- Institute for Chemistry and Biology of the Marine Environment, Carl-von-Ossietzky University of Oldenburg, Oldenburg, Germany
Dinoroseobacter shibae DFL 12T is a metabolically versatile member of the world-wide abundant Roseobacter clade. As an epibiont of dinoflagellates D. shibae is subjected to rigorous changes in oxygen availability. It has been shown that it loses up to 90% of its intracellular ATP when exposed to anoxic conditions. Yet, D. shibae regenerates its ATP level quickly when oxygen becomes available again. In the present study we focused on the bioenergetic aspects of the quick recovery and hypothesized that the proton-motive force decreases during anoxia and gets restored upon re-aeration. Therefore, we analyzed ΔpH and the membrane potential (ΔΨ) during the oxic-anoxic transitions. To visualize changes of ΔΨ we used fluorescence microscopy and the carbocyanine dyes DiOC2 (3; 3,3′-Diethyloxacarbocyanine Iodide) and JC-10. In control experiments the ΔΨ-decreasing effects of the chemiosmotic inhibitors CCCP (carbonyl cyanide m-chlorophenyl hydrazone), TCS (3,3′,4′,5-tetrachlorosalicylanilide) and gramicidin were tested on D. shibae and Gram-negative and -positive control bacteria (Escherichia coli and Micrococcus luteus). We found that ΔpH is not affected by short-term anoxia and does not contribute to the quick ATP regeneration in D. shibae. By contrast, ΔΨ was increased during anoxia, which was astonishing since none of the control organisms behaved that way. Our study shows physiological and bioenergetical aspects comparing to previous studies on transcriptomic responses to the transition from aerobic to nitrate respiration in D. shibae. For the lifestyle as an epibiont of a dinoflagellate, the ability to stand phases of temporary oxygen depletion is beneficial. With a boosted ΔΨ, the cells are able to give their ATP regeneration a flying start, once oxygen is available again.
Introduction
Dinoroseobacter shibae is a member of the metabolically versatile Roseobacter clade and can be found in marine and fresh water habitats world-wide (Kolber et al., 2001; Béjà et al., 2002). It belongs to the aerobic anoxygenically phototrophic bacteria. Accordingly, it was documented that light supports proton translocation and therefore contributes to the ATP regeneration in D. shibae (Holert et al., 2011). However, the cells are able to switch from an aerobic to anaerobic lifestyle (Piekarski et al., 2009). As epibionts of dinoflagellates (Biebl et al., 2005) that can shuttle between oxic and anoxic conditions (Wagner-Döbler et al., 2010), this versatility may be helpful.
The oxic-anoxic shift causes dramatic changes of the cell physiology. Laass et al. (2014) reported that D. shibae undergoes a metabolic crisis during the transition from aerobic to anaerobic growth with nitrate. Transcriptomic analysis showed that protein biosynthesis in D. shibae is reduced during this phase. The energy balance is disturbed and accumulation of TCA-cycle intermediates can be observed until the cells adapt to nitrate as electron acceptor (Laass et al., 2014). Comparably, it was found that the cells lose up to 90% of their ATP when incubated anoxically for 2 h. However, they show remarkably fast ATP regeneration upon re-aeration and exposure to light. Within only 40 s the cells recovered by producing up to 12 mM of intracellular ATP (Holert et al., 2011, see also our experiments in Supplement 1).
The mechanisms behind the fast recovery of the energy charge after anoxia are not clear yet. In the present study we addressed the bioenergetics of short-term anoxia and the quick ATP recovery in D. shibae. We hypothesized that the proton-motive force is involved, assuming that it would be low in de-energized cells and increase during recovery. Therefore we analyzed ΔpH and ΔΨ during oxic-anoxic transitions. Membrane-potential disrupting ionophores and some other bacteria were used for control experiments. Analysis of the intracellular pH was performed via butanol permeabilization after Scholes and Mitchell (1970). Two carbocyanine dyes were used to visualize the membrane potential by fluorescence microscopy. As hypothesized we found changes of the proton-motive force, but quite different from our expectations.
