- The Key Laboratory for Silviculture and Conservation of Ministry of Education, College of Forestry, Beijing Forestry University, Beijing, China
Verticillium dahliae, a ubiquitous phytopathogenic fungus, forms resting structures, known as microsclerotia that play crucial roles in Verticillium wilt diseases. VdHog1, a mitogen-activated protein kinase (MAPK), controls microsclerotia formation, virulence, and stress response in V. dahliae. In this study, we present detailed evidence that the conserved upstream component of VdHog1, VdPbs2, is a key regulator of microsclerotia formation, oxidative stress and fungicide response and plant virulence in V. dahliae. We identified VdPbs2, homologous to the yeast MAPK kinase Pbs2. Similar to the VdHog1 deletion mutant, VdPbs2 deletion strains exhibited delayed melanin synthesis and reduced formation of microsclerotia. When exposed to stresses, VdPbs2 mutants were more sensitive than the wild type to osmotic agents and peroxide, but more resistant to inhibitors of cell wall synthesis and some fungicides. Finally, VdPbs2 deletion mutants exhibited reduced virulence on smoke tree and tobacco seedlings. When taken together, we implicate that VdPbs2 and VdHog1 function in a cascade that regulates microsclerotia formation and virulence, but not all VdHog1 dependent functions are VdPbs2 regulated. This study thus provides novel insights into the signal transduction mechanisms that regulate microsclerotia formation and pathogenesis in this fungus.
Introduction
The mitogen-activated protein kinase (MAPK) signaling pathways are involved in integrating multiple extracellular and intracellular signals to regulate transcription of specific genes that help the cell adapt to the conditions in eukaryotic cells (Gustin et al., 1998; Widmann et al., 1999). MAPK cascades consist of MAPK kinase kinases (MEKK or MAPKKK), MAPK kinases (MEK or MAPKK), and MAPK. The MAPK is activated by MEK, which is activated in turn by MEK kinase (Widmann et al., 1999). Activated MAPKs can then phosphorylate downstream substrates, affecting their biochemical properties and leading to specific output responses (Hamel et al., 2012). In Saccharomyces cerevisiae five MAPK pathways work in coordination, and in some cases independently, to regulate mating, invasive growth, cell wall integrity, ascospore formation and hyperosmoregulation (Gustin et al., 1998; Levin, 2005; Roman et al., 2007; Zhao et al., 2007).
Upon stress (osmotic, oxidative, acid and heat, etc), the high osmolarity glycerol (HOG) pathway is activated and the stress-activated MAPK Hog1 is phosphorylated (Brewster and Gustin, 2014). This pathway is initiated by two upstream branches, Sln1 and Sho1, and they converge at the Pbs2 MAPKK and are able to activate Pbs2, which then phosphorylates the MAPK Hog1 (Brewster et al., 1993; O’Rourke and Herskowitz, 2004; Roman et al., 2007). The activated Hog1 translocates into the nucleus and then regulates gene expression through several transcription factors, Hot1, Sko1,Smp1, Msn2, and Msn4 (Estruch and Carlson, 1993; Schüller et al., 1994; Gorner et al., 1998; Proft and Serrano, 1999; Rep et al., 2000; de Nadal et al., 2003). In particular, HOG pathway plays an important and somewhat specialized role in sensing stress conditions and activating gene expression, enabling the cell to resist the toxic effects of stress, survive and ultimately grow under adverse conditions (Gustin et al., 1998; Widmann et al., 1999).
Hog1 and its homologs in filamentous fungi are referred to as stress-activated MAPKs. Besides osmoregulation, homologs of Hog1 in pathogenic fungi are involved in pathogenesis and response to various stresses (Xu, 2000; Zhao et al., 2007; Hamel et al., 2012). In Mycosphaerella graminicola, strains lacking Hog1 homolog are impaired in pathogenicity (Mehrabi et al., 2006). In Botrytis cinerea, SAK1 (Hog1 homolog) deletion mutants are unable to penetrate plant tissues (Segmuller et al., 2007). In oomycete Phytophthora sojae, silencing-mutants fail to colonize soybean (Li et al., 2010). However, some Hog1 homologs are dispensable for virulence, including Magnaporthe oryzae OSM1 (Dixon et al., 1999), Bipolaris oryzae SRM1 (Moriwaki et al., 2006), and Colletotrichum orbiculare Osc1 (Kojima et al., 2004). In several fungal species, it has also been reported that HOG pathway contributes to resistance to a variety of fungicides (Zhang et al., 2002; Kojima et al., 2004). Pbs2, as the specific activator of Hog1, affects the response to hyperosmotic stress (Posas and Saito, 1997). Similar to Hog1, Pbs2 has proved to be involved in multiple stress responses in S. cerevisiae (Akhtar et al., 1997; Lai et al., 1997; Gustin et al., 1998). Furthermore, the Pbs2-Hog1 module controls stress response, differentiation and virulence in pathogenic fungi. For example, in Cryptococcus neoformans, Candida albicans, and Cryphonectria parasitica, Pbs2 deletion mutants are hypersensitive to osmotic shock, high temperature, oxidative stress, and the antifungal drug fludioxonil, and attenuated in virulence (Arana et al., 2005; Bahn et al., 2005; Moretti et al., 2014).
Verticillium dahliae, a soil-borne plant pathogenic fungus, is responsible for Verticillium wilt diseases in more than 200 dicotyledonous plant species worldwide (Klosterman et al., 2011; Klimes et al., 2015). Notably, the microsclerotia with melanized particles in the interhyphal spaces confer resistance to UV irradiation, temperature extremes, enzymatic lysis, and fungicidal activities of the host plant (Gordee and Porter, 1961; Griffiths, 1970; Gessler et al., 2014). The high tolerance of microsclerotia allows the pathogen to survive under unfavorable conditions and prevents from chemical fungicides, and is thus an important aspect of pathogen fitness (Griffiths, 1970; Klosterman et al., 2009). Under optimal conditions, microsclerotia germinate to form hyphae in the soil, and penetrate the plant roots, where the fungus colonizes the xylem tissue of the plant vascular system. As disease progress, V. dahliae produces microsclerotia in dying plant tissues, which returned to the soil to initiate new primary infections. Because of their pivotal roles in pathogen survival and developmental processes, both of which linked to virulence, the microsclerotia are considered important targets for disease control (Gordee and Porter, 1961; Coley-Smith and Cooke, 1971; Duressa et al., 2013). Thus, elucidation of molecular mechanisms, especially the signal transduction pathways that regulate the development of microsclerotia, is essential for the development of novel control strategies.
Recently, dozens of genes that regulate microsclerotial development and virulence have been identified and functionally characterized in V. dahliae. Many of these genes are involved in MAP kinase signaling (Msb, VMK1 and Hog1) (Rauyaree et al., 2005; Tian et al., 2014; Wang et al., 2016), cAMP-PKA-mediated signaling (VdPKAC1) (Tzima et al., 2010), G protein signaling (VGB) (Tzima et al., 2012), and other associated genetic networks (Duressa et al., 2013; Hu et al., 2014; Xiong et al., 2014; Klimes et al., 2015). Besides, transcription factors such as VdCrz1 and VdMcm1 were reported lately (Xiong et al., 2015, 2016). Although functional genomics of V. dahliae facilitates to uncover the molecular basis of microsclerotia formation, little else is known about the signal pathways involved in microsclerotia formation. Studies on genes of HOG pathway in V. dahliae have shown the essential role in expressing certain pathogenicity-related traits. Mutants lacking the transmembrane mucin Msb exhibit significant reductions in invasive growth, adhesive capacity, conidiation, and microsclerotia formation (Tian et al., 2014). In addition, our previous report showed that deletion of VdHog1 delays microsclerotia formation, decreased virulence and heightened sensitivity to hyperosmotic stress (Wang et al., 2016).
In this study, we present evidence that VdPbs2 regulates microsclerotia formation, stress responses and pathogenicity in V. dahliae. The VdPbs2 deletion mutant exhibited delayed microsclerotia formation and reduced virulence on smoke tree and tobacco seedlings. Furthermore, deletion of VdPbs2 also increased sensitivity to osmotic agents, while increasing resistance to some fungicides and compounds that interfered with cell wall synthesis. Taken together, these results indicate that the VdPbs2-VdHog1 module is important for microsclerotia formation, stress response and plant virulence in V. dahliae.
Materials and Methods
Fungal Strains and Growth Conditions
Verticillium dahliae wild type XS11 was isolated from a smoke tree, Cotinus coggygria in Fragrant Hills Park, Beijing (Wang et al., 2013). The spores of the wild type and its derivative mutants and its complementation strains were stored in 15% (v/v) glycerin at -80°C. To acquire conidia, all strains were activated and cultured on potato dextrose agar medium (PDA, containing 200 g of potato, 20 g of glucose, and 15 g of agar per liter) at 25°C and then collected after 7 days for generation of fresh hyphae, germination tests, and etc. For all stress assay, strains were cultured on solid complete medium (CM, 50 ml of 20× nitrate salts, 1 ml of 1000× trace elements, 10 g of glucose, 2 g of peptone, 1 g of yeast extract, 1 g of casamino acids, and 1 ml of vitamin solution per liter). To test sensitivity to osmotic stress, all strains were grown for 24 days on CM containing 0.8 M NaCl and 1.2 M sorbitol. For cell wall stress assay, all strains were grown on CM with 20 μg/ml Calcofluor White (CFW) (Sigma-Aldrich) and 50 μg/ml Congo Red (CR) (Sigma-Aldrich) for 3 and 7 days, respectively. For oxidative stress, agar diffusion tests were performed to measure the sensitivity of strains to H2O2, the same spore suspension (105 spores/ml) of each strain were spread on PDA plates, and filter paper discs containing H2O2 (6, 12, and 18 mM) were placed in the center of each plate. The inhibition zone was determined after 3 days post inoculation (dpi). For fungicides assay, four different fungicides, such as 5 μg/ml difenoconazole (Sigma-Aldrich), 2 μg/ml chlorothalonil (Sigma-Aldrich), 10 μg/ml fludioxonil (Sigma-Aldrich), and 5 μg/ml iprodione (Sigma-Aldrich) were used. Three independent experiments of three replicates each were performed. To observe microsclerotia formation, conidia were sprayed onto the cellulose membrane (Ø = 80 mm; pore size = 0.22 μm) overlaid on solid basal medium (10 g of glucose, 0.2 g of sodium nitrate, 0.52 g of KCl, 0.52 g of MgSO4.7H2O, 1.52 g of KH2PO4, 3 μmol thiamine HCl, 0.1 μmol biotin, and 15 g of agar per liter). The microsclerotia formation were observed and photographed after incubation for every 48 h intervals. At 7 dpi, the observations were conducted every 7 days. All experiments were repeated at least three times.
Bioinformatics Analysis
Information regarding VdPbs2 was obtained from JGI1. Homologs of VdPbs2 were identified using BLASTP searches of home databases of other fungal species (Broad Institute and Joint Genome Institute). Multiple sequence alignments were conducted using ClustalX 2.0 (Larkin et al., 2007). The phylogenetic tree was constructed using Mega6.0 (Tamura et al., 2013) with the Neighbor Joining algorithm under default settings and 1000 bootstrap replications.
