be that phages bind to the host cells and can therefore not be detected. Additionally, bacteria have several phage defense mechanisms which could reduce the P100 numbers. Moreover, microbial load in smear water may also provide non-specific binding sites (Garcia et al., 2008). In food stuffs or raw milk, phages can be entrapped by charge or hydrophobic interactions and it is known that some phages are inactivated by raw milk and bovine whey proteins (Gerba, 1984; Gill et al., 2006b, 2010). Non-specific binding and consequently phage trapping could also occur in smear water. Furthermore, proteolytic activity of the smear water could affect the infectivity and integrity of phage particles (Guenther and Loessner, 2011). Another reason might be bacteriocins, which are known to be produced by lactic acid bacteria that could be found in smear water during dairy product processing. Bacteriocin sakacin P may cause phenotypic and transcriptional changes in L. monocytogenes (Tessema et al., 2011), which could consequently effect the host–phage relationship. Interestingly, temperature differences did not distinctly change phage survival, although it is known that phages are generally more stable at lower temperatures (Adams, 1949; Gill et al., 2010).
Overall the data suggest that phage P100 is very stable under a variety of environmental conditions. Therefore, P100 is most likely able to remain infective for a long period of time in food production plants. Indeed, from another study we were able to detect phage P100 in a dairy plant 3 months after the cessation of ListexTM P100 use (Rossmanith et al., 2014).
Besides phage stability, attachment of the phage to its target is the next crucial step in the phage infection process. Chemical and physiological factors on the other hand can influence the efficacy of phage binding. It is known that bovine whey proteins, which are also likely to be present in cheese and smear water, affect host adsorption of phages (Denes and Wiedmann, 2014). However, our data indicate that binding of P100 to L. monocytogenes is not affected by smear water (Figure 2). In addition, high NaCl concentrations (up to 2 M), which are commonly encountered in brine baths, do not hinder P100 binding to its host. Moreover, our data indicate that P100 binding is not influenced at all the pH values tested and in the presence of detergents such as SDS or Lutensol AO 7.
Phage replication is known to depend mainly on the physiological state of the host and the burst size correlates with the growth rate of the bacteria (Chibani-Chennoufi et al., 2004; Denes and Wiedmann, 2014). However, the growth rate is dependent upon stress conditions, which will frequently be present in food processing environments and thus pathogens are normally not found undergoing exponential growth (Denes and Wiedmann, 2014). However, metabolically active bacteria are a requirement for successful use of bacteriophages for active control strategies. These active attempts rely on host cell metabolism, which is necessary for phage replication and consequently active disruption of the bacterial cell leading to release of progeny phages. Results of our study have shown that replication was highly impaired (delayed or even inhibited) by chemical parameters such as pH value, high salt concentrations and the presence of surfactants, especially SDS (Figure 3). These parameters mainly influence the metabolic condition and growth rate of host cells, therefore hindering active control strategies (Denes and Wiedmann, 2014).
Although replication is necessary in the active approach for successful phage treatments, passive approaches in contrast do not depend on replication (Guenther et al., 2009; Hudson et al., 2010). In this case efficacy as defined by reduced bacterial cell counts is a relevant criterion for assessment and the fourth factor that was investigated in this study. Passive strategies are based on high phage concentrations (MOI ≥ 10; Kasman et al., 2002). On one hand phage infection should then lead to a change in host synthetic machinery resulting in arrest of host replication and a bacteriostatic effect. On the other hand a scenario termed “lysis from without” has been described (Abedon, 2011). This refers to infection of one bacteria cell with a high number of phages leading to its passive lysis. The supplier of ListexTM P100 recommends an application of 1 × 108 PFU/ml or 1 ml per 100 cm2 (= 2 × 1011 PFU/100 cm2; http://www.listex.eu/wp-content/uploads/Listex-Application-Data-Sheet-Cheese.pdf, accessed on 2016-05-16 and www.listex.eu/cheese-and-Listeria, accessed on 2015-03-07). Because in general the contamination rate of L. monocytogenes in food production pants is relatively low (maximum 107 CFU/ml L. monocytogenes were reported in outbreak cases; (Farber and Losos, 1988)), the recommended phage concentrations are likely to be consistent with a passive treatment strategy. Therefore, we examined the effect of chemicals on the efficacy of phage treatments using infective doses of MOIs of at least 10. Results (Figures 4–6; Supplementary Material) indicated that bacterial growth is inhibited and delayed by the environmental conditions and enhanced the efficacy of phage treatment. This observation is in agreement with other authors who were able to show that phage treatments are more efficacious in combination with conventional disinfection and sanitation measures (Montanez-Izquierdo et al., 2012). Synergetic effects were, for instance, reported in combination with the bacteriocin nisin on melons and apples (Leverentz et al., 2003). However, they were not obtained on beef (Dykes and Moorhead, 2002). Furthermore, in this study the efficacy of phage P100 in Fraser broth was similar as in TSB. Fraser broth is frequently used for selective enrichment of L. monocytogenes. These results indicate that the presence of phages (which were applied in the production plant) can influence growth dependent standard monitoring methods and might even cause false negative results.