Materials and Methods
Bacterial Strains and Cultivation
Dinoroseobacter shibae DFL 12T (Biebl et al., 2005) was grown in artificial seawater medium (SWM) with 10 mM succinate as sole electron and carbon source (Supplement 2 for all media). Cells were cultivated in a diurnal light/dark rhythm (12 h/12 h, 12 μmol photons m-2 s-1) in a shaker at 25°C and 125 rpm (Soora and Cypionka, 2013). Control organisms Escherichia coli (strain K12) and Micrococcus luteus (strain E10-2, Batzke et al., 2007) were grown on LB medium at 33°C.
Determination of the Intracellular pH (pHi)
The determination of the intracellular pH was performed after Scholes and Mitchell (1970) via permeabilization of the membrane with 5% (vol/vol) butanol. Cells were harvested by centrifugation (150 ml culture, 10.000 × g, 10 min, 4°C, Beckman J2-HS), the supernatant was discarded and the pellet was resuspended in 5 ml of non-buffered solution (0.3 M NaCl, pH 7). The measurement was performed in a 5 ml glass tube with an OD436 of 20. For the determination of the pHi under anoxic conditions the glass tube was closed with a rubber stopper and flushed with N2 for 2 h. A pH electrode (type Inlab Micro, Mettler Toledo) was immersed and the suspension was stirred. The data were logged (AD converter ADC-16, pico Technology) with the software MPwin (version 2008.08.25, Cypionka, 2005). For permeabilization 250 μl of butanol were added into the stirred suspension. From the pH changes after butanol addition at different outer pH values, the pHi could be determined.
Application of Short-term Anoxia and Subsequent Re-aeration
Exponentially growing cells (5 ml) were taken directly for ΔΨ analysis. Additionally, two more aliquots (each 5 ml) were transferred into glass tubes closed by rubber stoppers. The tubes were permanently protected from light by aluminum foil during further treatment. Both tubes were flushed with N2 to quickly establish anoxic conditions. After 2 h incubation at 25°C, one of the tubes was flushed for 2 min with air under light exposure (420 μmol photons m-2 s-1) to allow recovery of the cells. The other tube was kept anoxic and dark. Afterward cells from both tubes were analyzed for their ΔΨ and compared to the untreated cells from before.
Analysis of the Membrane Potential
The membrane potential was assessed via fluorescence microscopy with two ΔΨ-sensitive dyes, 3,3′-Diethyloxacarbocyanine Iodide [DiOC2(3), TCI, Germany] and JC-10 (AAT Bioquest, USA) using the same procedure. These lipophilic dyes form self-aggregates upon membrane uptake, which results in a color shift from green to red fluorescence. The appropriate concentrations of the dyes were figured out in a separate set of experiments, and were found to be 150 μM for D. shibae and E. coli, and 50 μM for M. luteus.
The staining procedure was performed with cells from three different energetic states; untreated cells from the exponential growth phase, cells that had been kept anoxic and dark for 2 h, and cells that had been re-aerated and exposed to light after anoxic and dark incubation.
During the following staining process, all cells were also protected from light to prevent photobleaching of the fluorescent dyes and unwanted, light-induced recovery of the cells. Therefore, 500 μl of the respective cells (OD436 2.0) were transferred to 2 ml reaction tubes each, mixed with 500 μl PBS buffer and stained with DiOC2(3) for 15 min. For the untreated cells complete depletion of oxygen by respiration during staining could be excluded, even for maximum respiration rates (Holert et al., 2011). For the anoxically incubated cells anoxic conditions were maintained during staining by using N2-flushed PBS buffer and dye.