Targeted Disruption of VdPbs2 and Mutant Complementation
To delete VdPbs2 in the genome of V. dahliae, we used the split-marker method. First, the 1476 bp upstream (5′) and 1494 bp downstream (3′) flanking sequences of VdPbs2 were amplified with primer pairs LY105/LY106 and LY107/LY108, respectively (Supplementary Table S1). The geneticin-resistance cassette was amplified with the Geneticinfor/Geneticinrev primers for deletion, which include approximately 20 bp that overlaps with the 5′ and 3′ flanking sequences, respectively. The two deletion cassettes resulting from fusion PCR with primer pairs LY105/Geneticinrev and Geneticinfor/LY108 (Supplementary Table S1) were used for protoplast transformation after sequencing. To obtain ΔVdPbs2 complementation strains, the 3804 bp segment and the VdPbs2-GFP fusion construct were constructed containing the native promoter and coding region of VdPbs2. The 3804 bp segment for native complementation, amplified with primer pair LY109/LY166 (Supplementary Table S1) is used to restore the defects of ΔVdPbs2 mutant. The VdPbs2-GFP fusion plasmid was constructed as follows. Firstly, a 3.76 kb genomic fragment was amplified with the primer pair LY105/LY167 (Supplementary Table S1), including the native promoter and the full VdPbs2 open reading frame region. Then, it was inserted into the pKD5-GFP digested with SmaI. Confirmations were performed using PCR with the primer pairs LY137/LY165-RB, restriction digestion and sequencing. Finally, the native complementary segments of VdPbs2 and VdPbs2-GFP fusion constructs were transformed along with a hygromycin-resistance cassette into ΔVdPbs2 protoplasts using the PEG method (Wang et al., 2013). All transformants were verified using external screening primer pair LY137/LY138 and internal screening primers pair LY145/LY146 (Supplementary Table S1). The ΔVdPbs2/Pbs2GFP strain was preliminarily screened for GFP fluorescence and then verified using the external screening primer pair LY137/LY138 and the internal screening primer pair LY145/LY146 (Supplementary Table S1). Finally, southern blotting was performed to confirm the deletion of VdPbs2 with the DIG High Prime DNA Labeling and Detection Starter Kit I in accordance with the manufacturers’ protocol (Roche, Germany). The genomic DNA of wild type and the deletion of VdPbs2 strain was digested with KpnI and hybridized with a probe amplified from the V. dahliae strain XS11 genomic DNA with LY170up/LY170down (Supplementary Table S1) and labeled with the DIG primer.
RNA Extraction and Quantitative Real-Time PCR
Fresh mycelium of ΔVdPbs2 mutants and wild type were cultured in CM at 25°C for 5 days and collected with single-layer miracloth. Mycelia were subjected to RNA extraction using TRIzol reagent (Invitrogen) and purified with the RNA Mini Kit (Ambion). RNA integrity was confirmed by agarose gel electrophoresis. Reverse-transcription PCR was performed with Oligo-DT and SuperScript III reverse transcriptase (Invitrogen). Quantitative real-time PCR (qRT-PCR) was performed with SuperReal Premix Plus (TIANGEN, China) on an ABI 7500 real-time PCR system (Applied Biosystems, USA). The β-tubulin of V. dahliae is used as an internal reference. Relative expression levels were calculated using the ΔΔCT method (Livak and Schmittgen, 2001). All primers used in this study are listed in Supplementary Table S1.
Pathogenicity Assays
To test the ability of penetration of ΔVdPbs2 mutant, spores were dropped onto onion epidermis at the concentration of 104 conidia/ml. At 32 hpi, the penetration was observed after staining with aniline blue under light microscopy (DM2500, Leica). To determine the pathogenicity of the ΔVdPbs2 mutant, spores were filtered from liquid CM after 10 days of cultivation and then diluted to 106/ml with distilled water. One-year-old smoke tree seedlings were selected for inoculation and soaked in the conidia suspension for 10 min. The seedlings were then replanted in autoclaved soil and observed at regular intervals. To determine whether specific strains could invade the seedlings, the seedling stems were clipped into tiny fragments for isolation 14 days after inoculation (Xiong et al., 2015). Tobacco seedlings were also used for virulence tests using the same methods. The height of tobacco seedlings were measured at 30 dpi.
Microscopic Observation and Localization of VdPbs2
To analyze the response to stress, mycelium of the wild type and VdPbs2 deletion mutant were inoculated in the CM with 0.8 M NaCl for 4 days, then myceliua were collected for observation. Pictures were taken using the microscope (Leica DM 2500). To analyze of subcellular localization of VdPbs2, conidia and hyphae were collected from liquid CM. Then the fluorescence of mycelium and conidia treated with 0.8 M NaCl for 2 h were observed. The pictures were acquired using a Leica SP5 confocal laser-scanning microscope. A diode laser, Argon/2 (458, 477, 488, 496, 514 nm) was used, and the fluorescence filters were EX 488; EM 510/40. The quantification of image fluorescence was performed using the Adobe Photoshop software.
Statistical Analysis
The melanized area fraction was measured using ImageJ2 under the default settings (all the threshold of image was 42.589) (Papadopulos et al., 2007). Data were expressed as mean values ± standard error of the mean. Statistical analyses were performed by using Student’s t-test. A p-value < 0.05 was considered as statistically significant.
Results
Generation of the VdPbs2 Mutant
To investigate whether the other component of HOG signaling pathway affects the physiology and morphology of V. dahliae, we identified the homolog of S. cerevisiae Pbs2 in the V. dahliae genome database. A gene encoding a MAPK kinase (VDAG_02783) was designated as VdPbs2. The protein contains two kinase motifs (residues 258–280 and 325–563) and a tyrosine kinase domain, Pkinase_Tyr (residues 322–559, marked with dashed lines in Supplementary Figure S1). Subsequent phylogenetic analysis and amino-acid sequence alignments revealed that VdPbs2 has high sequence similarity with Pbs2 homologs in other fungi, particularly those in V. alfalfae and N. crassa. Moreover, RNA-Seq revealed that expression levels of VdPbs2 increase during microsclerotial development at 60 h, 72 h, 96 h, and 14 days in XS11 strain (Xiong et al., 2014).
Two deletion mutants (ΔVdPbs2-22 and ΔVdPbs2-32) were verified by PCR and Southern blots (Supplementary Figure S2). The complemented strain ΔVdPbs2/Pbs2GFP was confirmed to harbor the full-length VdPbs2 gene (Supplementary Figure S2) and restore phenotypes of the ΔVdPbs2 mutant (Supplementary Figure S3). The results showed that the deletion mutants and complementation strain (ΔVdPbs2/Pbs2 and ΔVdPbs2/Pbs2GFP) were successfully generated.
VdPbs2 is Involved in Microsclerotia Formation and Melanin Biosynthesis
To investigate the role of VdPbs2 in microsclerotia formation, we first paid our attention to the connection between VdPbs2 function and axenic growth on plate media. Similar to the ΔVdHog1 mutant, ΔVdPbs2 mutants exhibited no significant difference in growth rate but delayed to form microsclerotia on PDA compared with the wild type (Figure 1A). Few melanized microsclerotia can form in the ΔVdPbs2 mutant; by contrast, abundant melanized microsclerotia were produced in the wild type and the ΔVdPbs2/Pbs2 strain (Figure 1A). To determine the influence of VdPbs2 on microsclerotia in detail, we observed the microsclerotia formation on BM. The wild type and the ΔVdPbs2/Pbs2 strain started to accumulate a small amount of melanized microsclerotia at 3 dpi, however, a small number of melanized microsclerotia were observed in the ΔVdPbs2 mutant at 7 dpi (Figure 1B). Furthermore, at 24 dpi, the ΔVdPbs2 and ΔVdHog1 strains still had significant defects in microsclerotia formation, and the melanized area fraction of each strain revealed the deficiency in the melanin accumulation in ΔVdPbs2 and ΔVdHog1 mutants when compared with wild type and the ΔVdPbs2/Pbs2 strain (Figures 1C,D). Strikingly, the melanized microsclerotia were significantly less in the ΔVdHog1 mutant than that of in the ΔVdPbs2 mutant (Figure 1), indicating that VdHog1 may play a more prominent role in the formation of melanized microsclerotia.
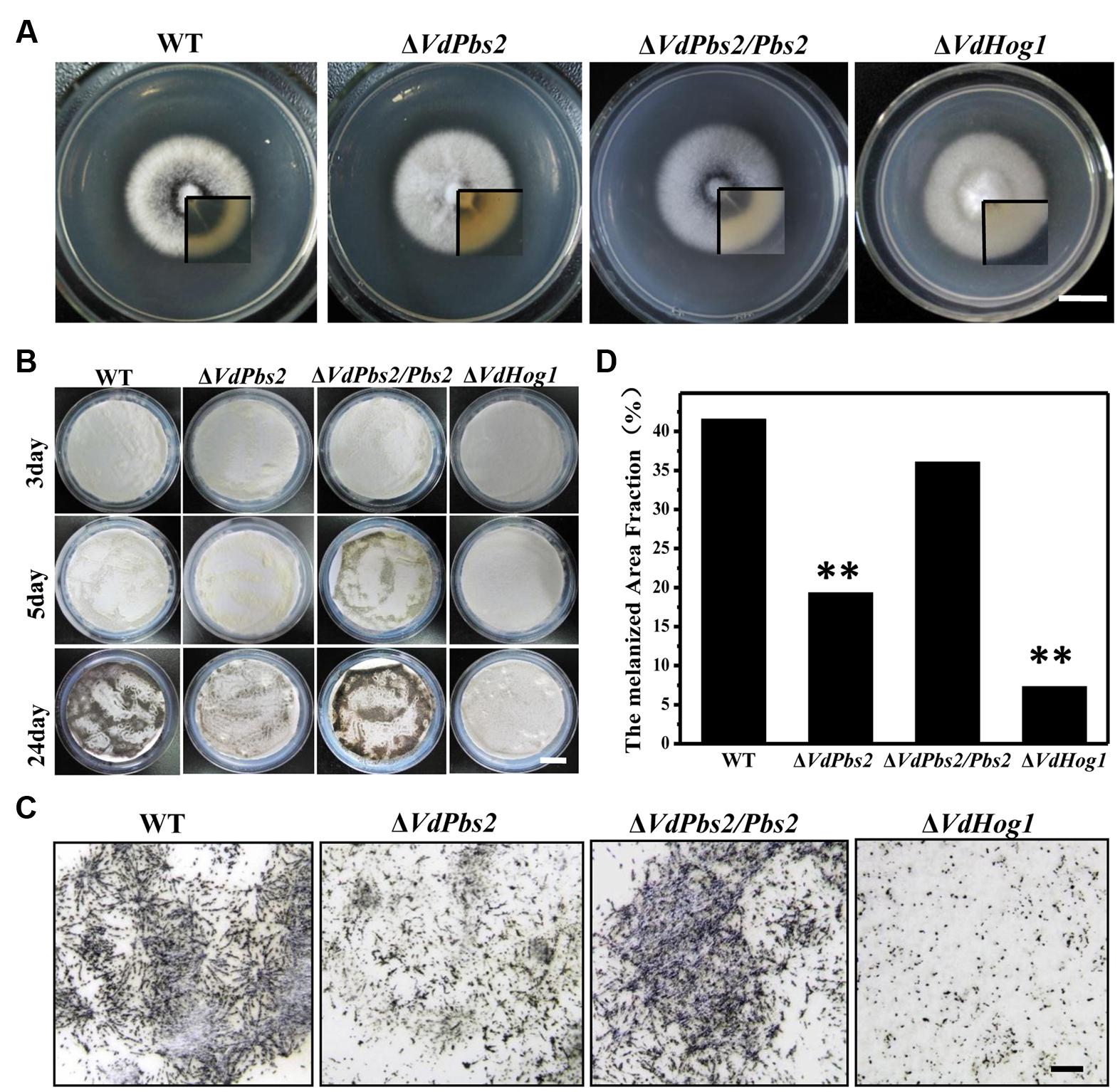
FIGURE 1. Loss of VdPbs2 leads to reduced microsclerotia formation. (A) Colony morphology of the wild type, ΔVdPbs2, ΔVdPbs2/Pbs2 and ΔVdHog1 grown on PDA for 8 days. The inset shows colony from the opposite view. (B) Microsclerotia formation of the individual strain on cellulose membrane placed onto basal medium plates, and incubated at 25°C at 3, 5, and 24 days. Conidia from each strain were sprayed on the cellulose membrane at a concentration of 105 conidia/ml. (C) Microscopic observation of microsclerotia formation of the above four strains at 24 dpi. Scale bar = 1 mm. (D) Melanized area fractions in the colony were counted by ImageJ. Asterisk indicates significant difference at P < 0.01.
