- 1School of Biotechnology and Biomolecular Sciences, University of New South Wales, Sydney, NSW, Australia
- 2School of Chemistry, University of New South Wales, Sydney, NSW, Australia
N-acetyl glucosamine, the monomer of chitin, is an abundant source of carbon and nitrogen in nature as it is the main component and breakdown product of many structural polymers. Some bacteria use N-acyl-L-homoserine lactone (AHL) mediated quorum sensing (QS) to regulate chitinase production in order to catalyze the cleavage of chitin polymers into water soluble N-acetyl-D-glucosamine (NAG) monomers. In this study, the impact of NAG on QS activities of LuxR, LasR, and CviR regulated gene expression was investigated by examining the effect of NAG on QS regulated green fluorescent protein (GFP), violacein and extracellular chitinase expression. It was discovered that NAG inhibits AHL dependent gene transcription in AHL reporter strains within the range of 50–80% reduction at low millimolar concentrations (0.25–5 mM). Evidence is presented supporting a role for both competitive inhibition at the AHL binding site of LuxR type transcriptional regulators and catabolite repression. Further, this study shows that NAG down-regulates CviR induced violacein production while simultaneously up-regulating CviR dependent extracellular enzymes, suggesting that an unknown NAG dependent regulatory component influences phenotype expression. The quorum sensing inhibiting activity of NAG also adds to the list of compounds with known quorum sensing inhibiting activities.
Introduction
Chitin, a polymer of linked amino sugar subunits (β-1,4-linked N-acetyl-D-glucosamine; NAG), is the second most abundant biological polymer on Earth (most abundant in the marine environment) with global production estimated at 1011 tons per year (Gooday, 1990; Souza et al., 2011). The fact that chitin does not accumulate in the environment indicates that chitin biodegradation (turnover) rates are as immense as production rates and that chitin biodegradation plays a major role in nutrient cycling (linking carbon and nitrogen cycles) and hence maintenance of life globally (Gooday, 1990). Extracellular chitinase enzymes catalyze the cleavage of the chitin polymer into water soluble oligomers and thereafter into dimers [(NAG)2] and monomers (NAG) that are taken up by microorganisms and catabolised intracellularly (Gooday, 1990; Keyhani and Roseman, 1999). Regulation of chitinase gene expression is complex and varied between even closely related bacterial strains, but chitinase genes are generally upregulated upon detection of chitin or chitin degradation products and downregulated by catabolite repression (Ulhoa and Peberdy, 1991; Techkarnjanaruk et al., 1997; Keyhani and Roseman, 1999). Additionally, chitinase production is controlled in some instances by N-acyl-L-homoserine lactone (AHL) mediated quorum sensing (Chernin et al., 1998; Hentzer et al., 2002; Christensen et al., 2003; Defoirdt et al., 2010; Chong et al., 2012).
It is common for many bacteria to regularly encounter NAG, the monomer of the chitin polymer, in various environments given the abundance of NAG sources in nature (Park and Uehara, 2008). Therefore, bacterial and eukaryotic cells developed mechanisms by which NAG regulates virulence properties of microbes and host cells. For example, NAG inhibits the production of type-1-fimbriae in Escherichia coli, thereby promoting infection (Sohanpal et al., 2004). NAG also modulates production of antimicrobials and toxins in Pseudomonas aeruginosa PA14 via a peptidoglycan sensing protein (Korgaonkar et al., 2013). Additionally, NAG induces chitinase activity in Pseudoalteromonas and Vibrio species while it reduces chitinase activity in Serratia marcescens and Trichoderma harzianum in a strain dependent manner (Monreal and Reese, 1969; Ulhoa and Peberdy, 1991; Chernin et al., 1998; Bhowmick et al., 2007).
N-Acyl-L-Homoserine Lactones mediated quorum sensing (QS) is a gene expression mechanism involving a transcriptional link between the extracellular accumulation of AHL and genes encoding phenotypes such as biofilm formation, bioluminescence, virulence factor production and exoenzyme production (Bassler, 1999; Williams et al., 2007). AHL production (AHL synthase, LuxI homologue) and response (AHL response regulator, LuxR homologue) is widespread amongst the α, β, and γ Proteobacteria (Manefield and Turner, 2002), which are generally highly abundant in nature. Recent studies have shown that AHL-mediated QS activity is not restricted to Proteobacteria but also observed in archaea and marine bacteria including Flavobacterium spp. and Tenacibaculum maritimum which belong to Bacteroidetes (Romero et al., 2010; Zhang et al., 2012; Twigg et al., 2014). AHL producing bacteria have been isolated from a wide variety of environments including soil, plants, animals, fresh water, marine water, and activated sludge in wastewater treatment plants.
QS mechanisms are attractive targets to interfere with bacterial virulence. It is evident that disruption of QS activities of pathogenic bacteria significantly reduces their virulence activities (LaSarre and Federle, 2013). Therefore, many studies have focussed on designing synthetic QS agonists and antagonists for inhibition of QS activities dependent on LuxR type regulators (Welsh et al., 2015; Nizalapur et al., 2016). Moreover, several types of QS inhibitor compounds are known to be produced by eukaryotes and prokaryotes. These compounds target the QS systems by different mechanisms including enzymatic degradation of AHLs, interference with the stability or function of AHL receptor and/or synthase proteins and inhibition of the production of AHLs (Givskov et al., 1996; Zhang et al., 2002; Gao et al., 2003; Kalia, 2013). In addition to known QS inhibitors, recent studies point out the importance of nutritional conditions on regulation of QS system within Gram-negative bacteria. It has been reported that specific carbon sources mediate QS activity and swarming motility in P. aeruginosa biofilm formation (Shrout et al., 2006). Similarly, glucose treated Aeromonas hydrophila cultures downregulate QS regulated phenomena such as biofilm formation and virulence (Jahid et al., 2013). On the other hand, starvation regulates QS signaling and represses cell growth in P. aeruginosa and Bacillus subtilis which triggers entry into the stationary phase (You et al., 1998; Lazazzera et al., 1999). The cylic AMP (cAMP) cAMP receptor protein (CRP) regulates numerous genes as both an activator and repressor (Kolb et al., 1993). Its role in regulating carbon metabolism through carbon catabolite repression of different bacteria such as E. coli and B. subtilis has been well characterized (Gorke and Stulke, 2008). It is also reported that CRP is required to control transcription of luxI/luxR homolog genes which encode QS signal receptor (Albus et al., 1997; Dunlap, 1999; Liang et al., 2007).
The objective of this study was to investigate the relationship between the chitin monomer (NAG) and quorum sensing activities of AHL reporter strains, and for this purpose, cultures were treated with various concentrations of NAG and the effect of NAG was compared to the related molecules glucose and glucosamine (GlcN). The role of catabolite repression was also examined.
Materials and Methods
Bacterial Strains and Culture Conditions
The bacterial strains used in this study are shown in Supplementary Table S1. Media supplements to AB minimal media (Clark and Maaløe, 1967) used for culturing were as follow; 0.2% tryptone, 0.4% yeast extract for Chromobacterium violaceum CV026, 0.2% tryptone, 0.1% lactate for E. coli MT102, and 0.2% tryptone, 0.1% yeast extract for P. aeruginosa MH602. Strains were grown at either 30°C (strain CV026) or 37°C (strains MT102 and MH602) with shaking at 150 rpm. N-acyl-L-homoserine lactones including N-hexanoyl-L-homoserine lactone (HHL), N-(3-oxo-hexanoyl)-L-homoserine lactone (OHHL), N-(3-oxo-dodecanoyl)-L-homoserine lactone (OdDHL; Sigma-Aldrich) were dissolved in MeOH and stored as 20 mM stocks at -20°C. Growth experiments commenced at an OD600 of 0.01 with reading taken at 30-min intervals using a multiwell plate reader (Ensight Plate Reader, PerkinElmer).
Screening of AHL-Mediated GFP(ASV) Production
AHL dependent GFP expressing E. coli MT102 (pJBA132) and P. aeruginosa MH602 strains were cultured overnight with appropriate AB media described above. Strains were diluted (1:15) and 100-μl aliquots were dispensed to flat bottom 96-well plate wells (Sarstedt Australia). E. coli MT102 cultures were supplemented with 10 nM OHHL and air dried prior to addition of cultures. No OHHL was added into negative control culture. Cultures were also supplemented with NAG, GlcN or glucose within the range of 0.25–2 mM. Positive control culture was supplemented with no extra carbon sources. Plates were sealed with self-adhesive microplate sealers (TopSeal-A, PerkinElmer) to allow air diffusion and to prevent condensation. Cultures were incubated at 37°C with shaking at 150 rpm overnight. Fluorescence (excitation, 485 nm; emission, 535 nm) and OD600 of cultures were measured by a multiwell plate reader (Ensight Plate Reader, PerkinElmer). The ratio of the fluorescence to OD600 (Fluorescence/OD600) represented the unit fluorescence activity. All cultures were prepared in triplicates and experiments were repeated at least three times.
Violacein Extraction and Quantification
Violacein production by C. violaceum CV026 was quantified as previously described (Blosser and Gray, 2000). Briefly, cultures were supplemented with 100 nM HHL and treated with NAG or GlcN within the range of 0.5–5 mM. All cultures were incubated at 30°C with shaking at 150 rpm over 20 h. Supernatants from 800 μL aliquots were discarded and cells were lysed by adding 200 μl of 10% sodium dodecyl sulfate and incubated at 25°C for 5 min. Violacein was extracted by adding 1 ml of water-saturated butanol and absorbance was taken at 585 nm. Negative control culture was treated with no HHL and carbon source. Positive control culture was supplemented with no extra carbon source. The OD660 of each culture was measured prior to violacein extraction. Absorbance readings were taken by a multiwell plate reader (Ensight Plate Reader, PerkinElmer). The ratio of the violacein absorbance (585 nm) to OD660 represented the unit violacein activity. All cultures were prepared in triplicates and experiments were repeated at least three times.
Growth Experiments
C. violaceum CV026, E. coli MT102 and P. aeruginosa MH602 strains were cultured in AB medium supplemented with 0.01% yeast extract and 20 mM of NAG, GlcN or glucose. Control cultures lacked an additional carbon source. Initial OD600 of all cultures was adjusted to 0,01 and optical density readings for MT102 and MH602 were taken at 30-min intervals whereas the OD600 of CV026 was measured after 20 h incubation by using a multiwell plate reader (Ensight Plate Reader, PerkinElmer).