As a result of their double-layered cell membrane Gram-negative organisms like D. shibae tend to take up cyanine dyes worse than Gram-positives (Novo et al., 1999). To overcome this 1 mM EDTA was added prior to staining, which significantly improved the staining results for the Gram-negative strains D. shibae and E. coli (Dumas et al., 2010) by weakening the outer bacterial membrane (Alakomi et al., 2006). The Gram-positive strain M. luteus was used for control experiments.
After staining, 10 μl of the cell suspension was transferred onto object microscope slides and analyzed under a fluorescence microscope (Leica DMRB, Leitz) at excitation wavelength of 450–490 nm, dichroic filter 510 nm, longpass filter 515 nm (Leica-Nr.: 513808). At this excitation the normally green fluorescence (488–530 nm) shifts to red (>600 nm) when the dye is taken up by the cells due to their membrane potential (Novo et al., 1999). Pictures were taken with an EOS 600 D camera using the EOS utility software (ver. 2.10.2.0). Final image processing was performed with PICOLAY (Cypionka et al., 2016). The quantification of the % of red stained cells was done with CountThem (Cypionka, 2011).
ΔΨ-Dissipating Agents
Carbonyl cyanide m-chlorophenyl hydrazone (CCCP), 3,3′,4′,5-tetrachlorosalicylanilide (TCS) and gramicidin were used for the disruption of ΔΨ. Samples were treated as described before and subsequently analyzed with fluorescent microscopy. The ionophores were added prior to staining and the samples were incubated 5 min at room temperature in the dark. Applied concentrations were 2 μM CCCP, 2 μM TCS, and 5 μg/ml gramicidin. In control experiments, the solvent DMSO did not have any influence without the inhibitors.
Results
The Intracellular pH of Dinoroseobacter shibae Is Independent of the Energetic State
In order to determine the intracellular pH (pHi) of D. shibae we used butanol (5% vol/vol) for membrane permeabilization. Butanol treatment resulted in alkalinization of the suspension when the initial pH was below 7.2 and in acidification of the suspension at pH values above 7.4. For the initial pH of 7.3 only minimal changes were observed after butanol treatment, varying slightly between repeated experiments (Figure 1). Thus, the pHi was determined to lie between 7.2 and 7.4. This result was identical for all D. shibae cells, including untreated-, anoxically incubated- and subsequently re-aerated cells. This indicates that the pHi in D. shibae is independent of the energetic state. For E. coli a pHi between 7.5 and 7.6 was determined with the same technique, confirming literature values (Wilks and Slonczewski, 2007).