Consistent with reduced melanin accumulation in the ΔVdPbs2 mutant, genes associated with melanin synthesis were expressed at significantly lower levels in ΔVdPbs2 mutant (Figure 2A). Notably, of five melanin-related genes, four genes (VDAG_00190, VDAG_03665, VDAG_03393, and VDAG_00183) were more than 50-fold down-regulated in ΔVdPbs2 mutant compared with the wild type and the ΔVdPbs2/Pbs2 complementation strain (Figure 2A). The result was consistent with expression profiles of these genes in ΔVdHog1 mutant (Wang et al., 2016). Furthermore, we tested the expression analysis and subcellular localization of VdPbs2 fused with GFP under the control native promoter of VdPbs2. The results demonstrated that VdPbs2 was significantly upregulated during microsclerotia formation and green fluorescence remained a higher level at the early stage of microsclerotia formation (Figures 2B,C). Taken together, these observations indicate VdPbs2 is required for melanized microsclerotia formation via the Hog1-mediated pathway.
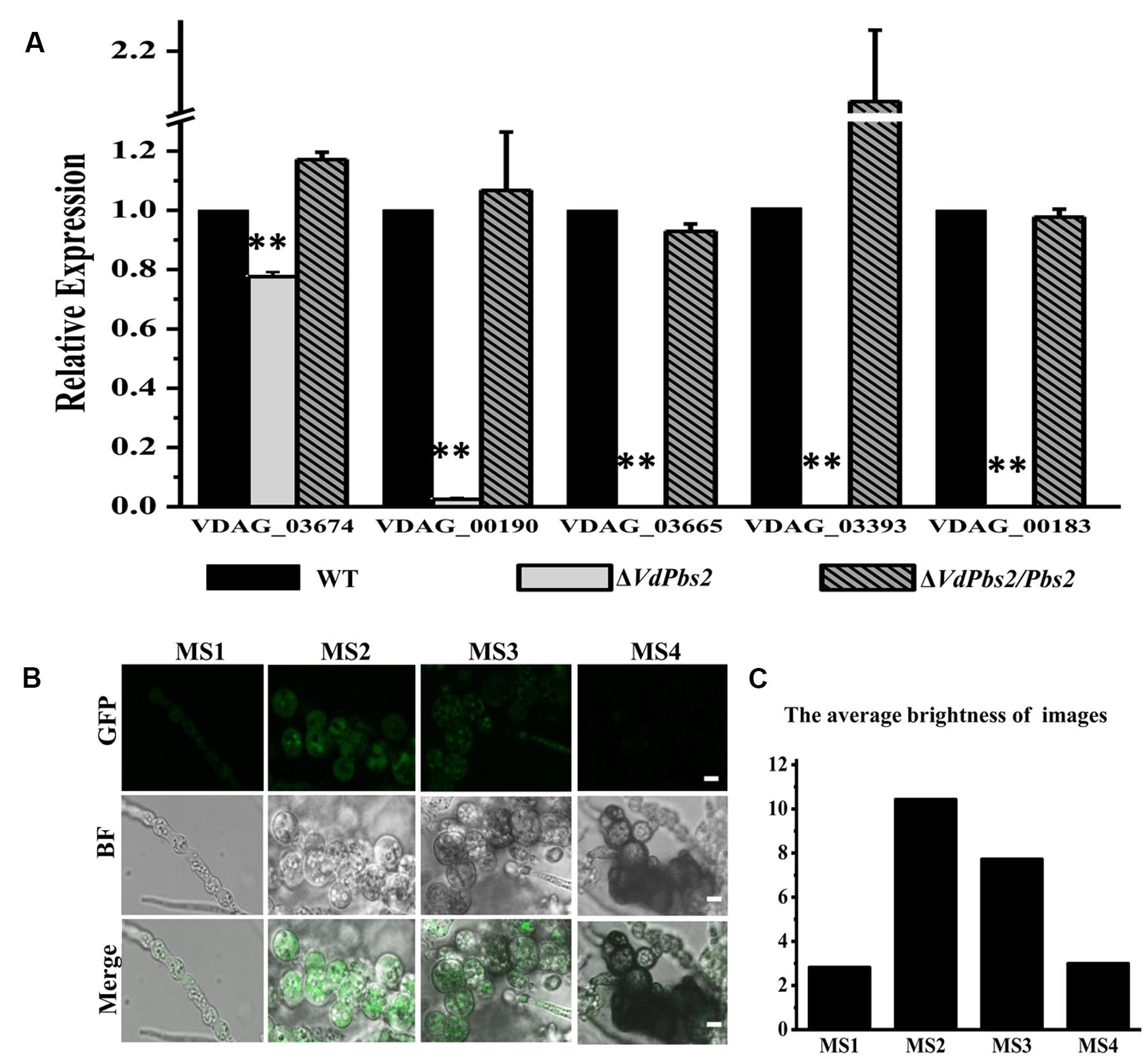
FIGURE 2. The expression of genes involved in melanin biosynthesis in the VdPbs2 mutant. (A) Expression of five melanin related genes (VDAG_03674, VDAG_00190, VDAG_03665, VDAG_03393 and VDAG_00183) during microsclerotia formation. The β-tubulin was used as an internal reference gene. Total RNA was directly extracted from mycelium of the wild type, ΔVdPbs2, and ΔVdPbs2/Pbs2 grown on PDA plates for 8 days. Error bar represents standard deviation. Asterisk indicates significant difference at P < 0.01. (B) Expression patterns of VdPbs2-GFP during microsclerotia development. GFP expression driven by the native promoter of VdPbs was examined using fluorescence microscope. Spores were cultivated in CM liquid for 4 days. MS1-MS4 represents four typical stages during the entire process of microsclerotia formation at 60 (mycelium at the early stage of inflation), 72 (mycelium inflated completely but without melanin accumulation), 96 h (inflated mycelium with the slight accumulation of melanin), and 14 days (inflated mycelium with the massive accumulation of melanin). Scale bar = 10 μm. (C) The quantification of images fluorescence correlated with Figure 3B. The average brightness of image was performed using the Adobe Photoshop software.
Deletion of VdPbs2 Impairs Fungal Growth under Osmotic Stress Conditions
To investigate the function of VdPbs2 in the response to hyperosmotic stress, strains were grown on CM supplemented with 0.8 M NaCl and 1.2 M sorbitol, respectively. When grown on minimal media containing 0.8 M NaCl and 1.2 M sorbitol, respectively, ΔVdPbs2 mutant, compared to the wild type and the ΔVdPbs2/Pbs2 strain was dramatically reduced for growth, which was similar to ΔVdHog1 mutant (Figures 3A,B). Besides, clear hyphal lysis occurred in both ΔVdPbs2 and ΔVdHog1 mutants indicated by hyphae deformities visible on the above media (Figure 3C). As shown in Figures 3D,E, cytoplasmic distribution of VdPbs2 was clearly observed after treated with 0.8 M NaCl. Collectively, the results suggested that VdPbs2-VdHog1 module contributes to the response to osmotic stress in V. dahliae.