RNA Transcript Analysis by qRT-PCR
Transcriptional differences of QS regulated genes were analyzed by qRT-PCR as follows. Total RNA was extracted from cultures at mid-logarithmic growth phase using RNeasy Mini Kit (QIAGEN). Synthesis of cDNA with primers listed in Supplementary Table S1 was performed with AVM Reverse Transcriptase (Promega) according to manufacturer’s instructions. Resulting cDNA was used as template in subsequent qPCR amplification of gfp(ASV) and violacein biosynthetic genes including vioA, vioC, vioD. Specific transcripts were verified with primers (Supplementary Table S1) designed using the NCBI primer-designing tool. Transcript abundance was log5 transformed and normalized to the 16S rRNA transcript abundance.
Ligand-Receptor Docking Studies
Possible binding sites and poses of the compounds within quorum sensing receptors LasR, TraR, and CviR (Zhang et al., 2002; Bottomley et al., 2007; Chen et al., 2011) were predicted by docking compounds into receptors LasR (PDB code, 2UV0), TraR (PDB code, 1L3L) and CviR (PDB codes, 3QP4, 3QP5) using Ligand Docking (GOLD; Cambridge Crystallography Data Centre, UK) in its implementation through the Discovery Studio (Accelrys) interface. Hydrogen atoms were added to all ligands and the receptor prior to performing the docking runs. All ligands were also minimized under the CHARMm forcefield. The binding pocket was defined from the binding site of the agonist or antagonist in the crystal structure. The number of docking runs was set to 10, the “Detect Cavity” and “Early Termination” options were set to be “False.” All other parameters were left at their default values. Gold scores, hydrogen bonds, and π-interactions of the ligands were analyzed for the first pose with the highest Gold score.
Chitinase Activity Assays
Extracellular chitinase activity was monitored through a commercially available chitinase assay kit (CS0980, Sigma-Aldrich). Cultures were grown overnight in AB medium supplemented with 0.2% tryptone, 0.1% yeast extract. Chitin flakes (1%) were supplemented to growth medium when it is required. Cell-free supernatants of cultures were incubated with 4-Nitrophenyl-N-acetyl-β-D-glucosaminide (1 mg/ml) and chitinase units were calculated according to manufacturer’s instructions. Results represent the chitinase units/per cell. Absorbance (405 nm) was measured by a multiwell plate reader (Ensight Plate Reader, PerkinElmer). All cultures were prepared in triplicates.
Statistical Analysis
GraphPad Prism 5 software was used to apply Student’s t-test where necessary to determine the significant differences observed in the assays. P-value lower than 0.01 (P < 0.01) indicated significant differences.
Results
The Chitin Monomer N-Acetylglucosamine Inhibits QS Activity
The impact of NAG on AHL-mediated QS was analyzed with CviR-based C. violaceum CV026, LasR-based P. aeruginosa MH602 and LuxR-based E. coli MT102 reporter strains. AHL reporter strains were treated with NAG ranging from 0.25 mM to 5 mM. AHL deficient strains were supplemented with AHLs required for optimum QS activity (100 nM HHL and 10 nM OHHL for CV026 and MT102, respectively). AHL dependent reporter activity of all three strains was significantly (P < 0.01) reduced in the presence of NAG (Figure 1).
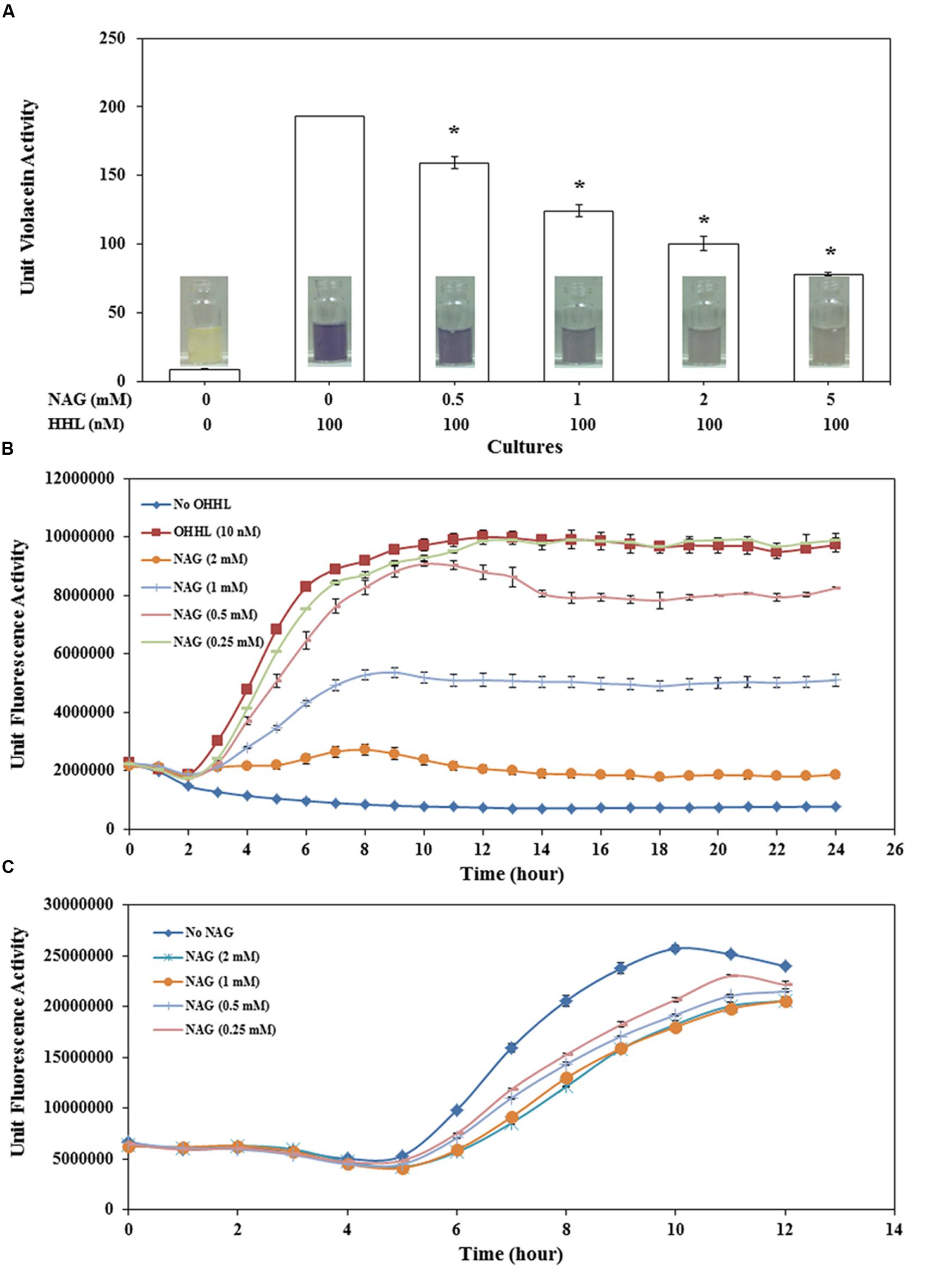
FIGURE 1. Concentration dependent effect of NAG on (A) CviR dependent violacein production by C. violaceum CV026 with violacein extracted after 20 h incubation, (B) LuxR dependent specific fluorescence activity of E. coli MT102 (pJBA132) over time in the presence of 10 nM OHHL and (C) LasR dependent specific fluorescence activity of P. aeruginosa MH602. All cultures were in triplicate. Error bars represent standard deviation. Asterisks indicate the significant differences in comparison to control samples (P < 0.01).
Violacein production by CV026 decreased in a concentration-dependent manner with 0.5 mM NAG reducing production by ∼18% and 5 mM reducing production by ∼60% (Figure 1A). Similar results were observed with the C. violaceum wild type strain (data not shown). QS activity of E. coli MT102 exhibited higher sensitivity to NAG in comparison to CV026 (Figure 1B). The minimum and maximum inhibitory concentrations of NAG on LuxR-based QS activity were 0.5 mM (∼15.2% inhibiton) and 2 mM (∼81% inhibition), respectively. No recovery was observed in QS activities of both E. coli MT102 and C. violaceum CV026 over time. In P. aeruginosa MH602, maximum inhibition (47%) was observed at mid-log growth phase (after 7 h incubation) in 2 mM NAG supplemented cultures. The minimum inhibitory concentrations were in the 0.25–0.5 mM range (Figure 1C). In contrast to strains MT102 and CV026, inhibition of QS activity in strain MH602 reversed at the early stationary growth phase. Isothermal titration calorimetry (ITC) was used to test for a direct interaction between AHLs and NAG. No interactions were observed (data not shown). Millimolar concentrations of NAG are commonly observed in nature suggesting that in such environments AHL-mediated gene expression will be repressed (Gorke and Stulke, 2008; Eisenreich et al., 2010; Frimmersdorf et al., 2010; Jagmann et al., 2012).
The impact of NAG on growth rates of analyzed strains was examined. All three strains grew to cell concentrations similar to untreated cultures (Figure 2). This indicates that NAG does not inhibit AHL mediated gene expression through a general toxicity or global inhibition of gene expression.