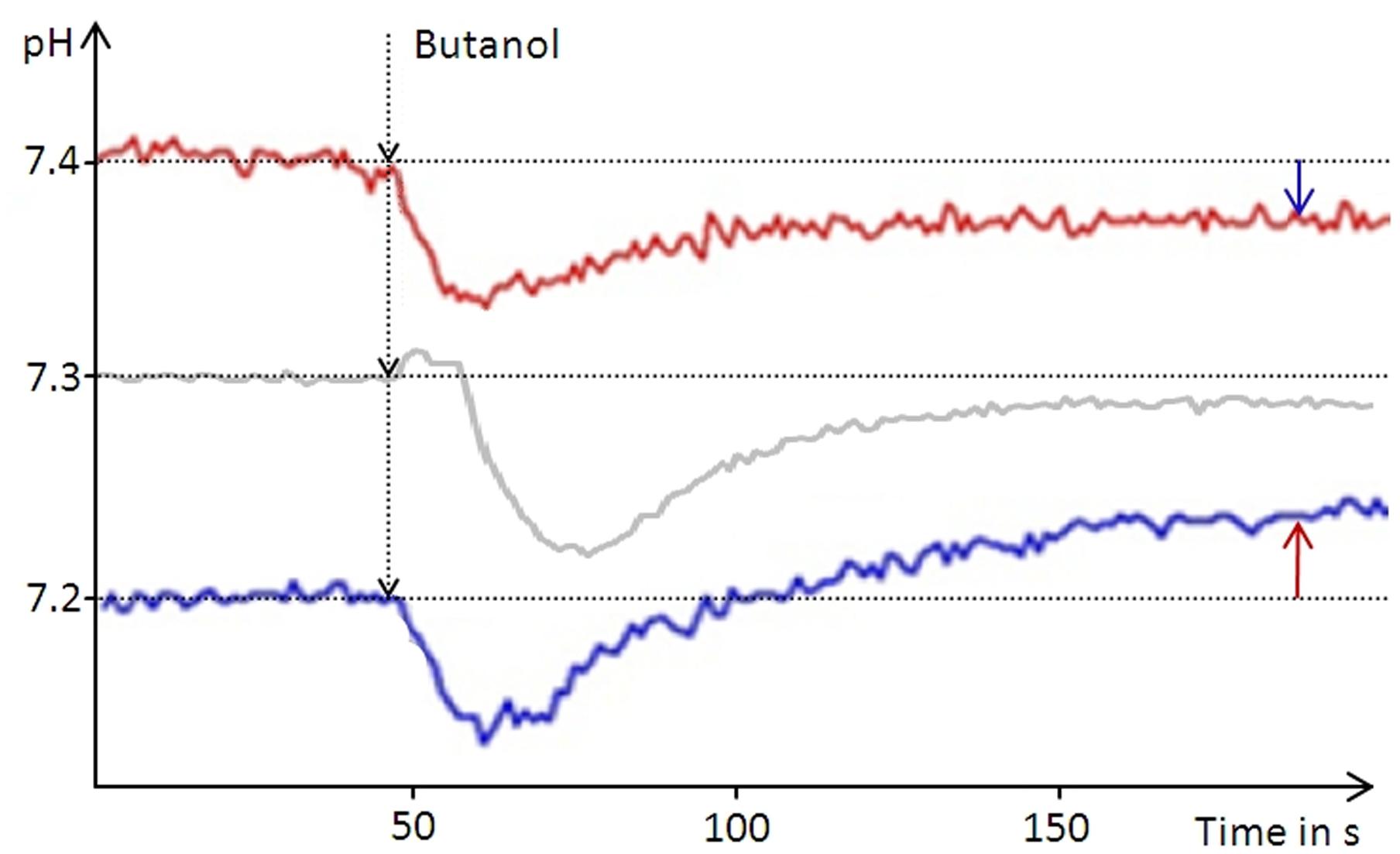
FIGURE 1. Narrowing down the intracellular pH of D. shibae. The pH of an unbuffered cell suspension was constantly recorded during butanol treatment leading to leakage of the cells. Arrows indicate the direction of the pH change from the respective starting pH of the suspension (red graph 7.4, blue graph 7.2) toward the intracellular pH of 7.3. At the starting pH of 7.3 the pH change is very small and can vary between the experiments.
Short-term Anoxia Causes a Boost of the Membrane Potential of Dinoroseobacter shibae
The membrane potential was visualized with two fluorescent carbocyanine dyes, DiOC2(3) and JC-10. The accumulation of DiOC2(3) underwent major changes between oxygen availability and anoxia. Freshly harvested cells of D. shibae stained moderately red, indicating the standard state of the membrane potential (Figure 2A). After anoxic incubation, cells stained more intensely red indicating increased ΔΨ (Figure 2B). Cells that were aerated after anoxic incubation, again showed moderate dye uptake and red fluorescence, similar to untreated cells (Figure 2C). A quantitative measure by image analysis shows that the amount of red stained cells increases about 2.5-fold during anoxia (Figure 3). These results were confirmed with the alternative fluorescent dye JC-10, although the fluorescence was a little weaker in intensity as with DiOC2(3) (Supplement 3). This shows that the uptake and intracellular accumulation of ΔΨ-dependent dyes in D. shibae changes significantly between oxic and anoxic conditions suggesting an increased ΔΨ in anoxic cells. The outcome of the staining was independent of the presents or absence of succinate, which was used as the cells sole electron and carbon source during cultivation. Furthermore, the increase of ΔΨ during anoxia could be observed for varying external pH values. With a pH of 7.5, the growth medium of D. shibae is more alkaline than the pHi (near 7.3). We obtained the same results with cells that were grown and tested at pH 7.0. Even at an external pH of 6.5, which is slightly below the pH optimum of D. shibae (Biebl et al., 2005), the effect was still observed. However, these cells appeared more orange than red. (Supplement 4).