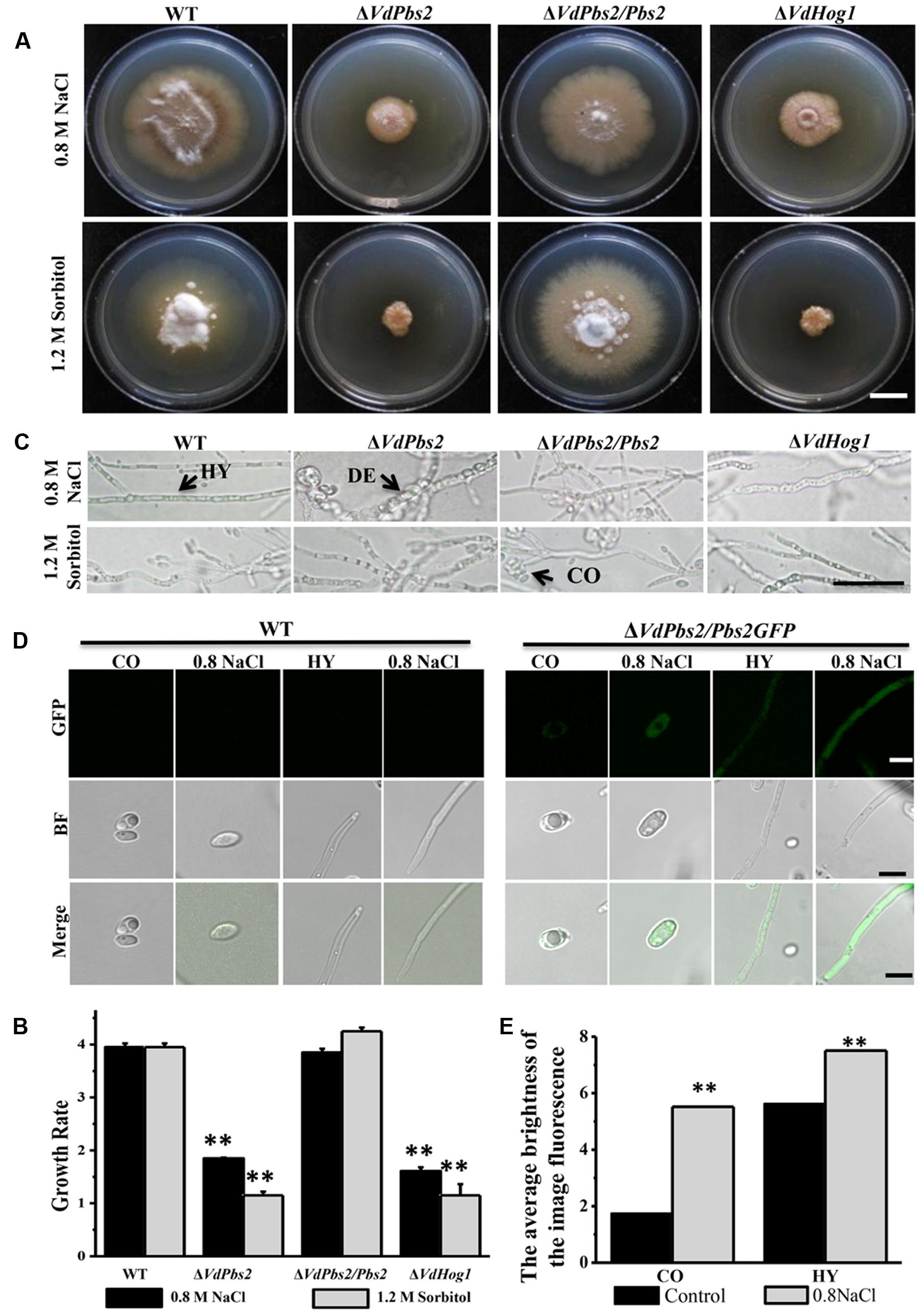
FIGURE 3. Deletion of VdPbs2 impairs fungal growth under osmotic stress with hyphal lysis. (A) Colony morphology of the wild type, ΔVdPbs2, ΔVdPbs2/Pbs2, and ΔVdHog1 grown at 25°C for 20 days on CM containing 0.8 M NaCl and 1.2 M sorbitol, respectively. Scale bar = 1 cm. (B) The growth rate of the individual strain on CM under osmotic agents. All assays were performed in triplicate. Error bars represent standard deviations. Asterisk indicates significant difference at P < 0.01. (C) Hyphal morphology of the four above strains treated by 0.8 M NaCl and 1.2 M sorbitol, respectively. Under hyperosmotic conditions, the mycelium of the mutant was deformed. HY = hyphae, CO = conidia, DE = deformity. Scale bar = 10 μm. (D) Expression pattern of VdPbs2-GFP in response to osmotic stress at conidia and hyphae. The conidia and hyphae of ΔVdPbs2/Pbs2GFP strains were treated with 0.8 M NaCl for 2 h compared with that of the wild type. HY = hyphae, CO = conidia. Scale bar = 5 μm. (E) The quantification of images fluorescence correlated with (D) in the ΔVdPbs2/Pbs2GFP strain.
Loss of VdPbs2 Increases Resistance to Cell Wall Stress
To determine whether deletion of VdPbs2 affects the response to cell wall stress in V. dahliae, we tested cell viability of the ΔVdPbs2 mutant under cell wall stressors such as CFW and CR. Conidia (105 conidia/ml and 106 conidia/ml) of ΔVdPbs2, ΔVdHog1, wild type, and ΔVdPbs2/Pbs2 strain were spotted on CM media containing CFW (20 μg/ml) and CR (50 μg/ml), respectively. Enhanced growth on media with CFW (20 μg/ml) and CR (50 μg/ml), respectively, was observed for ΔVdPbs2 and ΔVdHog1 mutants. By contrast, reduced growth was observed for the wild type and ΔVdPbs2/Pbs2 strain (Figures 4A,B), suggesting VdPbs2 and VdHog1 are involved in the response to cell wall stress.
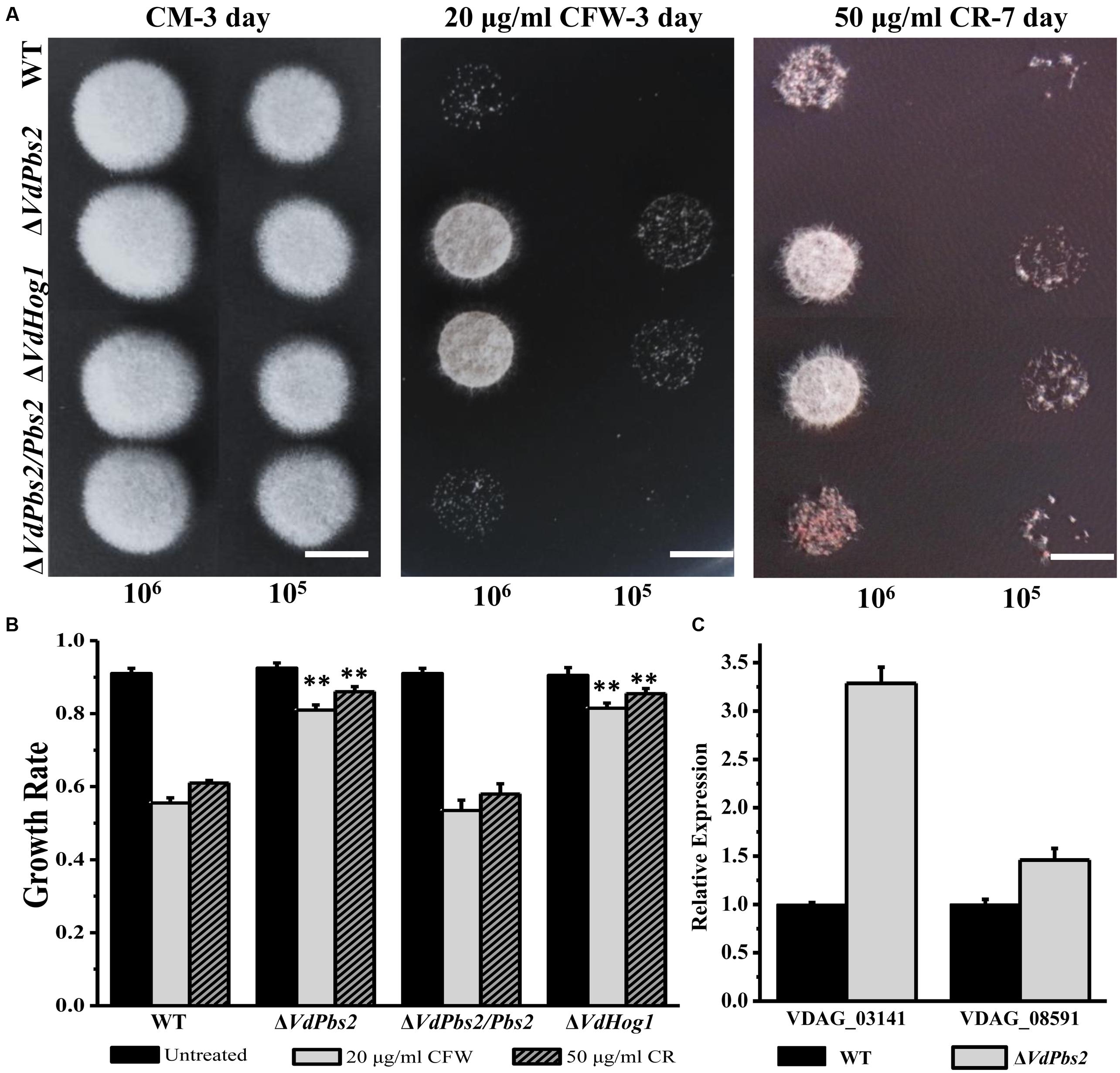
FIGURE 4. Loss of VdPbs2 increases resistance to cell wall stress. (A) Stress responses of wild type, ΔVdPbs2, ΔVdPbs2/Pbs2, and ΔVdHog1 strains on CM containing 20 μg/ml CFW and 50 μg/ml CR, respectively. Images were taken at 3 dpi for CFW and 7 dpi for CR. In all assays, the plates were inoculated with conidial solution of wild type, ΔVdPbs2, ΔVdPbs2/Pbs2, and ΔVdHog1 strains. Conidial suspension (105/ml and 106 /ml) of the individual strain were spotted on CM media containing the indicated concentration CFW and CR, Scale bar = 0.5 cm. (B) Relative growth of wild type, ΔVdPbs2, ΔVdPbs2/Pbs2, and ΔVdHog1 strains treated by the indicated cell stress. Error bar represents standard deviation. Asterisk indicates significant difference at P < 0.01. (C) The expression of two genes (VDAG_08591 and VDAG_03141) involved in chitin synthesis was increased in the ΔVdPbs2 mutant. Error bars indicate standard deviations derived from three independent experiments consisting of three replicas each.
We next sought more evidence for a functional connection between VdPbs2 and cell wall assembly. To determine the expression profiles of genes encoding chitin synthase, we used qPCR to analyze RNA extracted from wild type and ΔVdPbs2 mutant strains grown in liquid shake CM for 5 days. Loss of VdPbs2 function induced the expression of chitin synthase genes (VDAG_08591 and VDAG_03141) compared to wild type (Figure 4C). Thus, genes for chitin synthase are misregulated in ΔVdPbs2 mutant when compared with wild type, accounting for enhanced resistance to cell wall stressors. Summarily, these results demonstrate that VdPbs2 may negatively regulate cell wall synthesis.
VdPbs2 is Essential for the Oxidative Stress Response
To evaluate the responses of the ΔVdPbs2 mutant to oxidative stress, the inhibition zone was measured on the media containing H2O2. As shown in Figures 5A,B, the ΔVdPbs2 mutant exhibited the larger inhibition zones than the wild type and the ΔVdPbs2/Pbs2 strain at a different concentration of H2O2 suggesting that VdPbs2 is required for H2O2 detoxification. In addition, consistent with our previous observations, loss of VdHog1 did not abolish oxidative sensitivity in V. dahliae (Figure 5A). Furthermore, based on sequence homology, we identified genes encoding H2O2 detoxification in V. dahliae. Transcript analysis revealed that three genes (VDAG_08724, VDAG_03661, and VDAG_06340) were consistently down-regulated in the ΔVdPbs2 mutant compared to that of the wild type after treated with 1 mM H2O2 for 30 min (Figure 5C). Thus, VdPbs2 is essential for the oxidative stress response, but not VdHog1.