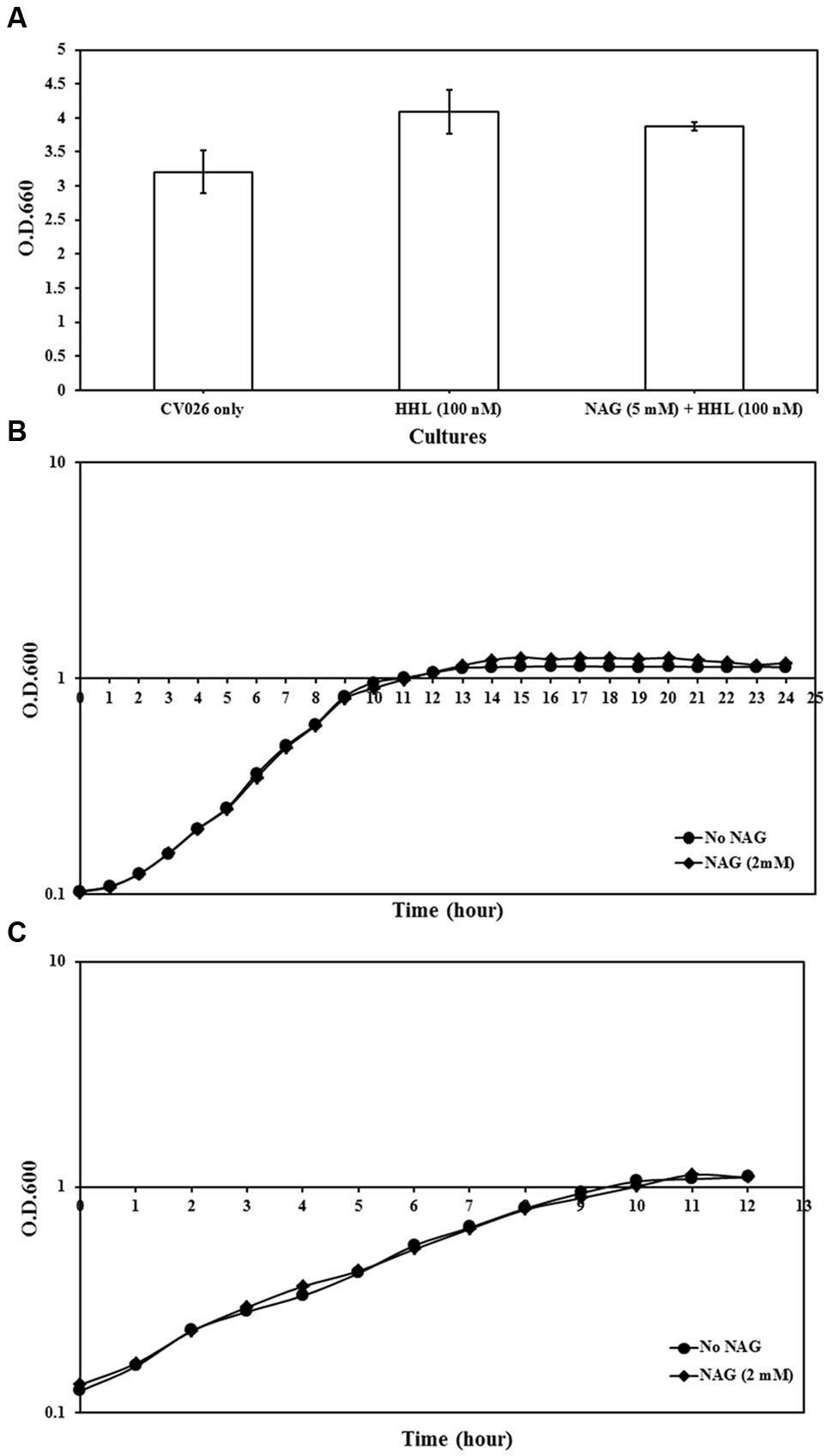
FIGURE 2. Growth analysis of reporter strains cultured in AB minimal medium supplemented with media supplements and appropriate concentrations of NAG. (A) OD600 of C. violaceum CV026 measured after 20 h incubation, (B) growth curve of E. coli MT102 measured at 1-h intervals during 24 h and (C) growth curve of P. aeruginosa MH602 measured at 1-h intervals during 12 h. Control cultures were supplemented with no NAG. All cultures were in triplicates. Error bars represent standard deviation.
N-Acetylglucosamine Downregulates Transcription of QS Regulated Genes
To determine if NAG was exerting its effect on the AHL bioassays at a transcriptional level, three mRNA transcripts encoding violacein production in C. violaceum CV026 (vioA, vioC, and vioD) and the mRNA transcript encoding the green fluorescent protein in E. coli (pJBA132) and P. aeruginosa MH602 were quantified in the presence and absence of NAG. RNA was extracted from cultures in mid-log growth phase and specific transcripts quantified by RT-qPCR. Transcription levels of vioA, vioC, and vioD were reduced twofold, fourfold, and eightfold, respectively, by 5 mM NAG (Figure 3A) corresponding reasonably well to the threefold reduction in violacein production at this concentration (Figure 1A). Similarly, transcription of gfp(ASV) in P. aeruginosa MH602 was reduced twofold by 1 mM NAG (Figure 3C) corresponding well to the 1.6-fold decrease in fluorescence observed after an 8 h incubation at this concentration (Figure 1C). With E. coli MT102 (pJBA132) the twofold drop in fluorescence was associated with a 200-fold drop in transcript abundance in the presence of 1 mM NAG (Figure 3B). The reason for this is unclear. It is possible that GFP activity is compensated for at a post-transcriptional level. Overall, these data suggest that NAG down-regulates transcription from AHL dependent promoters.
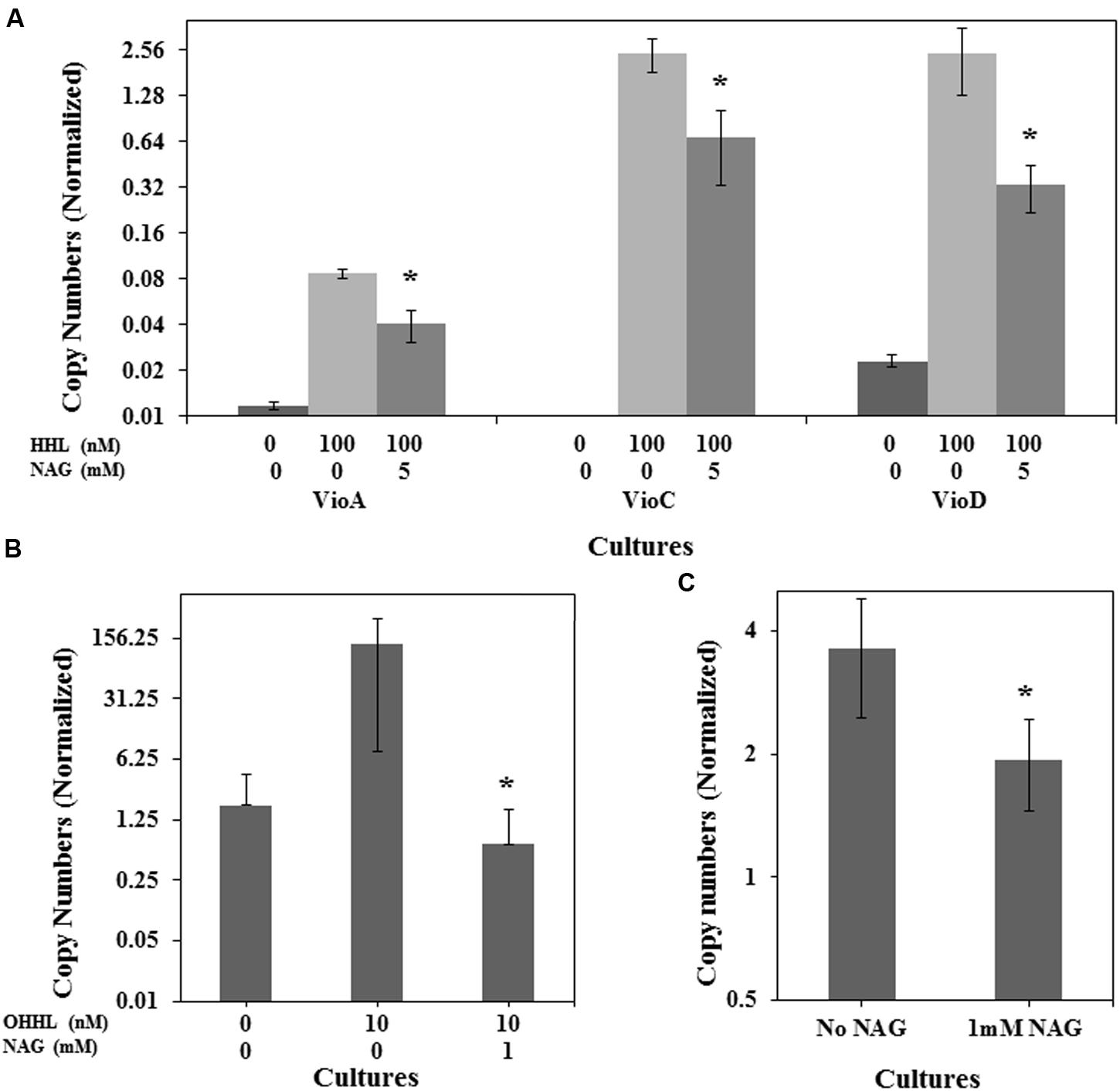
FIGURE 3. NAG down regulates QS regulated transcriptional activity. Transcriptional levels of (A) the C. violaceum CV026 CviR-HHL regulated vioA, vioC, and vioD genes in the presence of 100 nM HHL and 5 mM NAG, (B) the E. coli MT102 LuxR-OHHL regulated gfp(ASV) gene in the presence of 10 nM OHHL and 1 mM NAG and (C) the P. aeruginosa MH602 LasR-OdDHL regulated gfp(ASV) gene in the presence of 1 mM NAG. Cultures were sampled at mid-log growth phase for mRNA extractions. All cultures were in triplicate. Error bars represent standard deviation. Asterisks indicate the significant differences in comparison to control samples (P < 0.01).
N-Acetylglucosamine Imposed Inhibition Compared with Glucose and Glucosamine
Transcription of many QS regulated genes is also controlled by catabolite repression via cAMP and the CRP (Dunlap and Greenberg, 1988; Albus et al., 1997; Liang et al., 2007). To examine the susceptibility of the QS assays to catabolite repression the impact of related molecules GlcN and glucose on AHL mediated gene expression was also examined. The impact of glucose, GlcN and NAG on QS activity of CV026, MT102, and MH602 is represented in Figures 4A–C, respectively. Whilst GlcN and glucose displayed inhibitory activity the effects were reporter strain specific and less pronounced (Figure 4). For example the effect of glucose was similar to NAG in the LuxR dependent assay (Figure 4B) while no inhibition was observed in the LasR dependent assay (Figure 4C) suggesting catabolite repression plays a role in NAG inhibition of LuxR dependent transcription but not in LasR dependent transcription. This result is correlated with the fact that glucose is a non-preferred carbon source in P. aeruginosa and uptake of glucose has no effect on cAMP levels in P. aeruginosa (Rojo, 2010). Overall the inhibitory effects of GlcN were weak compared to NAG, with no effect observed in the LasR dependent (Figure 4C) assay and limited effects (6–17% reduction) observed in the LuxR and CviR dependent assays at the highest concentration tested (2 mM; Figures 4A,B). These data suggest that NAG exerts an effect on the activity of AHL dependent promoters through catabolite repression but that catabolite repression does not explain the inhibitory effect of NAG completely.