In our study, growth was monitored over 24 h. As proliferation of Listeria is generally slow at 4°C, long-term experiments (120 days) were also conducted to consider the effect of temperature. Results (Figure 7) of these experiments indicate that the highest reduction in L. monocytogenes counts was achieved at 4°C. However, at all temperatures tested, re-growth was observed. This is an interesting observation in respect of resistance development, which was confirmed by testing single colonies. At all tested temperatures, L. monocytogenes isolates were found that had a reduced sensitivity to phage P100 (Figure 7; Supplementary Table S1). This finding is in accordance with Guenther and Loessner (2011) who detected phage-insensitive clones when phage A511 was used for 22 days for the treatment of red mold cheese during ripening. In contrast, no phage insensitive clones were detected when they used another L. monocytogenes strain. In our study we used L. monocytogenes EGDe, because it is a frequently used model strain for L. monocytogenes serotype 1/2a, one of the most important food-borne serotype. Nevertheless, it is possible that some of our data are not directly applicable to all L. monocytogenes strains. However, in food production plants or natural environments there is always heterogeneity of bacteria and stress-resistant variants can be found (Abee et al., 2016). For example, in general L. monocytogenes serotypes 4 and 1/2 were more susceptible to phages compared to strains in other serotypes, however is was reported that in food production plants within one serotype both, phage susceptible and phage insensitive isolates, can be found (Vongkamjan et al., 2012, 2013; Fister et al., 2016). The development of phage resistant variants can be caused by different reasons. It is known that a single point mutation can result in changes of the P100 attachment site, leading to phage resistant L. monocytogenes (Prof. Loessner, personal communication). Moreover, alterations in the physiological status of bacteria can lead to transcriptional responses that ultimately lead to cell wall structure changes that reduce the susceptibility of the cell to the phage. Additionally, the permanent presence of phages in the production plant is likely to support development of phage-resistant bacteria (Hagens and Loessner, 2010; Hudson et al., 2010).
In summary, our data show that phage P100 is stable under most conditions typically encountered in dairy-production environments. Results also demonstrate that phage attachment is possible under all tested conditions. In contrast, phage replication, which is necessary for all active control strategies, is dependent upon host cell growth, which is reduced by factors such as the presence of detergents, extreme pH values or high salt concentrations. Our results demonstrate that high numbers of phages in combination with environmental conditions that limit growth of bacteria are most effective. The use of bacteriophages for biocontrol in food production plants has to be critically considered case by case. On one hand the efficacy of phage treatments could be reduced by the development of phage resistances. Moreover, growth-dependent microbiological standard monitoring methods (like growth in Fraser broth) can be influenced by the presence of phages.
Author Contributions
Conception and design of the work: SF, DS, MW, PR; Acquisition of data: SF, CR; Analysis and Interpretation of data: SF, AKW, PR; Drafting the work: CR, SF, PR; Revision of the manuscript: SF, AKW, DS, MW, PR; All authors approved the version to be published in Frontiers in Microbiology and agreed to be accountable for all aspects of the work.