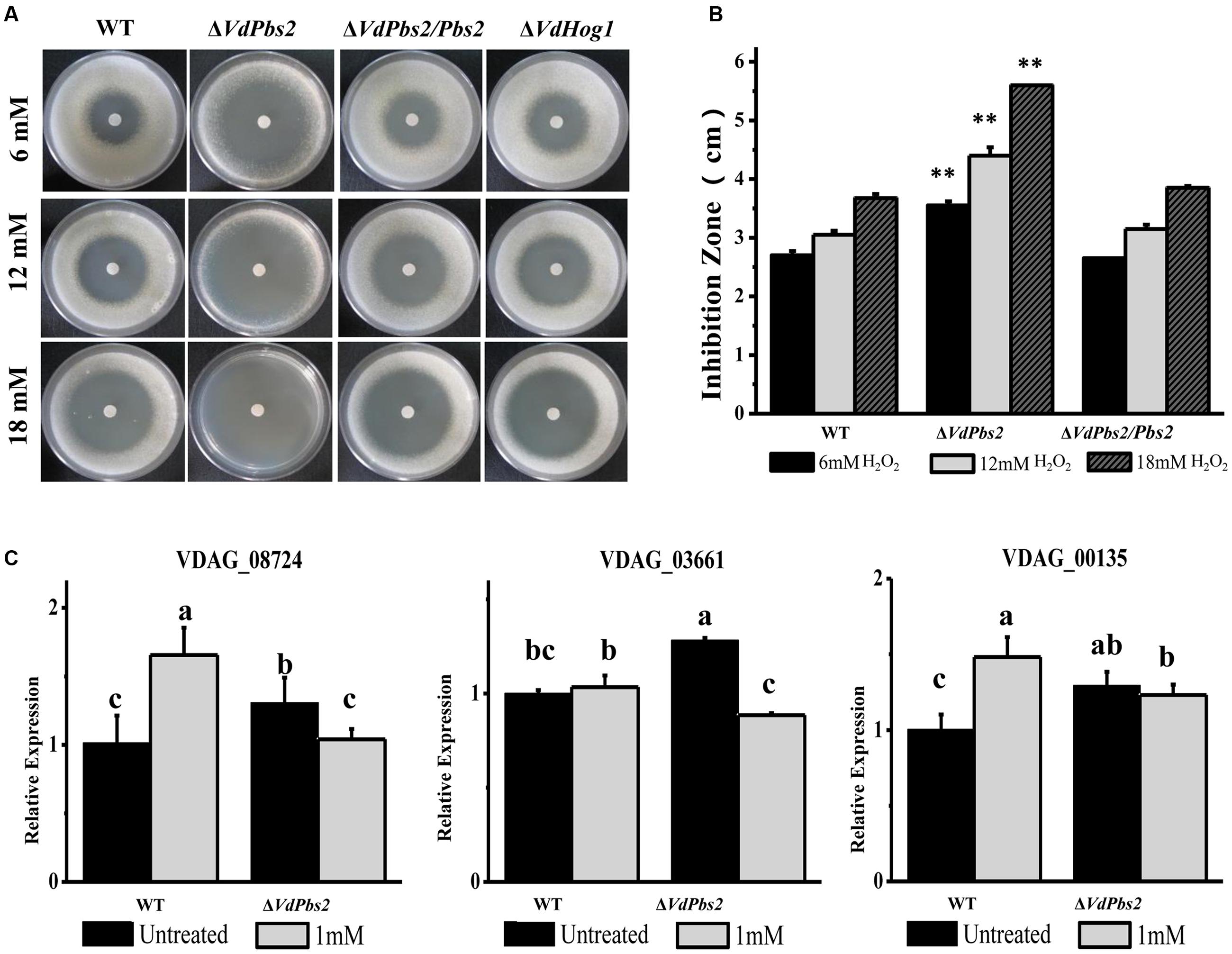
FIGURE 5. VdPbs2 contributes to the oxidative stress response. (A) The ΔVdPbs2 and ΔVdHog1 mutants were compared with the wild type and the ΔVdPbs2/Pbs2 strain. Equal conidial suspension (105 spores/ml) of each strain was sprayed on PDA plates. Sterile filter paper disks with 5 mm diameters were placed in the center of the plates, and 10 μL of 6, 12, and 18 mM H2O2 were added to each paper disk, respectively. The plates were incubated at 28°C for 4 days and the inhibition zones were measured Scale bar = 1 cm. (B) Zones of growth inhibition in (A) were quantified. Error bars represent standard deviation. Asterisk indicates significant difference at P < 0.01. (C) Downregulation of genes related to peroxidase in the ΔVdPbs2 mutant. Relative expression levels of three genes (VDAG_08724, VDAG_03661 and VDAG_06340), which encode peroxidases, were determined by qRT-PCR using the RNA from mycelium treated with 1 mM H2O2 for 30 min. Error bars represent standard deviation.
VdPbs2 Deletion Mutants Exhibit Distinct Responses to Different Fungicides
VdHog1 deletion mutant is highly resistant to the fungicide fludioxonil (Wang et al., 2016). To determine if deletion of VdPbs2 affects the response to fungicides, we tested the sensitivity of the ΔVdPbs2 mutant to various fungicides. Similar to the response of the ΔVdHog1 mutant to fungicides, the ΔVdPbs2 mutant exhibited enhanced resistance to fludioxonil and iprodione and increased sensitivity to chlorothalonil and difenoconazole, respectively, when compared with the wild type and the ΔVdPbs2/Pbs2 strain (Figure 6), suggesting that VdPbs2 is involved in accumulation of osmoprotectant molecules of fungal cell in the response to fungicidal compounds.
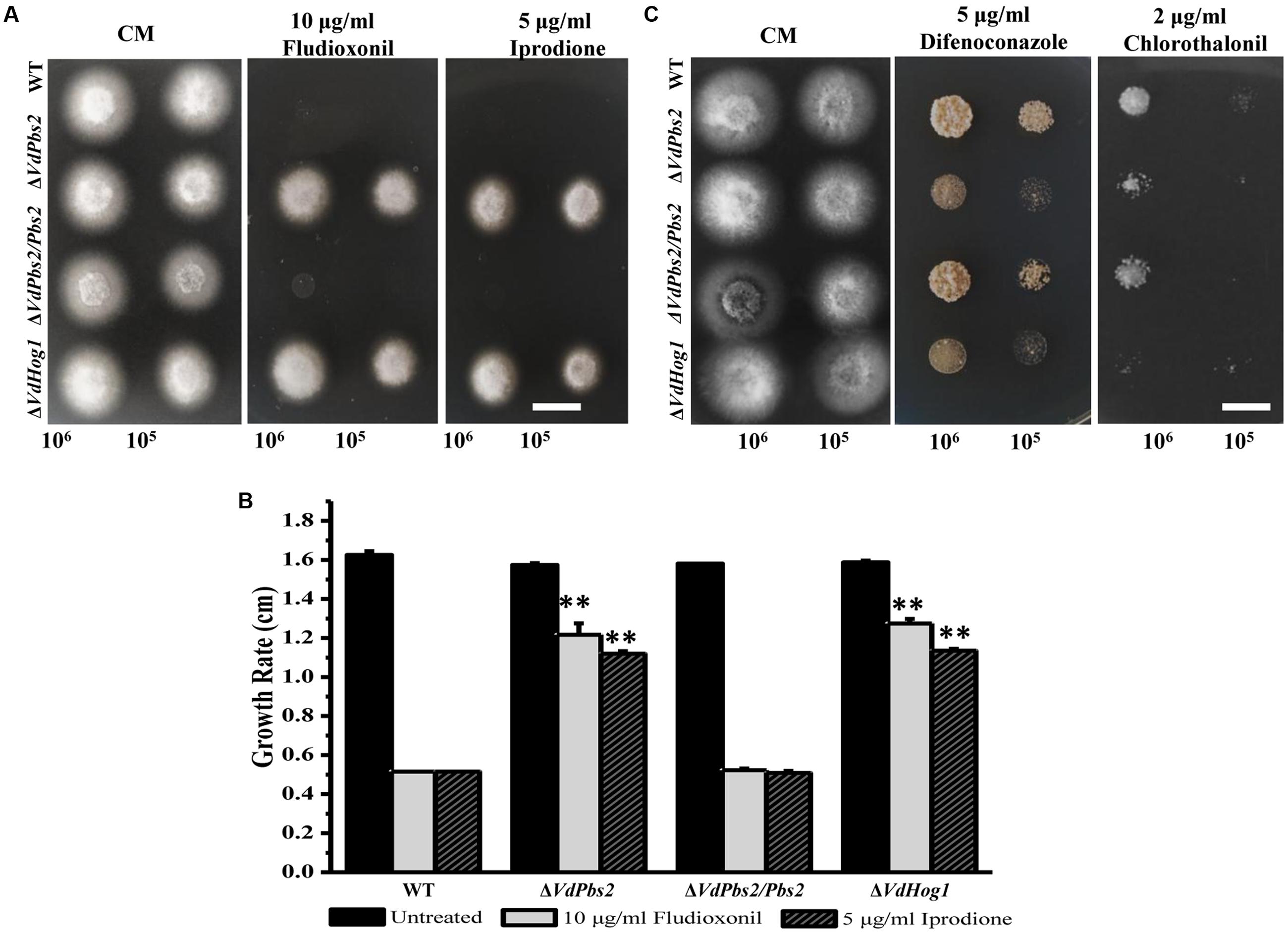
FIGURE 6. VdPbs2 deletion mutants exhibit distinct responses to different fungicides. (A) The ΔVdPbs2 and ΔVdHog1 mutants showed enhanced resistance to fludioxonil and iprodione. Conidial suspension (105/ml and 106 /ml) of the wild type, ΔVdPbs2 and ΔVdHog1, and the the ΔVdPbs2/Pbs2 were spotted on CM media with the indicated concentration of fludioxonil and iprodione, respectively. (B) The ΔVdPbs2 and ΔVdHog1 mutants exhibited more sensitivity to chlorothalonil and difenoconazole. Conidial suspension (105/ml and 106 /ml) of the above strains were spotted on CM media with the indicated concentration of chlorothalonil and difenoconazole, respectively. (C) Growth rate of the above strains on CM containing with the indicated concentration of fludioxonil and iprodione, respectively. Error bar represents standard deviation. Asterisk indicates significant difference at P < 0.01.
VdPbs2 is Required for Plant Infection
We next sought to address whether VdPbs2 plays a role in virulence in plants. We used seedlings of smoke tree and tobacco to carry out the virulence experiments. On both hosts, the ΔVdPbs2 mutant exhibited striking reduced virulence (Figures 7A,B) and only less 20% mortality of plants at 45 dpi (Figure 7D). By contrast, at 45 dpi, up to 80% mortality of which inoculated with the wild type and the ΔVdPbs2/Pbs2 strain showed clear wilt symptoms, including chlorosis (Figures 7A,B) and obviously reduced plant height (Figures 7B,C). Due to the limitations, we just further observed the penetration of the strain on onion epidermis. The wild type could infect epidermal cells and expand into the epidermal tissues, whereas the ΔVdPbs2 mutant hardly infects epidermal cells even though the mutants produced long germ tubes (Supplementary Figure S4). Together, these results indicated that VdPbs2 may be involved in the penetration process during plant infection.