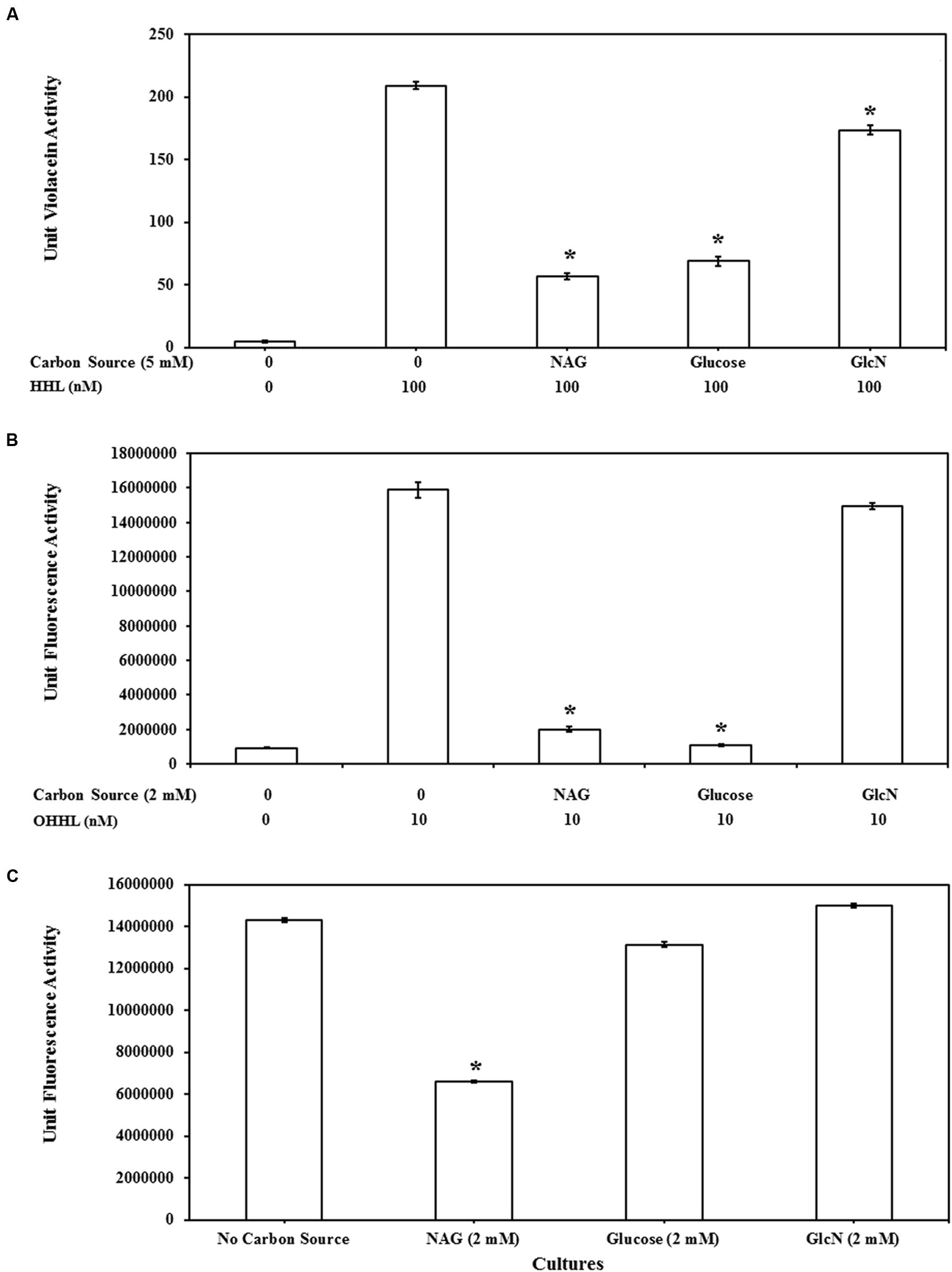
FIGURE 4. Comparison of QS inhibitory effects of NAG with glucosamine (GlcN) and glucose at 5 mM on (A) CviR dependent violacein production with exogenous addition of 100 nM HHL and violacein extraction after 20 h batch incubation, at 2 mM (B) LuxR dependent GFP production with exogenous addition of 10 nM OHHL and measurement of fluorescence at 20 h and (C) LasR dependent GFP production without exogenous AHL addition, fluorescence measurement 8 h. All cultures were in triplicate. Error bars represent standard deviation. Asterisks indicate the significant differences in comparison to control samples (P < 0.01).
Modeling Interactions with AHL Binding Sites
Bacterial cells import NAG through phosphotransferase systems that phosphorylate NAG as it enters the cell (Plumbridge, 1990). Whilst there are only vague structural similarities between AHLs and NAG (the amide bond and disposition of the ring oxygen) we considered the possibility of NAG and phosphorylated derivatives of NAG (NAG-1-phosphate and NAG-6-phosphate) interfering with AHL docking to LuxR type regulatory proteins. This might explain the inhibitory activities. With respect to size NAG has a molecular weight (mwt) of 221.21, phosphorylated NAG has a mwt of 301.19 and AHLs have mwts in the range of 213.23–297.39 (OHHL to OdDHL, respectively). With respect to polarity, the predicted log P-values (negative values indicate higher polarity) for NAG, NAG-1-phosphate and NAG-6-phosphateare -2.375, -2.850 and -3.699, respectively, whilst the log P-value for AHLs range from 0.19 for OHHL to 3.15 for OdDHL indicating NAG ligands are more water soluble or more polar than AHLs are.
Docking studies were performed using the AHL binding sites of LasR, TraR, and CviR (the latter in agonist and antagonist binding conformations) and cognate AHLs or NAG, phosphorylated NAG, glucose or glucosamine as ligands (Figure 5). Table 1 reports the Gold Score Fitness of the ligand-receptor docking relating to binding affinity and the interactions incumbent in the selected pose. Whilst the fitness scores of NAG, glucose, glucosamine and phosphorylated NAGs are lower than the cognate AHLs for each receptor protein surprisingly all compounds have affinity for AHL binding sites. For all three receptor proteins, the fitness score for phosphorylated NAG ligands are higher than for NAG, glucose and glucosamine. For reference, there are non-cognate AHLs that have lower Gold Fitness Scores than NAG for each of the AHL receptor proteins modeled (data not shown).
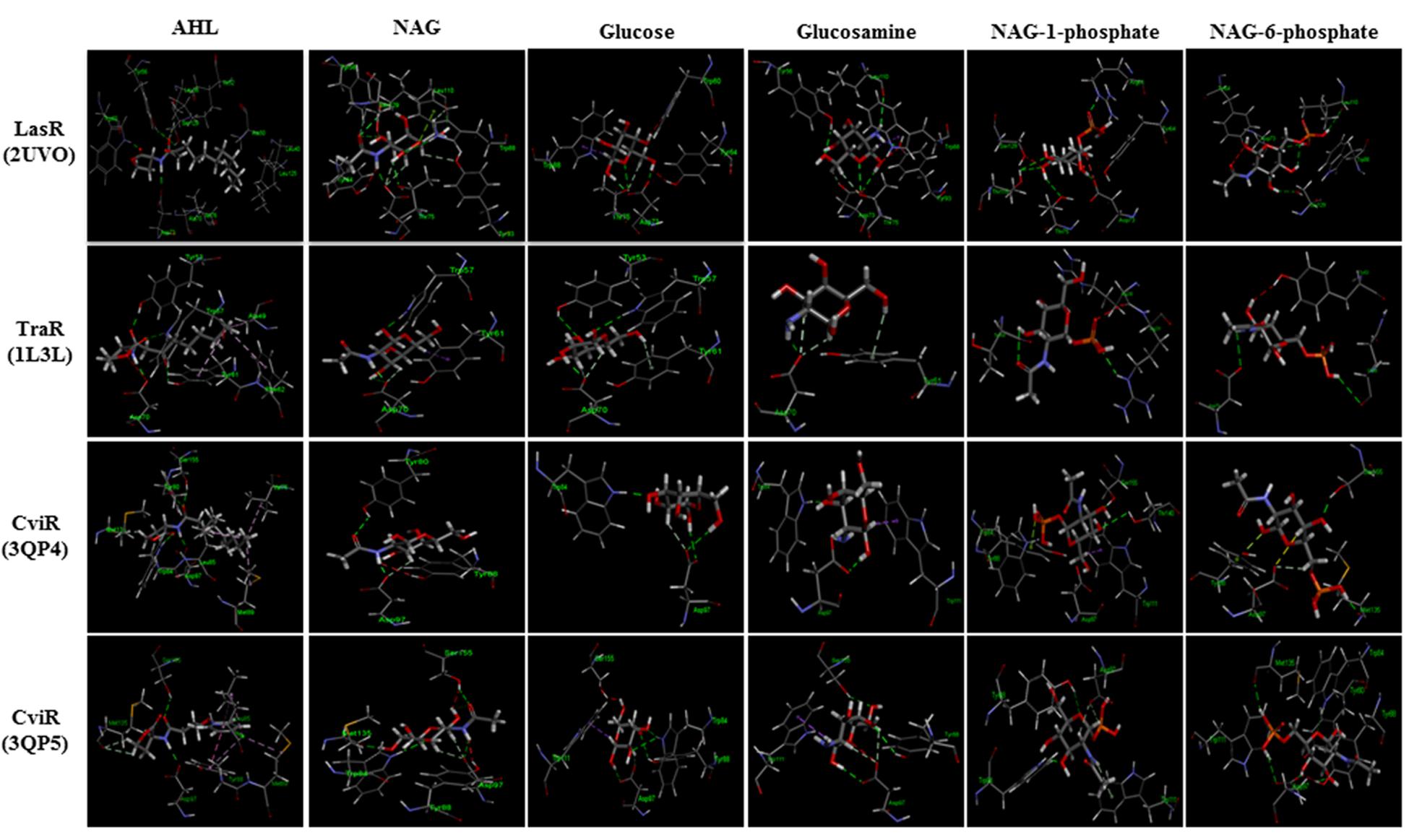
FIGURE 5. Modeled docking poses for AHL binding sites of LasR, TraR and CviR (the latter in agonist and antagonist binding conformations) and NAG, glucosamine, glucose, NAG-1-phosphate, NAG-6-phosphate or the cognate AHLs as ligands. All ligands could occupy the AHL binding pockets and showed hydrophilic interactions in general. Hydrogen bond interaction is shown as green dashed lines. NAG formed 40–80% of the hydrogen bonds formed by cognate AHLs and additional hydrogen bonds unique to NAG in the case of LasR.