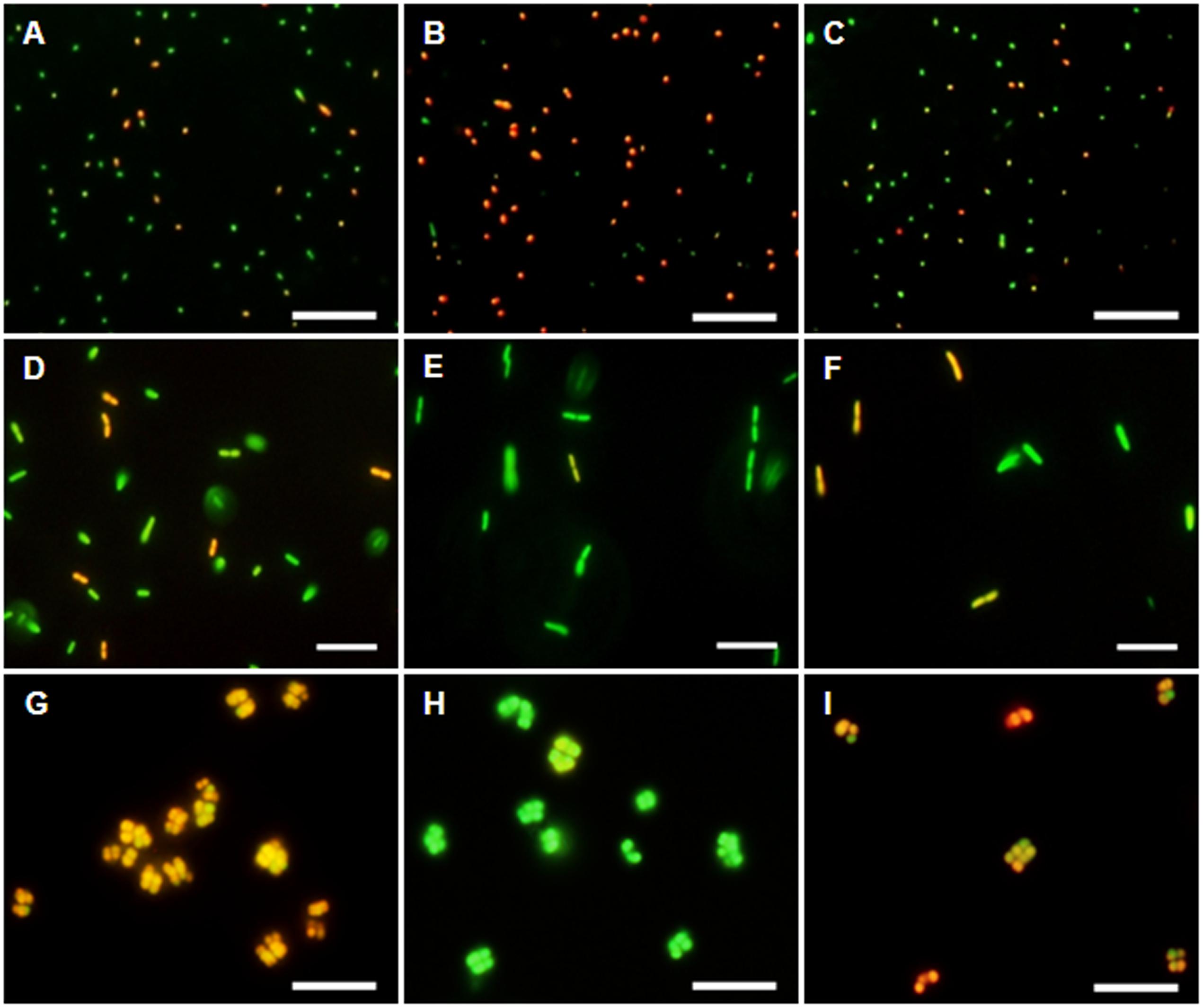
FIGURE 2. DiOC2(3)-uptake at different energetic states. (Left column) Untreated cells in exponential growth phase. (Middle column) Cells after 2 h of N2-gassing. (Right column) Cells after 2 h N2-gassing, aerated for 2 min subsequently. (A–C) Dye uptake of D. shibae increases after N2-gassing but declines after subsequent aeration. (D–F) Dye uptake of E. coli decreases after N2-gassing, but increases after subsequent aeration. (G–I) Dye uptake of M. luteus was strongest at the start, decreased drastically after N2-gassing, but increases after subsequent aeration. All scales: 10 μm.
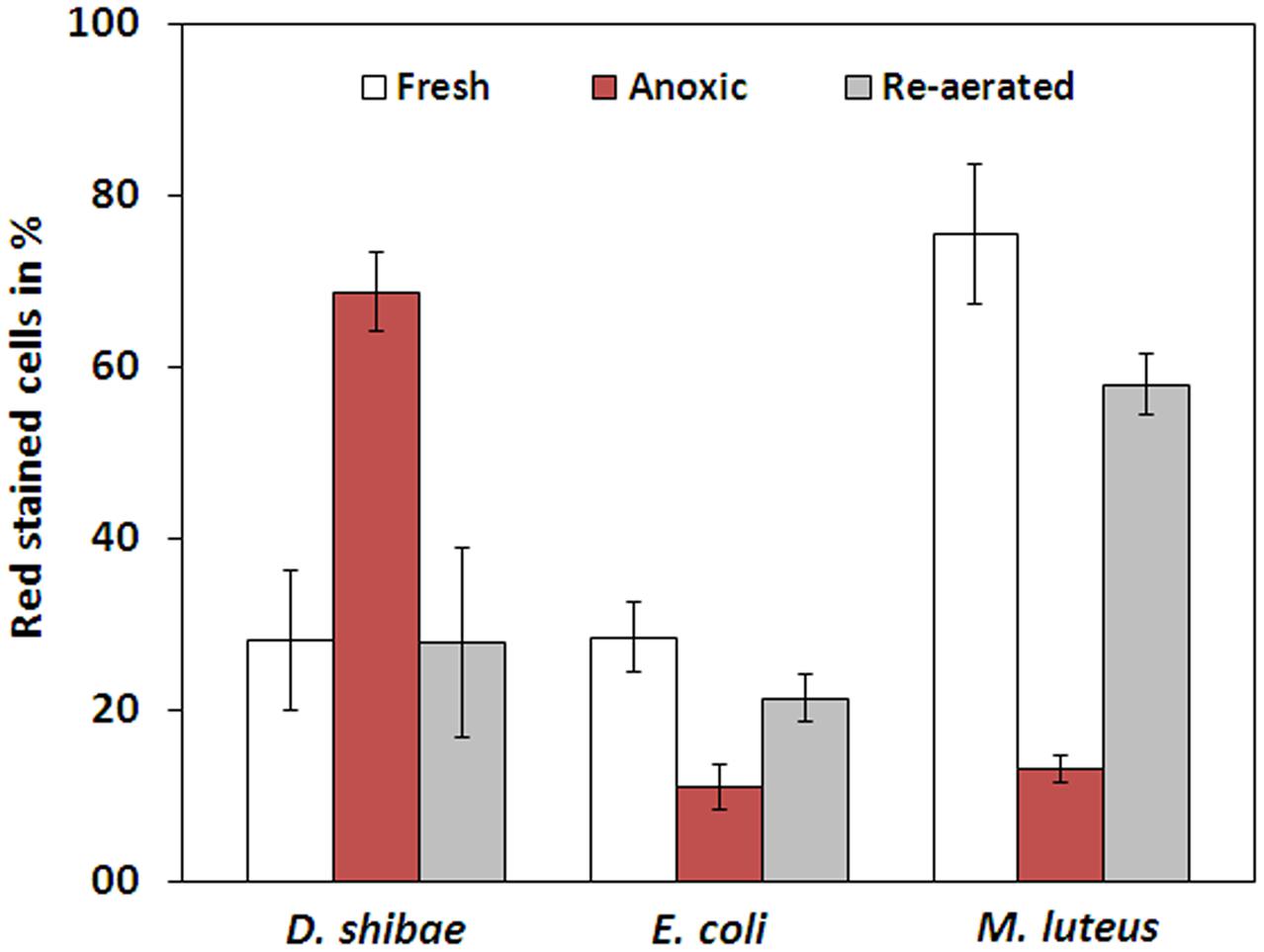
FIGURE 3. Fraction of red stained cells for fresh, anoxic, and re-aerated cells. D. shibae has 2.5-fold more red stained cells during anoxia, compared