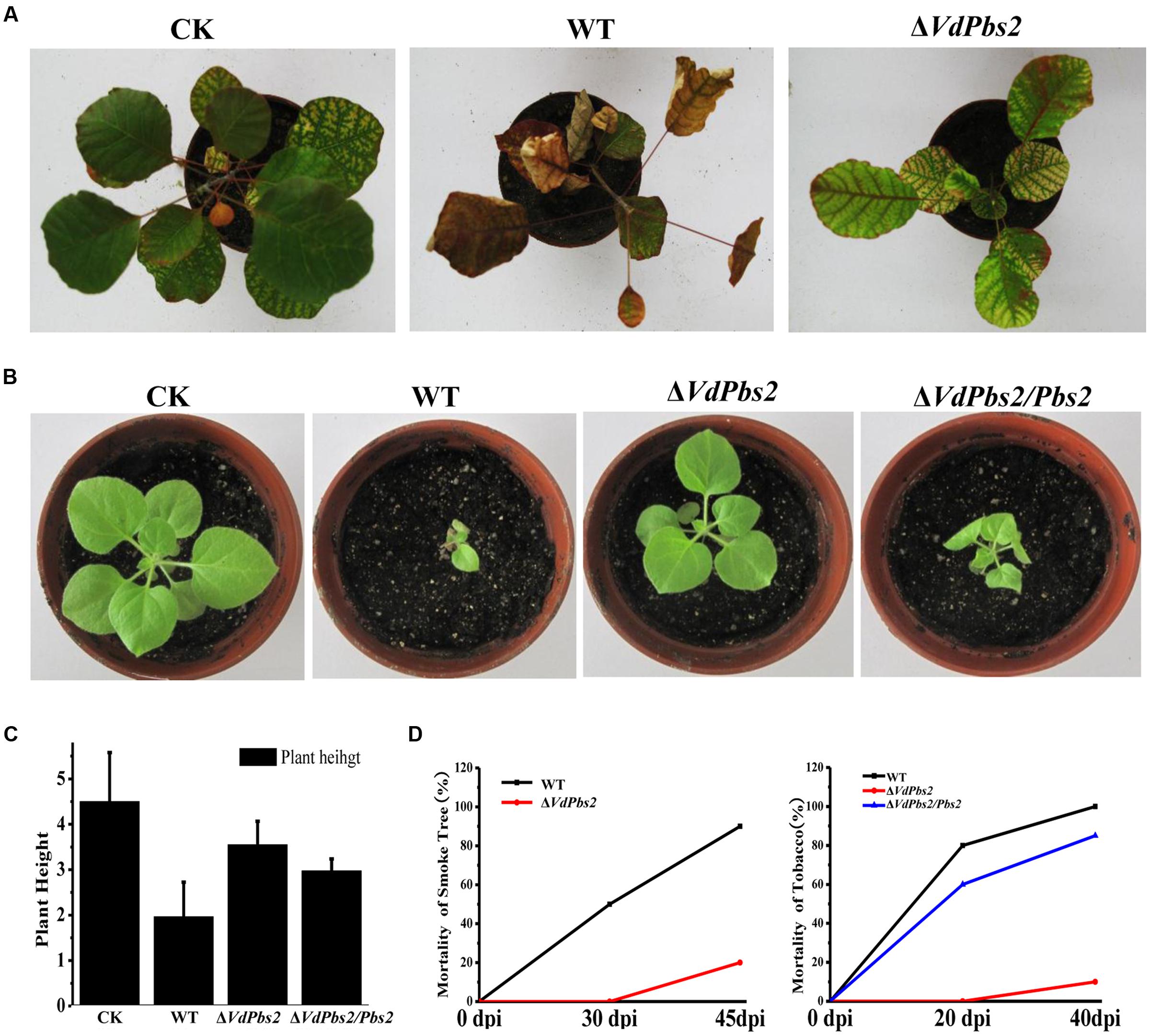
FIGURE 7. Reduced virulence of the VdPbs2 mutant on smoke tree and tobacco seedlings. (A) One-year-old smoke trees were inoculated with conidia concentration of 106/ml of the wild type and ΔVdPbs2 mutant. The pictures were taken at 45 dpi. Twenty seedlings were inoculated with each strain. (B) Two-month-old tobacco seedlings were inoculated with the same methods mentioned in (A). The assays were performed in triplicate. The pictures were taken at 40 dpi. (C) Height of tobacco seedlings inoculated with the above strains. The height of tobacco seedlings measured at 30 dpi. (D) The mortality of smoke tree (at 30, 45 dpi) and tobacco (at 20, 40 dpi) inoculated with the wild type, ΔVdPbs2 and ΔVdPbs2/Pbs2 strains.
Discussion
In this study, we investigated the role of VdPbs2 in the development of microsclerotia and pathogenicity in V. dahliae. Similar to the VdHog1 deletion mutant, VdPbs2 deletion mutants exhibited reduced microsclerotia formation, heightened sensitivity to osmotic stress, enhanced resistance to chemicals that interfered with cell wall synthesis and attenuated virulence on seedlings of smoke trees and tobacco. Strikingly, VdPbs2 plays a crucial role in the response to oxidative stress, whereas VdHog1 is dispensable for the response to oxidative stress. These results suggest that the module of VdPbs2-VdHog1 function in a signaling cascade that regulates stress response, developmental processes and pathogenicity in V. dahliae.
Microsclerotia with melanized particles in the interhyphal spaces confer resistance to adverse conditions (Gordee and Porter, 1961; Griffiths, 1970; Gessler et al., 2014). Genes involved in melanin biosynthesis in V. dahliae play crucial roles in the formation of fully functional microsclerotia (Griffiths, 1970; Wheeler et al., 1976; Xiong et al., 2014). In this study, VdPbs2 mutants exhibited significantly reduced microsclerotia formation (Figures 1A,B). In addition, five genes involved in melanin biosynthesis were also significantly downregulated in the ΔVdPbs2 mutant (Figure 2A). Although both VdHog1 and VdPbs2 were identified to positively regulate microsclerotia formation and melanin biosynthesis, VdHog1 has a stronger influence on melanized microsclerotia (Figures 1A–D). Accordingly, we speculate that VdHog1 possibly plays a more crucial role in the regulation of microsclerotia formation than VdPbs2. These findings emphasize that loss of Pbs2 and Hog1, the vital components of the HOG MAPK signal transduction pathway, delayed melanin synthesis and microsclerotia maturation in V. dahliae.
Mutants of VdPbs2 and VdHog1 also exhibited elevated sensitivity to osmotic stress, which identical with the other studies in yeast (Alonso-Monge et al., 2001), F. proliferatum (Adám et al., 2008), C. albicans (Alonso-Monge et al., 2009). Fungal cell wall mediates all signals between cells and their environment (Latge and Beauvais, 2014). Moreover, HOG signaling functionally participates in the maintenance of cell wall architecture (Garcia-Rodriguez et al., 2000) and C. albicans (Arana et al., 2005; Navarro-Garcia et al., 2005). In S. cerevisiae, there exist, two osmosensing signal transduction pathways, one is the HOG pathway and the other is the PKC-MAPK pathway, which respond to hypertonic and hypotonic shock, respectively (Davenport et al., 1995). Subsequently, some evidence revealed shared targets of the PKC1 pathway with high-osmolarity response routes (Alonso-Monge et al., 2001). The PKC-MAPK signaling pathway were reported to be vital to maintaining integrity of the cell and affected the location of cell wall components, the formation of melanin and responding to the osmotic and cell wall-inhibiting agents in pathogenic fungi (Davenport et al., 1995; Gerik et al., 2008), which indicated it is attractive targets for developing novel strategies to control pathogen. In C. albicans, Mkc1, the component of PKC-MAPK pathway, is phosphorylated under some stimuli and its function is partially dependent on the presence of Hog1 (Navarro-Garcia et al., 2005). Our study showed that both VdPbs2 and VdHog1 mutants exhibit altered susceptibility to CFW and CR, which inhibit fungal cell wall assembly by binding to chitin and β-1, 4-glucans, respectively. Furthermore, two genes related to chitin synthase were indeed significantly upregulated in the VdPbs2 deletion mutant. Therefore, we inferred that VdPbs2 and VdHog1 negatively regulate cell wall synthesis, thereby affecting some proteins involved in the PKC1 cascade, the major signaling pathway responsible for sensing cell integrity, suggesting potential cross-talk between the Hog1 and Mpk1 MAPK pathways. It has been reported in some filamentous fungi that Mkk1 played a critical role in the crosstalk between the PKC and HOG regulatory pathways (Li et al., 2012; Zheng et al., 2012; Yin et al., 2016).
Regarding the oxidative stress response, the roles of Pbs2 and Hog1 are more complicated. In S. cerevisiae, the HOG pathway is required for oxidative stress resistance, and extensive studies have defined the possible pathways by which Hog1 contributes to this phenomenon (Bilsland et al., 2004). In C. albicans, the Pbs2 deletion mutant exhibits a slight but reproducible increase in oxidative stress sensitivity and under such stress it loses viability faster than the Hog1 mutant, suggesting that both Pbs2 and Hog1 have additional (and separate) roles in this stress response (Arana et al., 2005). In V. dahliae, Pbs2 and Hog1 played different roles in the response to H2O2, similar to the situation in C. albicans. The main difference between the species is that, in V. dahliae, VdHog1 seems to be redundant rather than essential. Obviously, VdPbs2 plays a crucial role in the response to oxidative stress.
The ΔVdPbs2 mutant also exhibited elevated resistance to fungicides, such as iprodione and fludioxonil, which was consistent with the ΔVdHog1 mutant in previous studies (Wang et al., 2016). Similar results were obtained in N. crassa (Fujimura et al., 2003; Segmuller et al., 2007) and C. neoformans (Kojima et al., 2006). The mechanism underlying resistance to these fungicides may involve in overstimulation of the HOG pathway (Kojima et al., 2004; Motoyama et al., 2005; Hamel et al., 2012). All mutants, as well as the wild type, were clearly sensitive to chlorothalonil, a 14α-demethylase inhibitor that acts as a broad-spectrum fungicide. However, we observed no difference in sensitivity to 2-benzo imidazole methyl carbamate and thiophanate-methyl.
In V. dahliae, the HOG pathway plays a significant role in fungal virulence (Tian et al., 2014; Wang et al., 2016). Here, the VdPbs2 deletion mutant exhibited reduced virulence on smoke tree and tobacco seedlings. As we known, chitin is an essential structural component that confers rigidity to the fungal cell wall, allowing the cell to withstand chemical and physical challenges (Fesel and Zuccaro, 2015). Moreover, components of the cell wall are directly involved in colonization of host tissues and tissue damage (Lesage and Bussey, 2006; Oliveira-Garcia and Deising, 2013; Latge and Beauvais, 2014). As mentioned above, our results, VdPbs2 mutant showed a restricted ability to penetrate into onion epidermis might be influenced by the regulation of Pbs2 on the cell wall synthesis. Besides, the VdPbs2 deletion mutant exhibited sensitive to H2O2, which related to ROS during host–pathogen interactions (Mehdy, 1994; Torres et al., 2005; Huang et al., 2011). Accordingly, we concluded that the changes of the cell wall synthesis and the sensitive to H2O2 in the VdPbs2 mutants might contribute to its reduced virulence.