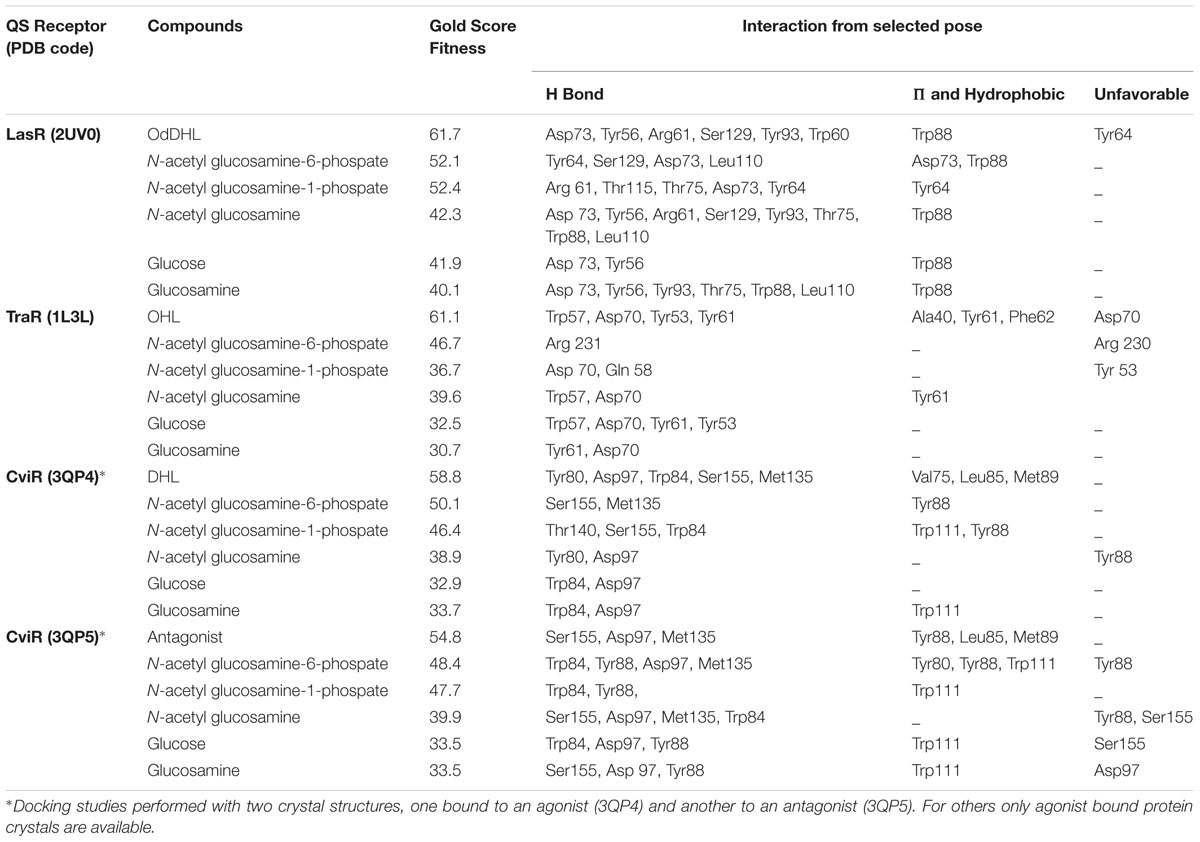
TABLE 1. Gold score fitness values and interactions for NAG and other ligands docking with LasR, TraR and CviR.
NAG-1-phosphate is predicted to form two out of the six hydrogen bonds (formed by OdDHL with the LasR AHL binding site in addition to three extra hydrogen bonds in the binding pocket. NAG-1-phosphate also forms one of four hydrogen bonds formed by N-octanoyl-L-homoserine lactone (OHL) in the TraR AHL binding site in addition to one extra bond and two of five or no shared hydrogen bonds formed by N-decanoyl-L-homoserine lactone (DHL) or an antagonist, respectively, with the CviR AHL binding site. The fitness score for NAG-6-phosphate is the highest among all analyzed ligands. NAG-6-phosphate is predicted to form two out of six hydrogen bonds formed by OdDHL with the LasR AHL binding site in addition to two extra hydrogen bonds. On the other hand, NAG-6-phosphate forms only one hydrogen bond (Arg 231) in the TraR binding site and has no shared hydrogen bonds formed by OHL in the binding pocket. NAG-6-phosphate also forms two of five or three of five hydrogen bonds formed by DHL or an antagonist, respectively, with the CviR AHL binding site. The results of this modeling investigation are congruent with our experimental data suggesting that low mM concentrations of NAG or phosphorylated NAG can interfere with transcription activated by LuxR type proteins.
N-Acetylglucosamine Upregulates Extracellular Chitinase Activity of C. violaceum CV026
C. violaceum CV026, which is known as an AHL synthase (cviI) deficient strain is reported to only produce chitinase activity on addition of AHLs (Chernin et al., 1998). It has also been reported that NAG can induce the expression of chitinase enzymes in different bacterial species. However, our results indicated that NAG inhibits QS-regulated violacein production of C. violaceum CV026. There is no report on the influence of NAG on extracellular chitinase activity in CV026. To test this, C. violaceum CV026 was cultured with various concentrations of NAG (0.5–5 mM) in the presence and absence of HHL (100 nM). Figure 6 shows significant (P < 0.01) upregulation of the chitinase activity by 2–5 mM NAG even in the absence of exogenous HHL. In order to test whether this response was specific to NAG, chitin (1%), glucose (5 mM) or GlcN (5 mM) were examined revealing no induction of chitinase activity in the absence of HHL. In the presence of HHL (100 nM) chitinase activity increased in response to chitin (1%) addition. The strongest chitinase activity was observed in the presence of both NAG (5 mM) and HHL (100 nM). Similar results were observed with the C. violaceum wild type strain treated with increasing concentrations (0.5–5 mM) of NAG (Supplementary Figure S1). In summary, HHL upregulates violacein production and chitinase activity, whilst NAG upregulates chitinase activity but downregulates violacein production.
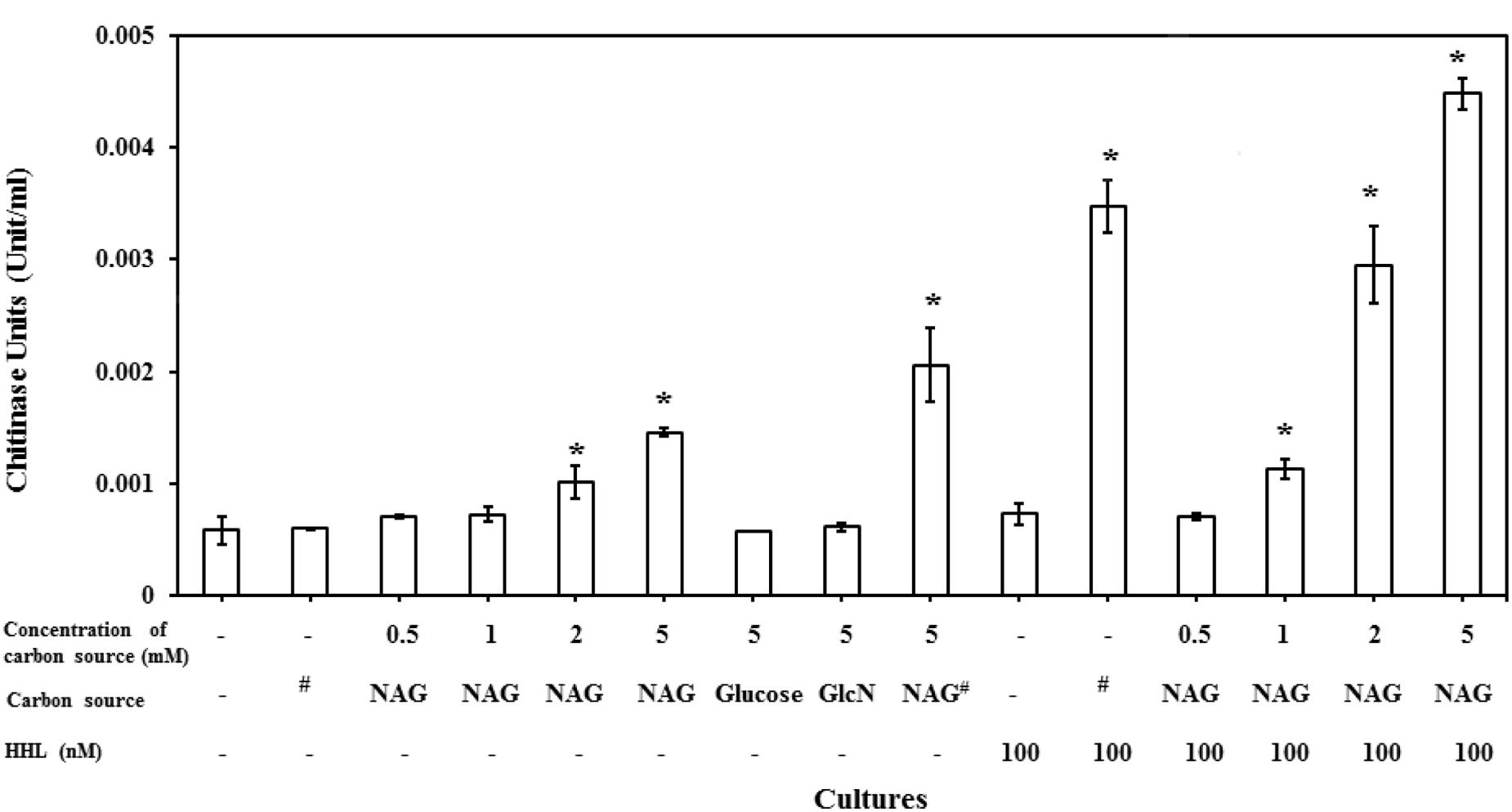
FIGURE 6. NAG regulates extracellular chitinase activity of bacteria. Concentration dependent effect of NAG on extracellular chitinase activity of C. violaceum (CV026) in the presence and absence of HHL 100 (nM). All cultures were in triplicates. Error bars represent standard deviation. Results represent the chitinase units/per cell. Asterisks indicate the significant differences in comparison to control samples (P < 0.01). “#” means chitin (1%).
Discussion
AHL dependent LuxR type proteins are one of many transcriptional regulators that are integrated to control expression of phenotypes in bacteria (Miller and Bassler, 2001). Other mechanisms that regulate transcription of genes influenced by LuxR type regulatory proteins include CRP, GroESL and Hnr (Shrout et al., 2006; Jahid et al., 2013). Results presented here suggest that NAG inhibits transcription of genes upregulated by AHL binding to LuxR, LasR and CviR based QS systems. NAG is the monomer of the second most abundant biopolymer on Earth and is also a major component of heterogeneous polysaccharides including murein and hyaluronic acid. It is commonly encountered in the environment in millimolar concentrations (Jagmann et al., 2012).
Several types of QS inhibitor compounds are produced by eukaryotes and prokaryotes and synthetic derivatives have shown QS inhibition activity (Wu et al., 2004). These compounds target QS systems by different mechanisms including inhibition of AHL production, enzymatic degradation of AHLs or interference with AHL binding or the stability of AHL receptors (Givskov et al., 1996; Zhang et al., 2002; Gao et al., 2003; Kalia, 2013; Chang et al., 2014). Evidence presented here suggests that NAG inhibits transcription of QS controlled genes via catabolite repression (CRP activity) and interference with some aspect of the QS mechanism (LuxR activity; Figure 7). Cellular uptake of NAG involves a phosphotransferase system that phosphorylates NAG as it enters the cell (Plumbridge, 1990). Therefore we speculate that phosphorylated NAG derivatives including NAG-1-phosphate and NAG-6-phosphate are likely responsible for the observed affects.