Funding
The financial support by the Austrian Federal Ministry of Science, Research and Economy and the National Foundation of Research, Technology and development is gratefully acknowledged.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01152
References
Abedon, S. T. (2012). Bacterial ‘immunity’ against bacteriophages. Bacteriophage 2, 50–54. doi: 10.4161/bact.18609
Abee, T., Koomen, J., Metselaar, K., Zwietering, M., and Besten, H. D. (2016). Impact of pathogen population heterogeneity and stress-resistant variants on food safety. Annu. Rev. Food Sci. Technol. 7, 439–456. doi: 10.1146/annurev-food-041715-033128
Adams, M. H. (1949). The stability of bacterial viruses in solutions of salts. J. Gen. Physiol. 32, 579–594. doi: 10.1085/jgp.32.5.579
Bachrach, G., Leizerovici-Zigmond, M., Zlotkin, A., Naor, R., and Steinberg, D. (2003). Bacteriophage isolation from human saliva. Lett. Appl. Microbiol. 36, 50–53. doi: 10.1046/j.1472-765X.2003.01262.x
Breitbart, M., and Rohwer, F. (2005). Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 13, 278–284. doi: 10.1016/j.tim.2005.04.003
Breitbart, M., Salamon, P., Andresen, B., Mahaffy, J. M., Segall, A. M., Mead, D., et al. (2002). Genomic analysis of uncultured marine viral communities. Proc. Natl. Acad. Sci. U.S.A. 99, 14250–14255. doi: 10.1073/pnas.202488399
Breitbart, M., Wegley, L., Leeds, S., Schoenfeld, T., and Rohwer, F. (2004). Phage community dynamics in hot springs. Appl. Environ. Microbiol. 70, 1633–1640. doi: 10.1128/AEM.70.3.1633-1640.2004
Bruttin, A., Desiere, F., D’amico, N., Guérin, J. P., Sidoti, J., Huni, B., et al. (1997). Molecular ecology of Streptococcus thermophilus bacteriophage infections in a cheese factory. Appl. Environ. Microbiol. 63, 3144–3150.
Carlton, R. M., Noordman, W. H., Biswas, B., De Meester, E. D., and Loessner, M. J. (2005). Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 43, 301–312. doi: 10.1016/j.yrtph.2005.08.005
Chan, B. K., Abedon, S. T., and Loc-Carrillo, C. (2013). Phage cocktails and the future of phage therapy. Future Microbiol. 8, 769–783. doi: 10.2217/fmb.13.47
Chaturongakul, S., and Ounjai, P. (2014). Phage-host interplay: examples from tailed phages and Gram-negative bacterial pathogens. Front. Microbiol. 5:442. doi: 10.3389/fmicb.2014.00442
Chibani-Chennoufi, S., Bruttin, A., Dillmann, M.-L., and Brüssow, H. (2004). Phage-host interaction: an ecological perspective. J. Bacteriol. 186, 3677–3686. doi: 10.1128/JB.186.12.3677-3686.2004
Denes, T., and Wiedmann, M. (2014). Environmental responses and phage susceptibility in foodborne pathogens: implications for improving applications in food safety. Curr. Opin. Biotechnol 26, 45–49. doi: 10.1016/j.copbio.2013.09.001
Dykes, G. A., and Moorhead, S. M. (2002). Combined antimicrobial effect of nisin and a listeriophage against Listeria monocytogenes in broth but not in buffer or on raw beef. Int. J. Food Microbiol. 73, 71–81. doi: 10.1016/S0168-1605(01)00710-3
EFSA (2009). Scientific opinion of the panel on biological hazards on a request from European Commisision on the use and mode of action of bacteriophages in food production. EFSA J. 1076, 1–26.
Farber, J. M., and Losos, J. Z. (1988). Listeria monocytogenes: a foodborne pathogen. CMAJ. 138, 413–418.
Fister, S., Fuchs, S., Stessl, B., Schoder, D., Wagner, M., and Rossmanith, P. (2016). Screening and characterisation of bacteriophage P100 insensitive Listeria monocytogenes isolates in Austrian dairy plants. Food Control 59, 108–117. doi: 10.1016/j.foodcont.2015.05.026
Fuhrman, J. A. (1999). Marine viruses and their biogeochemical and ecological effects. Nature 399, 541–548. doi: 10.1038/21119
Ganegama Arachchi, G. J., Cridge, A., Dias-Wanigasekera, B., Cruz, C., Mcintyre, L., Liu, R., et al. (2013a). Effectiveness of phages in the decontamination of Listeria monocytogenes adhered to clean stainless steel, stainless steel coated with fish protein, and as a biofilm. J. Ind. Microbiol. Biotechnol. 40, 1105–1116. doi: 10.1007/s10295-013-1313-3
Ganegama Arachchi, G. J., Flint, S. H., Mcintyre, L., Cruz, C. D., Dias-Wanigasekera, B. M., and Mutukumira, A. N. (2013b). A Study of Natural Lytic Listeria Phages with Decontaminating Properties for Use in Seafood Processing Plants. Degree of Doctor of Philosophy in Food Techniology thesis, Institute of Food, Nutriton and Human Health, Massey University, Auckland, 119.