to fresh cells and after re-aeration. E. coli has 2.5-fold and M. luteus even 5-fold less red stained cells during anoxia. All strains approach their original value after re-aeration.
Bacteria in Control Experiments Showed Stable- or Decreased ΔΨ during Anoxia
The observation of an increasing ΔΨ for D. shibae under anoxic conditions was unexpected. To clarify this behavior we analyzed the ΔΨ of other bacteria under the same conditions. E. coli revealed a decrease in ΔΨ during short-term anoxia (Figures 2D–F) with about 2.5 times less red stained cells (Figure 3). The strictly aerobe M. luteus (Woodward and Kell, 1991), which took up the dye very well from the start, showed a drastically decreased ΔΨ after N2-gassing (Figures 2G–I), with five times less red stained cells (Figure 3). Unlike D. shibae, none of the tested control organisms showed an increased ΔΨ during oxic or anoxic conditions.
Protonophore Treatment Proves ΔΨ-Depending Dye Uptake
Control experiments with protonophores were conducted to make sure the observed uptake of the dye is indeed ΔΨ-depending. In all tested organisms dye uptake was drastically decreased and red fluorescence was prevented almost completely when the cells were pre-incubated with the protonophores CCCP or TCS for 5 min (Figure 4). The channel-forming peptide gramicidin did not work as effective for the Gram-negative bacteria D. shibae and E. coli, but worked fine on the Gram-positive M. luteus. With gramicidin, the amount of D. shibae cells stained red/orange decreased from previously mentioned 69(±5)% to 41(±1)%. For M. luteus all cells appeared green with no visible dye aggregation.