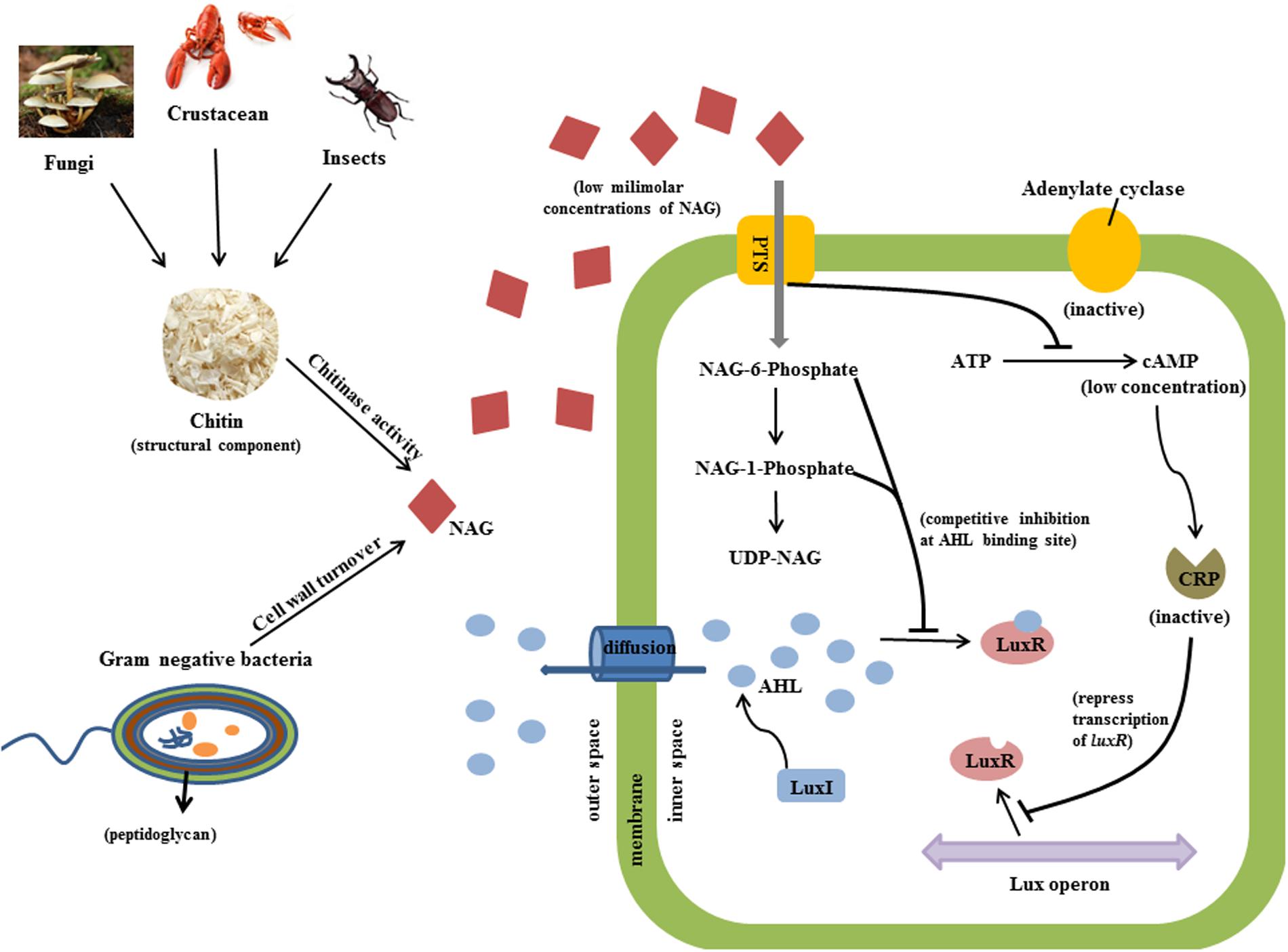
FIGURE 7. Predicted inhibitory mechanism of NAG on QS activity is illustrated. Chitinases cleave chitin from fungi, insects and crustacean and releases NAG molecules (red diamond). Peptidoglycan structure of bacteria is also an important source of NAG. Bacterial cells uptake NAG through membrane bounded phosphotransferases. High concentrations of NAG inhibit adenylate cyclase activity and thereby reduce the concentration of cAMP molecules which binds to CRP in order to induce transcription of luxR. Secondly, phosphorylated NAG molecules competitively bind to AHL binding site of LuxR protein at low milimolar concentrations and inhibit QS activity.
Phosphorylation of NAG could inhibit adenylate cyclase activity, which converts ATP into cAMP thereby influencing CRP transcriptional regulation activity (Kolb et al., 1993). The role of CRP in regulating carbon metabolism through carbon catabolite repression of E. coli and B. subtilis has been well characterized (Gorke and Stulke, 2008) but as mentioned above CRP also influences transcription of AHL dependent genes by binding to the same promoters of LuxR type proteins (Dunlap and Greenberg, 1988; Albus et al., 1997; Liang et al., 2007). Certainly, nutritional conditions have been shown to affect expression of QS regulated phenotypes (Shrout et al., 2006; Jahid et al., 2013). Assay inhibition by glucose and glucosamine presented here shows that the influence of catabolite repression is strain specific.
Molecular modeling is a commonly used and powerful technique to predict the binding affinity of a ligand to the binding site of a target protein where binding pockets have been defined by crystallography (Meng et al., 2011; Ferreira et al., 2015). It has been shown that molecular modeling is also an effective tool in the search for novel compounds with QS inhibitory activities (Yang et al., 2009; Ahumedo et al., 2010; Tan et al., 2013). From our docking studies, we found that NAG-1-phosphate and NAG-6-phosphate showed important hydrogen bonding interactions with LasR, TraR, and CviR proteins similar to their native AHL ligands. Receptor-ligand binding modeling suggests that NAG-1-phosphate and NAG-6-phosphate could inhibit transcription of AHL regulated genes by occupying the AHL binding site of LuxR type regulatory proteins. There are several non-AHL molecules that are capable of binding to AHL receptor proteins and interfering with native signal binding or increasing the turnover of receptor protein (LaSarre and Federle, 2013). For example, halogenated furanones are well known to compete with AHLs for binding to LuxR homologs resulting in a decrease in the concentration of the regulatory protein (Rasmussen et al., 2000; Manefield et al., 2002).
NAG can be utilized as a carbon/nitrogen source as well as regulate bacterial host colonization, production of virulence factors and biofilm formation (Chang et al., 2004; Korgaonkar and Whiteley, 2011; Jagmann et al., 2012; Kawada-Matsuo et al., 2012). In a previous study, transcriptome analysis to examine the gene expression levels of P. aeruginosa growing on NAG has shown that 5 mM NAG upregulates the abundance of quorum sensing repressor protein (QscR) sixfold (Korgaonkar and Whiteley, 2011). QscR is an orphan LuxR-type protein with no associated AHL synthase gene but responds to AHL molecules synthesized by LasI and RhlI (Chugani et al., 2001). Studies have shown that QscR negatively regulates the activities of both LasR and RhlR regulators through formation of heterodimers with receptor proteins and/or binding to their AHL molecules (Ledgham et al., 2003; Fuqua, 2006). Likewise, many other orphan LuxR-type regulatory genes are thought to exist within proteobacterial genomes and expression of these genes could also be regulated by NAG.
P. aeruginosa has a hierarchical QS-cascade between three separate QS circuits known as the Las, Rhl and PQS systems (Latifi et al., 1995; Pearson et al., 1995; Cao et al., 2001). Las and Rhl signaling systems regulate expression of OdDHL and BHL, respectively, while the PQS system regulates expression of the Pseudomonas quinolone signal molecule. It has been reported that low millimolar concentrations of NAG induce quinolone signal production in P. aeruginosa (Korgaonkar and Whiteley, 2011). Results presented in this study revealed that NAG inhibits the Las-dependent QS system, which is considered the master regulator of Rhl and PQS signaling systems (Pesci et al., 1997; McKnight et al., 2000). It is known that the QS regulatory cascade of P. aeruginosa is dependent on environmental and nutritional conditions (Dekimpe and Deziel, 2009; Cabeen, 2014). There are several examples of Las-independent regulation of Rhl and PQS mediated gene expression (D’Argenio et al., 2007; Bjarnsholt et al., 2010). For instance, starvation and phosphate-limitation induce transcriptional activation of Rhl and PQS in Las-deficient strains (Van Delden et al., 1998; Jensen et al., 2006).
NAG is widely used in dietary supplements, cosmetics and therapeutics development due to its unique features (Chen et al., 2010). It has been reported that NAG is a highly safe compound and intravenous injection of NAG (20 g) to the human body has no toxic effect and does not alter the blood glucose concentrations (Levin et al., 1961). Therefore, NAG is safely used as a nutritional supplement to treat diseases such as inflammatory bowel disease and osteoarthritis (Talent and Gracy, 1996; Kanazawa and Fukudo, 2006). On the other hand, there are many human pathogens such as P. aeruginosa, S. marcescens and V. cholera, which utilize QS to regulate their virulence activity (Zhu et al., 2002; Wei and Lai, 2006; Jimenez et al., 2012). The novel function of NAG on AHL-based QS activity can be used to develop therapeutic strategies to treat diseases associated with QS-mediated bacterial pathogenicity.
Additional tests investigated the relationship between NAG and HHL on extracellular chitinase activity of C. violaceum CV026. CV026 is reported to only produce chitinase and the purple pigment violacein on addition of AHLs (McClean et al., 1997; Chernin et al., 1998). Our results showed that NAG downregulates QS-regulated violacein production of C. violaceum CV026 while it upregulates QS-regulated extracellular chitinase activity. These results suggest that NAG influences extracellular chitinase activity through an unknown regulatory mechanism. This kind of inverse regulation has been recently reported on RhlR activity in P. aeruginosa which inversely modulates pyocyanin and rhamnolipid synthesis in response to non-native AHLs (Welsh et al., 2015).
The ability of bacteria to distinguish intra-cellular and inter-cellular NAG allows them to detect any possible threats from neighboring cells or pathogenic microorganisms (Konopka, 2012). Accordingly, bacteria could utilize NAG to switch on/off QS activity and modulate the production of QS-regulated phenotypes. This kind of a regulatory mechanism could also allow bacteria to avoid the cost of QS activity.
Conclusion
This study demonstrates the effect of NAG on three different types of LuxR based QS systems. Evidence is presented indicating that NAG inhibits the QS activities of all analyzed strains. NAG is shown to repress transcription of genes upregulated by AHLs. Our results point out the possible involvement of both catabolite repression and competitive inhibition of AHL binding sites of LuxR type receptor proteins by phosphorylated NAG molecules. Inverse effects of NAG on regulation of violacein and extracellular chitinase synthesis in C. violaceum indicate that NAG could inhibit one QS regulated phenotype while simultaneously inducing another. Therefore, therapeutic applications of NAG require extra caution when targeting a certain type of a LuxR type receptor in order to avoid overexpression of undesirable phenotypes. Further research on identification of the role of NAG on QS regulation will improve our understanding of how bacteria survive in mixed communities within a host or in the environment and may help us to design novel therapeutic strategies to interfere with AHL dependent bacterial pathogenicity.