Garcia, P., Martinez, B., Obeso, J. M., and Rodriguez, A. (2008). Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 47, 479–485. doi: 10.1111/j.1472-765X.2008.02458.x
Gerba, C. P. (1984). Applied and theoretical aspects of virus adsorption to surfaces. Adv. Appl. Microbiol. 30, 133–168. doi: 10.1016/S0065-2164(08)70054-6
Gill, J. J., Pacan, J. C., Carson, M. E., Leslie, K. E., Griffiths, M. W., and Sabour, P. M. (2006a). Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob. Agents Chemother. 50, 2912–2918. doi: 10.1128/AAC.01630-05
Gill, J. J., Sabour, P., and Griffiths, M. (2010). “Practical and theoretical considerations for the use of bacteriophages in food systems,” in Bacteriophages in the Control of Food-and Waterborne Pathogens, eds P. Sabour and M. Griffiths (Washington, DC: ASM Press), 217–235.
Gill, J. J., Sabour, P. M., Leslie, K. E., and Griffiths, M. W. (2006b). Bovine whey proteins inhibit the interaction of Staphylococcus aureus and bacteriophage K. J. Appl. Microbiol. 101, 377–386. doi: 10.1111/j.1365-2672.2006.02918.x
Guenther, S., Huwyler, D., Richard, S., and Loessner, M. J. (2009). Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl.Environ.Microbiol. 75, 93–100. doi: 10.1128/AEM.01711-08
Guenther, S., and Loessner, M. J. (2011). Bacteriophage biocontrol of Listeria monocytogenes on soft ripened white mold and red-smear cheeses. Bacteriophage 1, 94–100. doi: 10.4161/bact.1.2.15662
Hagens, S., and Loessner, M. J. (2007). Application of bacteriophages for detection and control of foodborne pathogens. Appl. Microbiol. Biotechnol. 76, 513–519. doi: 10.1007/s00253-007-1031-8
Hagens, S., and Loessner, M. J. (2010). Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr. Pharm. Biotechnol. 11, 58–68. doi: 10.2174/138920110790725429
Havelaar, A. H., and Hogeboom, W. M. (1984). A method for the enumeration of male-specific bacteriophages in sewage. J. Appl. Bacteriol. 56, 439–447. doi: 10.1111/j.1365-2672.1984.tb01372.x
Howett, M. K., Neely, E. B., Christensen, N. D., Wigdahl, B., Krebs, F. C., Malamud, D., et al. (1999). A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob. Agents Chemother. 43, 314–321.
Hudson, J. A., Mcintyre, L., Billington, C., Sabour, P., and Griffiths, M. (2010). “Application of bacteriophages to control pathogenic and spoilage bacteria in food processing and distribution,” in Bacteriophages in the Control of Food-and Waterborne Pathogens, eds P. M. Sabour and M. W. Griffiths (Washington, DC: American Society for Microbiology Press), 119–135.
Jończyk, E., Kłak, M., Międzybrodzki, R., and Górski, A. (2011). The influence of external factors on bacteriophages—review. Folia Microbiol. 56, 191–200. doi: 10.1007/s12223-011-0039-8
Kasman, L. M., Kasman, A., Westwater, C., Dolan, J., Schmidt, M. G., and Norris, J. S. (2002). Overcoming the phage replication threshold: a mathematical model with implications for phage therapy. J. Virol. 76, 5557–5564. doi: 10.1128/JVI.76.11.5557-5564.2002
Koutsoumanis, K. P., and Sofos, J. N. (2005). Effect of inoculum size on the combined temperature, pH and aw limits for growth of Listeria monocytogenes. Int. J. Food Microbiol. 104, 83–91. doi: 10.1016/j.ijfoodmicro.2005.01.010
Kropinski, A. M., Mazzocco, A., Waddell, T. E., Lingohr, E., and Johnson, R. P. (2009). “Enumeration of bacteriophages by double agar overlay plaque assay,” in Bacteriophages: Methods and Protocols: Isolation, Characterization, and Interactions, Vol. 1, eds A. M. K. Martha and R. J. Clokie (New York, NY: Humana Press), 69–76.