In summary, VdPbs2 in V. dahliae is highly similar to homologs in other fungal species and acts as a key regulator during microsclerotia formation. Furthermore, deletion of VdPbs2 has dramatic effects on cell wall synthesis, the response to stress and fungicide and virulence on smoke tree seedlings. Taken together, these results indicate that the Pbs2-Hog1 module is important for stress responses, developmental processes and pathogenicity in V. dahliae. Although components of the HOG MAPK signal transduction pathway in V. dahliae, VdMsb and VdHog1 were recently identified and characterized, the pathway awaits further characterization, especially regarding pathogenesis. Thorough investigations may yield a clear molecular mechanism for microsclerotia formation, which could be exploited in novel approaches to disease control.
Author Contributions
YW, CT, and LT designed the experiments. LT, JY, HZ, and DX performed the experiments and the data analyses. YW and LT prepared the figures and wrote the manuscript.
Funding
The research was supported by National Natural Science Foundation of China (31370013), to YW.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.01532
FIGURE S1 | Comparison of VdPbs2 with its homologs. (A) Phylogenetic tree of VdPbs2 and its homologs. The phylogenetic tree was constructed using MEGA 6.0 with full-length protein sequences. The numbers on the phylogenetic tree correspond to bootstrap values. (B) Amino-acid sequence alignment of Pbs2. Amino-acid sequence alignment of VdPbs2 (VDAG_02783) and its homologs from Saccharomyces cerevisiae (YJL128C), Neurospora crassa (NCU00587), Verticillium alfalfae (VDBG_02315), Aspergillus nidulans (AN0931.2), Botrytis cinerea (XP_001553220), Candida albicans (XP_716629), Ustilago maydis (UMAG_15092), and Magnaporthe oryzae (MGG_00800). Conserved residues are shaded: similar residues in light gray, identical residues in dark gray. Additionally, the main conserved Pkinase domain, Pkinase_Tyr (on the sites of 322–559), is marked in the box with a dashed line.
FIGURE S2 | Disruption of VdPbs2 in V. dahliae. (A) Construction of cassette for VdPbs2 gene disruption. The top and second lines show the two deletion cassettes, and the third line represents the open reading frame of VdPbs2 with the 5′ and 3′ flanking regions of the wild type (XS11). The available restriction sites used for the Southern blot in this assay are marked with black arrows on the two deletion cassettes. The 1989 bp VdPbs2 fragment (black box) was replaced by the resistance gene cassette (white box) after three homologous recombinations in the wild type. The two bottom cassettes were used for complementation and subcellular localization, respectively. P = probe. (B) Confirmation of gene replacement by PCR. A 2030 bp segment and no stripe were amplified in gene replacement mutants (ΔVdPbs2-22; ΔVdPbs2-32,) with external primers LY145/LY146 and internal primer pairs LY137/LY138), respectively, whereas the wild type exhibited 2788 and 327 bp bands. Using genomic DNA from the ΔVdPbs2/Pbs2 strain as a template, bands at 2030 bp and 327 bp were amplified using primer pair LY105/LY166. (C) Validation of gene replacement in the two VdPbs2 deletion mutants by Southern blotting. The 527 bp band demonstrates that the ΔVdPbs2-22 is a single-copy knockout. ΔVdPbs2-32 was a two-copy knockout (data not shown).
FIGURE S3 | The phenotypic assays of ΔVdPbs2/Pbs2 GFP strain. (A) ΔVdPbs2/Pbs2GFP strain restores the reduced microsclerotia formation on BM plates. (B)ΔVdPbs2/Pbs2GFP recovers the fungal growth under osmotic and cell wall inhibitor agents, respectively. (C) The growth rate of ΔVdPbs2/Pbs2GFP strain. These entire assays were performed in triplicate and same as the phenotype analysis of ΔVdPbs2 strains.
FIGURE S4 | Penetration of the VdPbs2 deletion strain into onion epidermis. (A) Penetration assays on onion epidermis revealed restricted penetration by ΔVdPbs2. Inoculations were performed with 104/ml conidia. Images were acquired at 36 hpi. (B) The percentage of penetration. The penetration of the ΔVdPbs2 and the wild type were performed after at 36 hpi.
TABLE S1 | PCR primers used in this study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Adám, A. L., Kohut, G., and Hornok, L. (2008). Fphog1, a HOG-type MAP kinase gene, is involved in multistress response in Fusarium proliferatum. J. Basic Microbiol 48, 151–159. doi: 10.1002/jobm.200700403
Akhtar, N., Blomberg, A., and Adler, L. (1997). Osmoregulation and protein expression in a pbs2delta mutant of Saccharomyces cerevisiae during adaptation to hypersaline stress. FEBS Lett. 403, 173–180. doi: 10.1016/S0014-5793(97)00048-3
Alonso-Monge, R., Carvaihlo, S., Nombela, C., Rial, E., and Pla, J. (2009). The Hog1 MAP kinase controls respiratory metabolism in the fungal pathogen Candida albicans. Microbiology 155, 413–423. doi: 10.1099/mic.0.023309-0
Alonso-Monge, R., Real, E., Wojda, I., Bebelman, J. P., Mager, W. H., and Siderius, M. (2001). Hyperosmotic stress response and regulation of cell wall integrity in Saccharomyces cerevisiae share common functional aspects. Mol. Microbiol. 41, 717–730. doi: 10.1046/j.1365-2958.2001.02549.x
Arana, D. M., Nombela, C., Alonso-Monge, R., and Pla, J. (2005). The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology 151(Pt. 4), 1033–1049. doi: 10.1099/mic.0.27723-0
Bahn, Y. S., Kojima, K., Cox, G. M., and Heitman, J. (2005). Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell 16, 2285–2300. doi: 10.1091/mbc.E04-11-0987
Bilsland, E., Molin, C., Swaminathan, S., Ramne, A., and Sunnerhagen, P. (2004). Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol. Microbiol. 53, 1743–1756. doi: 10.1111/j.13652958.2004.04238.x
Brewster, J. L., de Valoir, T., Dwyer, N. D., Winter, E., and Gustin, M. C. (1993). An osmosensing signal transduction pathway in yeast. Science 259, 1760–1763. doi: 10.1126/science.7681220
Brewster, J. L., and Gustin, M. C. (2014). Hog1: 20 years of discovery and impact. Sci. Signal. 7:re7. doi: 10.1126/scisignal.2005458
Coley-Smith, J. R., and Cooke, R. C. (1971). Survival and germination of fungal sclerotia. Annu. Rev. Phytopathol. 9, 65–92. doi: 10.1146/annurev.py.09.090171.000433
Davenport, K. R., Sohaskey, M., Kamada, Y., Levin, D. E., and Gustin, M. C. (1995). A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270, 30157–30161. doi: 10.1074/jbc.270.50.30157
de Nadal, E., Casadome, L., and Posas, F. (2003). Targeting the MEF2-like transcription factor Smp1 by the stress-activated Hog1 mitogen-activated protein kinase. Mol. Cell. Biol. 23, 229–237. doi: 10.1128/MCB.23.1.229-237.2003
Dixon, K. P., Xu, J. R., Smirnoff, N., and Talbot, N. J. (1999). Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 11, 2045–2058. doi: 10.1105/tpc.11.10.2045
Duressa, D., Anchieta, A., Chen, D., Klimes, A., Garcia-Pedrajas, M. D., Dobinson, K. F., et al. (2013). RNA-seq analyses of gene expression in the microsclerotia of Verticillium dahliae. BMC Genomics 14:607. doi: 10.1186/1471-2164-14-607
Estruch, F., and Carlson, M. (1993). Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 3872–3881. doi: 10.1128/MCB.13.7.3872
Fesel, P. H., and Zuccaro, A. (2015). beta-glucan: crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 90, 53–60. doi: 10.1016/j.fgb.2015.12.004
Fujimura, M., Ochiai, N., Oshima, M., Motoyama, T., Ichiishi, A., Usami, R., et al. (2003). Putative homologs of SSK22 MAPKK kinase and PBS2 MAPK kinase of Saccharomyces cerevisiae encoded by os-4 and os-5 genes for osmotic sensitivity and fungicide resistance in Neurospora crassa. Biosci. Biotechnol. Biochem. 67, 186–191. doi: 10.1271/bbb.67.186
Garcia-Rodriguez, L. J., Duran, A., and Roncero, C. (2000). Calcofluor antifungal action depends on chitin and a functional high-osmolarity glycerol response (HOG) pathway: evidence for a physiological role of the Saccharomyces cerevisiae HOG pathway under noninducing conditions. J. Bacteriol. 182, 2428–2437. doi: 10.1128/JB.182.9.2428-2437.2000
Gerik, K. J., Bhimireddy, S. R., Ryerse, J. S., Specht, C. A., and Lodge, J. K. (2008). PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot. Cell 7, 1685–1698. doi: 10.1128/ec.00146-08
Gessler, N. N., Egorova, A. S., and Belozerskaia, T. A. (2014). Melanin pigments of fungi under extreme environmental conditions (review). Prikl. Biokhim. Mikrobiol. 50, 125–134.
Gordee, R. S., and Porter, C. L. (1961). Structure, germination, and physiology of microsclerotia of Verticillium albo-atrum. Mycologia 53, 171–182. doi: 10.2307/3756235
Gorner, W., Durchschlag, E., Martinez-Pastor, M. T., Estruch, F., Ammerer, G., Hamilton, B., et al. (1998). Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12, 586–597. doi: 10.1101/gad.12.4.586
Griffiths, D. A. (1970). The fine structure of developing microsclerotia of Verticillium dahliae Kleb. Arch. Mikrobiol. 74, 207–212. doi: 10.1007/bf00408881
Gustin, M. C., Albertyn, J., Alexander, M., and Davenport, K. (1998). MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62, 1264–1300.