Author Contributions
ÖK, SK, NK, and MM planned the experiments. ÖK, ZU, SN, ML, SK, and SB performed the experiments. ÖK, ZU, SB, and MM contributed to interpretation of data. ÖK, ZU, and MM contributed in writing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
MM was supported by an Australian Research Council Future Fellowship (FT100100078).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01313
FIGURE S1 | Concentration dependent effect of NAG on extracellular chitinase activity of C. violaceum WT strain. All cultures were in triplicates. Error bars represent standard deviation. Results represent the chitinase units/per cell. Asterisks indicate the significant differences in comparison to control samples (P < 0.01).
References
Ahumedo, M., Díaz, A., and Vivas-Reyes, R. (2010). Theoretical and structural analysis of the active site of the transcriptional regulators LasR and TraR, using molecular docking methodology for identifying potential analogues of acyl homoserine lactones (AHLs) with anti-quorum sensing activity. Eur. J. Med. Chem. 45, 608–615. doi: 10.1016/j.ejmech.2009.11.004
Albus, A. M., Pesci, E. C., Runyen-Janecky, L. J., West, S. E., and Iglewski, B. H. (1997). Vfr controls quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179, 3928–3935.
Andersen, J. B., Heydorn, A., Hentzer, M., Eberl, L., Geisenberger, O., Christensen, B. B., et al. (2001). Gfp-based N-acyl homoserine-lactone sensor systems for detection of bacterial communication. Appl. Environ. Microbiol. 67, 575–585. doi: 10.1128/aem.67.2.575-585.2001
Bassler, B. L. (1999). How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2, 582–587. doi: 10.1016/S1369-5274(99)00025-9
Bhowmick, R., Ghosal, A., and Chatterjee, N. S. (2007). Effect of environmental factors on expression and activity of chitinase genes of Vibrios with special reference to Vibrio cholerae. J. Appl. Microbiol. 103, 97–108. doi: 10.1111/j.1365-2672.2006.03253.x
Bjarnsholt, T., Jensen, P. O., Jakobsen, T. H., Phipps, R., Nielsen, A. K., Rybtke, M. T., et al. (2010). Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS ONE 5:e10115. doi: 10.1371/journal.pone.0010115
Blosser, R. S., and Gray, K. M. (2000). Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 40, 47–55. doi: 10.1016/S0167-7012(99)00136-0
Bottomley, M. J., Muraglia, E., Bazzo, R., and Carfi, A. (2007). Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J. Biol. Chem. 282, 13592–13600. doi: 10.1074/jbc.M700556200
Cabeen, M. T. (2014). Stationary phase-specific virulence factor overproduction by a lasR mutant of Pseudomonas aeruginosa. PLoS ONE 9:e88743. doi: 10.1371/journal.pone.0088743
Cao, H., Krishnan, G., Goumnerov, B., Tsongalis, J., Tompkins, R., and Rahme, L. G. (2001). A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc. Natl. Acad. Sci. U.S.A. 98, 14613–14618. doi: 10.1073/pnas.251465298
Chang, C.-Y., Krishnan, T., Wang, H., Chen, Y., Yin, W.-F., Chong, Y.-M., et al. (2014). Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci. Rep 4:7245. doi: 10.1038/srep07245
Chang, D. E., Smalley, D. J., Tucker, D. L., Leatham, M. P., Norris, W. E., Stevenson, S. J., et al. (2004). Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. U.S.A. 101, 7427–7432. doi: 10.1073/pnas.0307888101
Chen, G., Swem, L. R., Swem, D. L., Stauff, D. L., O’Loughlin, C. T., Jeffrey, P. D., et al. (2011). A strategy for antagonizing quorum sensing. Mol. Cell 42, 199–209. doi: 10.1016/j.molcel.2011.04.003
Chen, J. K., Shen, C. R., and Liu, C. L. (2010). N-acetylglucosamine: production and applications. Mar Drugs 8, 2493–2516. doi: 10.3390/md8092493
Chernin, L. S., Winson, M. K., Thompson, J. M., Haran, S., Bycroft, B. W., Chet, I., et al. (1998). Chitinolytic activity in Chromobacterium violaceum: substrate analysis and regulation by quorum sensing. J. Bacteriol. 180, 4435–4441.
Chong, G., Kimyon, O., Rice, S. A., Kjelleberg, S., and Manefield, M. (2012). The presence and role of bacterial quorum sensing in activated sludge. Microb. Biotechnol. 5, 621–633. doi: 10.1111/j.1751-7915.2012.00348.x
Christensen, A. B., Riedel, K., Eberl, L., Flodgaard, L. R., Molin, S., Gram, L., et al. (2003). Quorum-sensing-directed protein expression in Serratia proteamaculans B5a. Microbiology 149, 471–483. doi: 10.1099/mic.0.25575-0
Chugani, S. A., Whiteley, M., Lee, K. M., D’Argenio, D., Manoil, C., and Greenberg, E. P. (2001). QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 98, 2752–2757. doi: 10.1073/pnas.051624298
Clark, D. J., and Maaløe, O. (1967). DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23, 99–112. doi: 10.1016/S0022-2836(67)80070-6
D’Argenio, D. A., Wu, M., Hoffman, L. R., Kulasekara, H. D., Deziel, E., Smith, E. E., et al. (2007). Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol. Microbiol. 64, 512–533. doi: 10.1111/j.1365-2958.2007.05678.x
Defoirdt, T., Darshanee Ruwandeepika, H. A., Karunasagar, I., Boon, N., and Bossier, P. (2010). Quorum sensing negatively regulates chitinase in Vibrio harveyi. Environ. Microbiol. Rep. 2, 44–49. doi: 10.1111/j.1758-2229.2009.00043.x
Dekimpe, V., and Deziel, E. (2009). Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology 155(Pt 3), 712–723. doi: 10.1099/mic.0.022764-0
Dunlap, P. V. (1999). Quorum regulation of luminescence in Vibrio fischeri. J. Mol. Microbiol. Biotechnol. 1, 5–12.
Dunlap, P. V., and Greenberg, E. P. (1988). Control of Vibrio fischeri lux gene transcription by a cyclic AMP receptor protein-luxR protein regulatory circuit. J. Bacteriol. 170, 4040–4046.
Eisenreich, W., Dandekar, T., Heesemann, J., and Goebel, W. (2010). Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat. Rev. Microbiol. 8, 401–412. doi: 10.1038/nrmicro2351
Ferreira, L., dos Santos, R., Oliva, G., and Andricopulo, A. (2015). Molecular docking and structure-based drug design strategies. Molecules 20, 13384–13421. doi: 10.3390/molecules200713384
Frimmersdorf, E., Horatzek, S., Pelnikevich, A., Wiehlmann, L., and Schomburg, D. (2010). How Pseudomonas aeruginosa adapts to various environments: a metabolomic approach. Environ. Microbiol. 12, 1734–1747. doi: 10.1111/j.1462-2920.2010.02253.x
Fuqua, C. (2006). The QscR quorum-sensing regulon of Pseudomonas aeruginosa: an orphan claims its identity. J. Bacteriol. 188, 3169–3171. doi: 10.1128/jb.188.9.3169-3171.2006
Gao, M., Teplitski, M., Robinson, J. B., and Bauer, W. D. (2003). Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol. Plant Microbe Interact. 16, 827–834. doi: 10.1094/MPMI.2003.16.9.827
Givskov, M., de Nys, R., Manefield, M., Gram, L., Maximilien, R., Eberl, L., et al. (1996). Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 178, 6618–6622.
Gooday, G. (1990). Physiology of microbial degradation of chitin and chitosan. Biodegradation 1, 177–190. doi: 10.1007/BF00058835
Gorke, B., and Stulke, J. (2008). Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6, 613–624. doi: 10.1038/nrmicro1932
Hentzer, M., Riedel, K., Rasmussen, T. B., Heydorn, A., Andersen, J. B., Parsek, M. R., et al. (2002). Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148, 87–102. doi: 10.1099/00221287-148-1-87
Jagmann, N., von Rekowski, K. S., and Philipp, B. (2012). Interactions of bacteria with different mechanisms for chitin degradation result in the formation of a mixed-species biofilm. FEMS Microbiol. Lett. 326, 69–75. doi: 10.1111/j.1574-6968.2011.02435.x
Jahid, I. K., Lee, N.-Y., Kim, A., and Ha, S.-D. (2013). Influence of glucose concentrations on biofilm formation, motility, exoprotease production, and quorum sensing in Aeromonas hydrophila. J. Food Prot. 76, 239–247. doi: 10.4315/0362-028X.JFP-12-321
Jensen, V., Lons, D., Zaoui, C., Bredenbruch, F., Meissner, A., Dieterich, G., et al. (2006). RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J. Bacteriol. 188, 8601–8606. doi: 10.1128/JB.01378-06
Jimenez, P. N., Koch, G., Thompson, J. A., Xavier, K. B., Cool, R. H., and Quax, W. J. (2012). The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 76, 46–65. doi: 10.1128/MMBR.05007-11
Kalia, V. C. (2013). Quorum sensing inhibitors: an overview. Biotechnol. Advan. 31, 224–245. doi: 10.1016/j.biotechadv.2012.10.004
Kanazawa, M., and Fukudo, S. (2006). Effects of fasting therapy on irritable bowel syndrome. Int. J. Behav. Med. 13, 214–220. doi: 10.1207/s15327558ijbm1303_4
Kawada-Matsuo, M., Mazda, Y., Oogai, Y., Kajiya, M., Kawai, T., Yamada, S., et al. (2012). GlmS and NagB regulate amino sugar metabolism in opposing directions and affect Streptococcus mutans virulence. PLoS ONE 7:e33382. doi: 10.1371/journal.pone.0033382
Keyhani, N. O., and Roseman, S. (1999). Physiological aspects of chitin catabolism in marine bacteria. Biochim. Biophys. Acta 1473, 108–122. doi: 10.1016/S0304-4165(99)00172-5
Kolb, A., Busby, S., Buc, H., Garges, S., and Adhya, S. (1993). Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62, 749–795. doi: 10.1146/annurev.bi.62.070193.003533
Konopka, J. B. (2012). N-acetylglucosamine functions in cell signaling. Scientifica 2012:15. doi: 10.6064/2012/489208
Korgaonkar, A., Trivedi, U., Rumbaugh, K. P., and Whiteley, M. (2013). Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc. Natl. Acad. Sci. U.S.A. 110, 1059–1064. doi: 10.1073/pnas.1214550110
Korgaonkar, A. K., and Whiteley, M. (2011). Pseudomonas aeruginosa enhances production of an antimicrobial in response to N-acetylglucosamine and peptidoglycan. J. Bacteriol. 193, 909–917. doi: 10.1128/jb.01175-10
LaSarre, B., and Federle, M. J. (2013). Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 77, 73–111. doi: 10.1128/MMBR.00046-12
Latifi, A., Winson, M. K., Foglino, M., Bycroft, B. W., Stewart, G. S. A. B., Lazdunski, A., et al. (1995). Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17, 333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x
Lazazzera, B. A., Kurtser, I. G., McQuade, R. S., and Grossman, A. D. (1999). An autoregulatory circuit affecting peptide signaling in Bacillus subtilis. J. Bacteriol. 181, 5193–5200.