Lasobras, J., Muniesa, M., Frias, J., Lucena, F., and Jofre, J. (1997). Relationship between the morphology of bacteriophages and their persistence in the environment. Water Sci. Technol. 35, 129–132. doi: 10.1016/S0273-1223(97)00247-3
Leverentz, B., Conway, W. S., Camp, M. J., Janisiewicz, W. J., Abuladze, T., Yang, M., et al. (2003). Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69, 4519–4526. doi: 10.1128/AEM.69.8.4519-4526.2003
Lu, Z., Breidt, F., Plengvidhya, V., and Fleming, H. P. (2003). Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 69, 3192–3202. doi: 10.1128/AEM.69.6.3192-3202.2003
Ly-Chatain, M. H. (2014). The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 5:51. doi: 10.3389/fmicb.2014.00051
Mahony, J., Mcauliffe, O., Ross, R. P., and Van, S. D. (2011). Bacteriophages as biocontrol agents of food pathogens. Curr. Opin. Biotechnol. 22, 157–163. doi: 10.1016/j.copbio.2010.10.008
Maura, D., and Debarbieux, L. (2011). Bacteriophages as twenty-first century antibacterial tools for food and medicine. Appl. Microbiol. Biotechnol. 90, 851–859. doi: 10.1007/s00253-011-3227-1
Mazzocco, A., Waddell, T., Lingohr, E., and Johnson, R. (2009). “Enumeration of bacteriophages using the small drop plaque assay system,” in Bacteriophages, eds M. J. Clokie and A. Kropinski (New York, NY: Humana Press), 81–85.
Meaden, S., and Koskella, B. (2013). Exploring the risks of phage application in the environment. Front. Microbiol. 4:358. doi: 10.3389/fmicb.2013.00358
Miller, R.V. (1998). “Methods for enumeration and characterisation of bacteriophages from environmental samples,” in Techniques in Microbial Ecology, eds R. S. Burlage, R. Atlas, D. Stahl, G. Geesey, and G. Sayler (New York, NY: Oxford University Press), 218–235.
Montañez-Izquierdo, V. Y., Salas-Vázquez, D. I., and Rodr<guez-Jerez, J. J. (2012). Use of epifluorescence microscopy to assess the effectiveness of phage P100 in ontrolling Listeria monocytogenes biofilms on stainless steel surfaces. Food Control 23, 470–477.
Nel, L., Wingfield, B. D., Van Der Meer, L. J., and Van Vuuren, H. (1987). Isolation and characterization of Leuconostoc oenos bacteriophages from wine and sugarcane. FEMS Microbiol. Lett. 44, 63–67. doi: 10.1111/j.1574-6968.1987.tb02243.x
O’Flaherty, S., Ross, R. P., and Coffey, A. (2009). Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol. Rev. 33, 801–819. doi: 10.1111/j.1574-6976.2009.00176.x
Oliveira, M., Viñas, I., Colàs, P., Anguera, M., Usall, J., and Abadias, M. (2014). Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiol. 38, 137–142. doi: 10.1016/j.fm.2013.08.018
Prigent, M., Leroy, M., Confalonieri, F., Dutertre, M., and Dubow, M. (2005). A diversity of bacteriophage forms and genomes can be isolated from the surface sands of the Sahara Desert. Extremophiles 9, 289–296. doi: 10.1007/s00792-005-0444-5
Pringsulaka, O., Patarasinpaiboon, N., Suwannasai, N., Atthakor, W., and Rangsiruji, A. (2011). Isolation and characterisation of a novel Podoviridae-phage infecting Weissella cibaria N 22 from Nham, a thai fermented pork sausage. Food Microbiol. 28, 518–525. doi: 10.1016/j.fm.2010.10.011
Rossmanith, P., Fister, S., Wagner, M., and Schoder, D. (2014). “Evaluation of an in-house laboratory for listeria self-monitoring of a dairy production plant,” in Proceedings of the European Symposium on Food Safety (Des Moines, IA: IAFP).