Hamel, L. P., Nicole, M. C., Duplessis, S., and Ellis, B. E. (2012). Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell 24, 1327–1351. doi: 10.1105/tpc.112.096156
Hu, D., Wang, C., Tao, F., Cui, Q., Xu, X., Shang, W., et al. (2014). Whole genome wide expression profiles on germination of Verticillium dahliae microsclerotia. PLoS ONE 9:e100046. doi: 10.1371/journal.pone.0100046
Huang, K., Czymmek, K. J., Caplan, J. L., Sweigard, J. A., and Donofrio, N. M. (2011). HYR1-mediated detoxification of reactive oxygen species is required for full virulence in the rice blast fungus. PLoS Pathog. 7:e1001335. doi: 10.1371/journal.ppat.1001335
Klimes, A., Dobinson, K. F., Thomma, B. P., and Klosterman, S. J. (2015). Genomics spurs rapid advances in our understanding of the biology of vascular wilt pathogens in the genus Verticillium. Annu. Rev. Phytopathol. 53, 181–198. doi: 10.1146/annurev-phyto-080614-120224
Klosterman, S. J., Atallah, Z. K., Vallad, G. E., and Subbarao, K. V. (2009). Diversity, pathogenicity, and management of verticillium species. Annu. Rev. Phytopathol. 47, 39–62. doi: 10.1146/annurev-phyto-080508-081748
Klosterman, S. J., Subbarao, K. V., Kang, S., Veronese, P., Gold, S. E., Thomma, B. P., et al. (2011). Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog. 7:e1002137. doi: 10.1371/journal.ppat.1002137
Kojima, K., Bahn, Y. S., and Heitman, J. (2006). Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology 152(Pt. 3), 591–604. doi: 10.1099/mic.0.28571-0
Kojima, K., Takano, Y., Yoshimi, A., Tanaka, C., Kikuchi, T., and Okuno, T. (2004). Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 53, 1785–1796. doi: 10.1111/j.1365-2958.2004.04244.x
Lai, M. H., Silverman, S. J., Gaughran, J. P., and Kirsch, D. R. (1997). Multiple copies of PBS2, MHP1 or LRE1 produce glucanase resistance and other cell wall effects in Saccharomyces cerevisiae. Yeast 13, 199–213. doi: 10.1002/(SICI)1097-0061(19970315)13:3<199::AID-YEA76>3.0.CO;2-Z
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. doi: 10.1093/bioinformatics/btm404
Latge, J. P., and Beauvais, A. (2014). Functional duality of the cell wall. Curr. Opin. Microbiol. 20, 111–117. doi: 10.1016/j.mib.2014.05.009
Lesage, G., and Bussey, H. (2006). Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70, 317–343. doi: 10.1128/mmbr.00038-05
Levin, D. E. (2005). Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69, 262–291. doi: 10.1128/MMBR.69.2.262-291.2005
Li, A., Wang, Y., Tao, K., Dong, S., Huang, Q., Dai, T., et al. (2010). PsSAK1, a stress-activated MAP kinase of Phytophthora sojae, is required for zoospore viability and infection of soybean. Mol. Plant Microbe Interact. 23, 1022–1031. doi: 10.1094/mpmi-23-8-1022
Li, G., Zhou, X., and Xu, J. R. (2012). Genetic control of infection-related development in Magnaporthe oryzae. Curr. Opin. Microbiol. 15, 678–684. doi: 10.1016/j.mib.2012.09.004
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Mehdy, M. C. (1994). Active oxygen species in plant defense against pathogens. Plant Physiol. 105, 467–472.
Mehrabi, R., Zwiers, L. H., de Waard, M. A., and Kema, G. H. (2006). MgHog1 regulates dimorphism and pathogenicity in the fungal wheat pathogen Mycosphaerella graminicola. Mol. Plant Microbe Interact. 19, 1262–1269. doi: 10.1094/mpmi-19-1262
Moretti, M., Rossi, M., Ciuffo, M., and Turina, M. (2014). Functional characterization of the three mitogen-activated protein kinase kinases (MAP2Ks) present in the Cryphonectria parasitica genome reveals the necessity of Cpkk1 and Cpkk2, but not Cpkk3, for pathogenesis on chestnut (Castanea spp.). Mol. Plant Pathol. 15, 500–512. doi: 10.1111/mpp.12111
Moriwaki, A., Kubo, E., Arase, S., and Kihara, J. (2006). Disruption of SRM1, a mitogen-activated protein kinase gene, affects sensitivity to osmotic and ultraviolet stressors in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol. Lett. 257, 253–261. doi: 10.1111/j.1574-6968.2006.00178.x
Motoyama, T., Kadokura, K., Ohira, T., Ichiishi, A., Fujimura, M., Yamaguchi, I., et al. (2005). A two-component histidine kinase of the rice blast fungus is involved in osmotic stress response and fungicide action. Fungal Genet. Biol. 42, 200–212. doi: 10.1016/j.fgb.2004.11.002
Navarro-Garcia, F., Eisman, B., Fiuza, S. M., Nombela, C., and Pla, J. (2005). The MAP kinase Mkc1p is activated under different stress conditions in Candida albicans. Microbiology 151(Pt. 8), 2737–2749. doi: 10.1099/mic.0.28038-0
Oliveira-Garcia, E., and Deising, H. B. (2013). Infection structure-specific expression of beta-1,3-glucan synthase is essential for pathogenicity of Colletotrichum graminicola and evasion of beta-glucan-triggered immunity in maize. Plant Cell 25, 2356–2378. doi: 10.1105/tpc.112.103499
O’Rourke, S. M., and Herskowitz, I. (2004). Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol. Biol. Cell 15, 532–542. doi: 10.1091/mbc.E03-07-0521
Papadopulos, F., Spinelli, M., Valente, S., Foroni, L., Orrico, C., Alviano, F., et al. (2007). Common tasks in microscopic and ultrastructural image analysis using ImageJ. Ultrastruct. Pathol. 31, 401–407. doi: 10.1080/01913120701719189
Posas, F., and Saito, H. (1997). Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science 276, 1702–1705. doi: 10.1126/science.276.5319.1702
Proft, M., and Serrano, R. (1999). Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in Saccharomyces cerevisiae: bZIP protein Sko1p confers HOG-dependent osmotic regulation. Mol. Cell. Biol. 19, 537–546. doi: 10.1128/MCB.19.1.537
Rauyaree, P., Ospina-Giraldo, M. D., Kang, S., Bhat, R. G., Subbarao, K. V., Grant, S. J., et al. (2005). Mutations in VMK1, a mitogen-activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Curr. Genet. 48, 109–116. doi: 10.1007/s00294-005-0586-0
Rep, M., Krantz, M., Thevelein, J. M., and Hohmann, S. (2000). The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 275, 8290–8300. doi: 10.1074/jbc.275.12.8290
Roman, E., Arana, D. M., Nombela, C., Alonso-Monge, R., and Pla, J. (2007). MAP kinase pathways as regulators of fungal virulence. Trends Microbiol. 15, 181–190. doi: 10.1016/j.tim.2007.02.001
Schüller, C., Brewster, J. L., Alexander, M. R., Gustin, M. C., and Ruis, H. (1994). The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 13, 4382–4389.
Segmuller, N., Ellendorf, U., Tudzynski, B., and Tudzynski, P. (2007). BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot. Cell 6, 211–221. doi: 10.1128/EC.00153-06
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Tian, L., Xu, J., Zhou, L., and Guo, W. (2014). VdMsb regulates virulence and microsclerotia production in the fungal plant pathogen Verticillium dahliae. Gene 550, 238–244. doi: 10.1016/j.gene.2014.08.035
Torres, M. A., Jones, J. D. G., and Dangl, J. L. (2005). Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 37, 1130–1134.
Tzima, A., Paplomatas, E. J., Rauyaree, P., and Kang, S. (2010). Roles of the catalytic subunit of cAMP-dependent protein kinase A in virulence and development of the soilborne plant pathogen Verticillium dahliae. Fungal Genet. Biol. 47, 406–415. doi: 10.1016/j.fgb.2010.01.007
Tzima, A. K., Paplomatas, E. J., Tsitsigiannis, D. I., and Kang, S. (2012). The G protein beta subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet. Biol. 49, 271–283. doi: 10.1016/j.fgb.2012.02.005
Wang, Y., Tian, L., Xiong, D., Klosterman, S. J., Xiao, S., and Tian, C. (2016). The mitogen-activated protein kinase gene, VdHog1, regulates osmotic stress response, microsclerotia formation and virulence in Verticillium dahliae. Fungal Genet. Biol. 88, 13–23. doi: 10.1016/j.fgb.2016.01.011
Wang, Y., Xiao, S., Xiong, D., and Tian, C. (2013). Genetic transformation, infection process and qPCR quantification of Verticillium dahliae on smoke-tree Cotinus coggygria. Australas. Plant Pathol. 42, 33–41. doi: 10.1007/s13313-012-0172-0
Wheeler, M. H., Tolmsoff, W. J., and Meola, S. (1976). Ultrastructure of melanin formation in Verticillium dahliae with (+)-scytalone as a biosynthetic intermediate. Can. J. Microbiol. 22, 702–711. doi: 10.1139/m76-103
Widmann, C., Gibson, S., Jarpe, M. B., and Johnson, G. L. (1999). Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180.
Xiong, D., Wang, Y., Ma, J., Klosterman, S. J., Xiao, S., and Tian, C. (2014). Deep mRNA sequencing reveals stage-specific transcriptome alterations during microsclerotia development in the smoke tree vascular wilt pathogen, Verticillium dahliae. BMC Genomics 15:324. doi: 10.1186/1471-2164-15-324
Xiong, D., Wang, Y., Tang, C., Fang, Y., Zou, J., and Tian, C. (2015). VdCrz1 is involved in microsclerotia formation and required for full virulence in Verticillium dahliae. Fungal Genet. Biol. 82, 201–212. doi: 10.1016/j.fgb.2015.07.011
Xiong, D., Wang, Y., Tian, L., and Tian, C. (2016). MADS-box transcription factor VdMcm1 regulates conidiation, microsclerotia formation, pathogenicity and secondary metabolism of Verticillium dahliae. Front. Microbiol. 7:1192. doi: 10.3389/fmicb.2016.01192
Xu, J. R. (2000). Map kinases in fungal pathogens. Fungal Genet. Biol. 31, 137–152. doi: 10.1006/fgbi.2000.1237
Yin, Z., Tang, W., Wang, J., Liu, X., Yang, L., Gao, C., et al. (2016). Phosphodiesterase MoPdeH targets MoMck1 of the conserved mitogen-activated protein (MAP) kinase signalling pathway to regulate cell wall integrity in rice blast fungus Magnaporthe oryzae. Mol. Plant Pathol. 17, 654–668. doi: 10.1111/mpp.12317
Zhang, Y., Lamm, R., Pillonel, C., Lam, S., and Xu, J. R. (2002). Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 68, 532–538. doi: 10.1128/AEM.68.2.532-538.2002
Zhao, X., Mehrabi, R., and Xu, J. R. (2007). Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell 6, 1701–1714. doi: 10.1128/EC.00216-07
Keywords: Verticillium dahliae, MAP kinase pathway, microsclerotia formation, stress responses, pathogenicity
Citation: Tian L, Wang Y, Yu J, Xiong D, Zhao H and Tian C (2016) The Mitogen-Activated Protein Kinase Kinase VdPbs2 of Verticillium dahliae Regulates Microsclerotia Formation, Stress Response, and Plant Infection. Front. Microbiol. 7:1532. doi: 10.3389/fmicb.2016.01532
Received: 30 June 2016; Accepted: 13 September 2016;
Published: 27 September 2016.
Edited by:
Vijai Kumar Gupta, National University of Ireland, Galway, IrelandReviewed by:
Akanksha Singh, Central Institute of Medicinal and Aromatic Plants (CSIR), IndiaDavid Turra, University of Cordoba, Spain
Copyright © 2016 Tian, Wang, Yu, Xiong, Zhao and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonglin Wang, ylwang@bjfu.edu.cn
 Longyan Tian
Longyan Tian Yonglin Wang
Yonglin Wang Jun Yu
Jun Yu Dianguang Xiong
Dianguang Xiong