Ledgham, F., Ventre, I., Soscia, C., Foglino, M., Sturgis, J. N., and Lazdunski, A. (2003). Interactions of the quorum sensing regulator QscR: interaction with itself and the other regulators of Pseudomonas aeruginosa LasR and RhlR. Mol. Microbiol. 48, 199–210. doi: 10.1046/j.1365-2958.2003.03423.x
Levin, R. M., Krieger, N. N., and Winzler, R. J. (1961). Glucosamine and acetylglucosamine tolerance in man. J. Lab. Clin. Med. 58, 927–932.
Liang, W., Pascual-Montano, A., Silva, A. J., and Benitez, J. A. (2007). The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology 153(Pt 9), 2964–2975. doi: 10.1099/mic.0.2007/006668-0
Manefield, M., Rasmussen, T. B., Henzter, M., Andersen, J. B., Steinberg, P., Kjelleberg, S., et al. (2002). Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148, 1119–1127. doi: 10.1099/00221287-148-4-1119
Manefield, M., and Turner, S. L. (2002). Quorum sensing in context: out of molecular biology and into microbial ecology. Microbiology 148, 3762–3764. doi: 10.1099/00221287-148-12-3762
McClean, K. H., Winson, M. K., Fish, L., Taylor, A., Chhabra, S. R., Camara, M., et al. (1997). Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143, 3703–3711. doi: 10.1099/00221287-143-12-3703
McKnight, S. L., Iglewski, B. H., and Pesci, E. C. (2000). The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 182, 2702–2708. doi: 10.1128/JB.182.10.2702-2708.2000
Meng, X.-Y., Zhang, H.-X., Mezei, M., and Cui, M. (2011). Molecular docking: a powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 7, 146–157. doi: 10.2174/157340911795677602
Miller, M. B., and Bassler, B. L. (2001). Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. doi: 10.1146/annurev.micro.55.1.165
Monreal, J., and Reese, E. T. (1969). The chitinase of Serratia marcescens. Can. J. Microbiol. 15, 689–696. doi: 10.1139/m69-122
Nizalapur, S., Kimyon, O., Biswas, N. N., Gardner, C. R., Griffith, R., Rice, S. A., et al. (2016). Design, synthesis and evaluation of N-aryl-glyoxamide derivatives as structurally novel bacterial quorum sensing inhibitors. Org. Biomol. Chem. 14, 680–693. doi: 10.1039/C5OB01973G
Park, J. T., and Uehara, T. (2008). How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiol. Mol. Biol. Rev. 72, 211–227. doi: 10.1128/mmbr.00027-07
Pearson, J. P., Passador, L., Iglewski, B. H., and Greenberg, E. P. (1995). A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 92, 1490–1494. doi: 10.1073/pnas.92.5.1490
Pesci, E. C., Pearson, J. P., Seed, P. C., and Iglewski, B. H. (1997). Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179, 3127–3132.
Plumbridge, J. A. (1990). Induction of the nag regulon of Escherichia coli by N-acetylglucosamine and glucosamine: role of the cyclic AMP-catabolite activator protein complex in expression of the regulon. J. Bacteriol. 172, 2728–2735.
Rasmussen, T. B., Manefield, M., Andersen, J. B., Eberl, L., Anthoni, U., Christophersen, C., et al. (2000). How Delisea pulchra furanones affect quorum sensing and swarming motility in Serratia liquefaciens MG1. Microbiology 146, 3237–3244. doi: 10.1099/00221287-146-12-3237
Rojo, F. (2010). Carbon catabolite repression in Pseudomonas: optimizing metabolic versatility and interactions with the environment. FEMS Microbiol. Rev. 34, 658–684. doi: 10.1111/j.1574-6976.2010.00218.x
Romero, M., Avendano-Herrera, R., Magarinos, B., Camara, M., and Otero, A. (2010). Acylhomoserine lactone production and degradation by the fish pathogen Tenacibaculum maritimum, a member of the cytophaga-flavobacterium-bacteroides (CFB) group. FEMS Microbiol. Lett. 304, 131–139. doi: 10.1111/j.1574-6968.2009.01889.x
Shrout, J. D., Chopp, D. L., Just, C. L., Hentzer, M., Givskov, M., and Parsek, M. R. (2006). The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 62, 1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x
Sohanpal, B. K., El-Labany, S., Lahooti, M., Plumbridge, J. A., and Blomfield, I. C. (2004). Integrated regulatory responses of fimB to N-acetylneuraminic (sialic) acid and GlcNAc in Escherichia coli K-12. Proc. Natl. Acad. Sci. U.S.A. 101, 16322–16327. doi: 10.1073/pnas.0405821101
Souza, C., Almeida, B., Colwell, R., and Rivera, I. G. (2011). The importance of chitin in the marine environment. Mar. Biotechnol. 13, 823–830. doi: 10.1007/s10126-011-9388-1
Talent, J. M., and Gracy, R. W. (1996). Pilot study of oral polymeric N-acetyl-d-glucosamine as a potential treatment for patients with osteoarthritis. Clin. Ther. 18, 1184–1190. doi: 10.1016/S0149-2918(96)80073-7
Tan, S. Y.-Y., Chua, S.-L., Chen, Y., Rice, S. A., Kjelleberg, S., Nielsen, T. E., et al. (2013). Identification of five structurally unrelated quorum-sensing inhibitors of Pseudomonas aeruginosa from a natural-derivative database. Antimicrob. Agents Chemother. 57, 5629–5641. doi: 10.1128/AAC.00955-13
Techkarnjanaruk, S., Pongpattanakitshote, S., and Goodman, A. E. (1997). Use of a promoterless lacZ gene insertion to investigate chitinase gene expression in the marine bacterium Pseudoalteromonas sp. strain S9. Appl. Environ. Microbiol. 63, 2989–2996.
Twigg, M. S., Tait, K., Williams, P., Atkinson, S., and Camara, M. (2014). Interference with the germination and growth of Ulva zoospores by quorum-sensing molecules from Ulva-associated epiphytic bacteria. Environ. Microbiol. 16, 445–453. doi: 10.1111/1462-2920.12203
Ulhoa, C. J., and Peberdy, J. F. (1991). Regulation of chitinase synthesis in Trichoderma harzianum. J. Gen. Microbiol. 137, 2163–2169. doi: 10.1099/00221287-137-9-2163
Van Delden, C., Pesci, E. C., Pearson, J. P., and Iglewski, B. H. (1998). Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum-sensing mutant. Infect. Immun. 66, 4499–4502.
Wei, J. R., and Lai, H. C. (2006). N-acylhomoserine lactone-dependent cell-to-cell communication and social behavior in the genus Serratia. Int. J. Med. Microbiol. 296, 117–124. doi: 10.1016/j.ijmm.2006.01.033
Welsh, M. A., Eibergen, N. R., Moore, J. D., and Blackwell, H. E. (2015). Small molecule disruption of quorum sensing cross-regulation in Pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J. Am. Chem. Soc. 137, 1510–1519. doi: 10.1021/ja5110798
Williams, P., Winzer, K., Chan, W. C., and Cámara, M. (2007). Look who’s talking: communication and quorum sensing in the bacterial world. Philos. Trans. R. Soc. B Biol. Sci. 362, 1119–1134. doi: 10.1098/rstb.2007.2039
Wu, H., Song, Z., Hentzer, M., Andersen, J. B., Molin, S., Givskov, M., et al. (2004). Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J. Antimicrob. Chemother. 53, 1054–1061. doi: 10.1093/jac/dkh223
Yang, L., Rybtke, M. T., Jakobsen, T. H., Hentzer, M., Bjarnsholt, T., Givskov, M., et al. (2009). Computer-aided identification of recognized drugs as Pseudomonas aeruginosa quorum-sensing inhibitors. Antimicrob. Agents Chemother. 53, 2432–2443. doi: 10.1128/aac.01283-08
You, Z., Fukushima, J., Tanaka, K., Kawamoto, S., and Okuda, K. (1998). Induction of entry into the stationary growth phase in Pseudomonas aeruginosa by N-acylhomoserine lactone. FEMS Microbiol. Lett. 164, 99–106. doi: 10.1111/j.1574-6968.1998.tb13073.x
Zhang, G., Zhang, F., Ding, G., Li, J., Guo, X., Zhu, J., et al. (2012). Acyl homoserine lactone-based quorum sensing in a methanogenic archaeon. ISME J. 6, 1336–1344. doi: 10.1038/ismej.2011.203
Zhang, H. B., Wang, L. H., and Zhang, L. H. (2002). Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. U.S.A. 99, 4638–4643. doi: 10.1073/pnas.022056699
Keywords: chitin, quorum sensing, acetyl glucosamine, N-acylhomoserine lactone, chitinase
Citation: Kimyon Ö, Ulutürk Zİ, Nizalapur S, Lee M, Kutty SK, Beckmann S, Kumar N and Manefield M (2016) N-Acetylglucosamine Inhibits LuxR, LasR and CviR Based Quorum Sensing Regulated Gene Expression Levels. Front. Microbiol. 7:1313. doi: 10.3389/fmicb.2016.01313
Received: 29 June 2016; Accepted: 09 August 2016;
Published: 23 August 2016.
Edited by:
Efstathios D. Giaouris, University of the Aegean, GreeceReviewed by:
Alexandra Lianou, Agricultural University of Athens, GreeceDurg Vijai Singh, Institute of Life Sciences, India
Chien-Yi Chang, Heriot-Watt University, UK
Copyright © 2016 Kimyon, Ulutürk, Nizalapur, Lee, Kutty, Beckmann, Kumar and Manefield. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mike Manefield, manefield@unsw.edu.au
 Önder Kimyon
Önder Kimyon Zehra İ. Ulutürk1
Zehra İ. Ulutürk1 Shashidhar Nizalapur
Shashidhar Nizalapur Matthew Lee
Matthew Lee Samuel K. Kutty
Samuel K. Kutty Sabrina Beckmann
Sabrina Beckmann Mike Manefield
Mike Manefield