Rossmanith, P., Krassnig, M., Wagner, M., and Hein, I. (2006). Detection of Listeria monocytogenes in food using a combined enrichment/real-time PCR method targeting the prfA gene. Res. Microbiol. 157, 763–771. doi: 10.1016/j.resmic.2006.03.003
Skurnik, M., Pajunen, M., and Kiljunen, S. (2007). Biotechnological challenges of phage therapy. Biotechnol. Lett. 29, 995–1003. doi: 10.1007/s10529-007-9346-1
Smułek, W., Kaczorek, E., Zgoła-Grzeskowiak, A., and Cybulski, Z. (2015). Impact of alkyl polyglucosides surfactant lutensol GD 70 on modification of bacterial cell surface properties. Water Air Soil Pollut. 226, 1–9.
Soni, K. A., Desai, M., Oladunjoye, A., Skrobot, F., and Nannapaneni, R. (2012). Reduction of Listeria monocytogenes in queso fresco cheese by a combination of listericidal and listeriostatic GRAS antimicrobials. Int. J. Food Microbiol. 155, 82–88. doi: 10.1016/j.ijfoodmicro.2012.01.010
Soni, K. A., Nannapaneni, R., and Hagens, S. (2010). Reduction of Listeria monocytogenes on the surface of fresh channel catfish fillets by bacteriophage Listex P100. Foodborne Pathog. Dis. 7, 427–434. doi: 10.1089/fpd.2009.0432
Swaminathan, B., and Gerner-Smidt, P. (2007). The epidemiology of human listeriosis. Microbes Infect. 9, 1236–1243. doi: 10.1016/j.micinf.2007.05.011
Tessema, G. T., Moretro, T., Snipen, L., Axelsson, L., and Naterstad, K. (2011). Global transcriptional analysis of spontaneous sakacin P-resistant mutant strains of Listeria monocytogenes during growth on different sugars. PLoS ONE 6:e16192. doi: 10.1371/journal.pone.0016192
Vongkamjan, K., Roof, S., Stasiewicz, M. J., and Wiedmann, M. (2013). Persistent Listeria monocytogenes subtypes isolated from a smoked fish processing facility included both phage susceptible and resistant isolates. Food Microbiol. 35, 38–48. doi: 10.1016/j.fm.2013.02.012
Vongkamjan, K., Switt, A. M., Den Bakker, H. C., Fortes, E. D., and Wiedmann, M. (2012). Silage collected from dairy farms harbors an abundance of listeriaphages with considerable host range and genome size diversity. Appl. Environ. Microbiol. 78, 8666–8675. doi: 10.1128/AEM.01859-12
Wendlinger, G., Loessner, M. J., and Scherer, S. (1996). Bacteriophage receptors on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology 142(Pt 4), 985–992. doi: 10.1099/00221287-142-4-985
Williamson, K. E., Wommack, K. E., and Radosevich, M. (2003). Sampling natural viral communities from soil for culture-independent analyses. Appl. Environ. Microbiol. 69, 6628–6633. doi: 10.1128/AEM.69.11.6628-6633.2003
Keywords: bacteriophage P100, host–phage interaction, environmental influence, resistance, L. monocytogenes, ListexTM P100
Citation: Fister S, Robben C, Witte AK, Schoder D, Wagner M and Rossmanith P (2016) Influence of Environmental Factors on Phage–Bacteria Interaction and on the Efficacy and Infectivity of Phage P100. Front. Microbiol. 7:1152. doi: 10.3389/fmicb.2016.01152
Received: 17 March 2016; Accepted: 11 July 2016;
Published: 28 July 2016.
Edited by:
Jean-Christophe Augustin, Ecole Nationale Vétérinaire d’Alfort, FranceReviewed by:
Eric Altermann, AgResearch, New ZealandLaurent Guillier, French Agency for Food, Environmental and Occupational Health & Safety, France
Copyright © 2016 Fister, Robben, Witte, Schoder, Wagner and Rossmanith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Rossmanith, peter.rossmanith@vetmeduni.ac.at
 Susanne Fister
Susanne Fister Christian Robben1
Christian Robben1 Anna K. Witte
Anna K. Witte Dagmar Schoder
Dagmar Schoder Martin Wagner
Martin Wagner