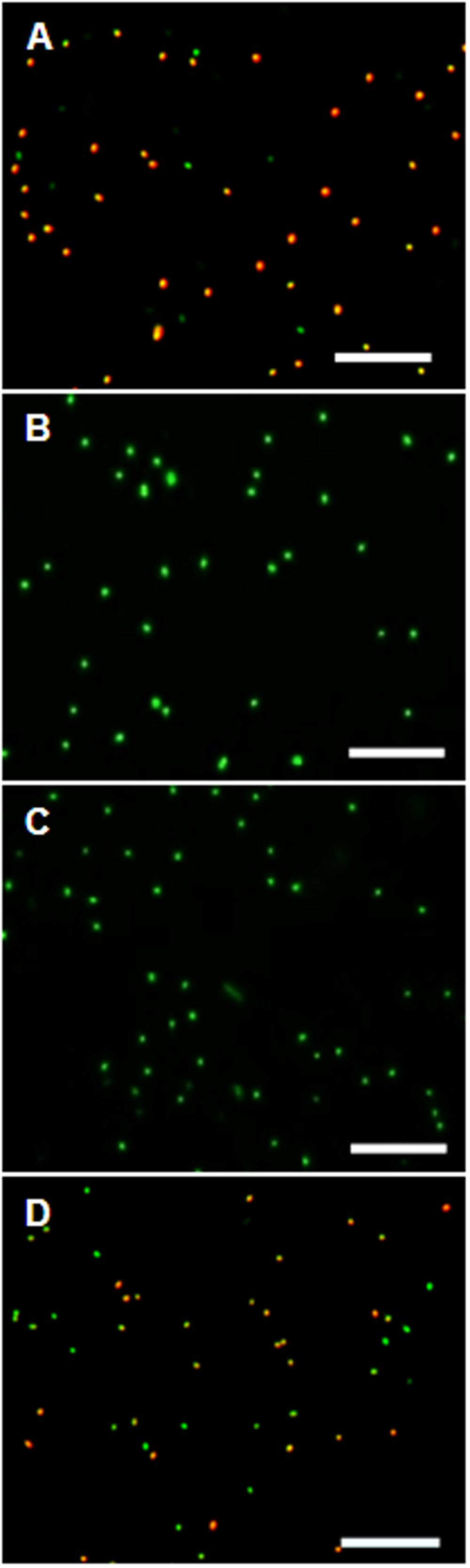
FIGURE 4. The effect of ΔΨ-dissipating agents on the DiOC2(3)-uptake of D. shibae cells. (A) D. shibae cells with increased membrane potential. (B) 2 μM CCCP. (C) 5 μM TCS. (D) 5 μg/ml gramicidin. All scales: 10 μm.
Discussion
In the present study, we show that the membrane potential ΔΨ of D. shibae is strongly influenced by short-term anoxia – although in an unexpected way – while ΔpH is not affected. The used carbocyanine dyes for ΔΨ-visualization are usually applied in combination with flow cytometry (Novo et al., 1999; Jiao et al., 2004; Zhen et al., 2014). We have shown that fluorescence microscopy can be a useful alternative, since it produces in vivo pictures of ΔΨ-caused dye-uptake (Supplement 5).
ΔpH Is Not Contributing to ATP Recovery
Different from our expectations, ΔpH is seemingly not contributing to the quick ATP recovery of D. shibae. Instead the intracellular pH was determined to be near 7.3, and was not affected from short-term anoxia and re-aeration. The intracellular pH was lower than that of the medium (pH 7.5), resulting even in a slightly reversed ΔpH. We conclude that ΔpH does not support proton-driven ATP synthesis in D. shibae. Instead, our results suggest that the proton-motive force relies mainly on ΔΨ.
Boosted Membrane Potential Supports ATP Regeneration
According to our expectation short-term anoxia had an impact on ΔΨ. However, D. shibae increased its membrane potential as reaction to short-term anoxia and the resulting ATP depletion, while ΔpH remained unchanged. An increasing membrane potential as a response to anoxia is unusual, as indicated by the analyzed control organisms. None of them showed a comparable reaction to short-term anoxia. It was shown before that D. shibae cells undergo a metabolic crisis during the absence of oxygen and metabolites start to accumulate (Laass et al., 2014). We show that this crisis is also reflected on a bioenergetic and physiological level. The previously documented drop in the ATP content (Holert et al., 2011) and the newly found increase in membrane potential are tied together and a result of the cells’ adaptation to anoxia. Like the metabolic crisis, the increased ΔΨ normalizes with re-aeration. However, it is still unclear how the process of increasing ΔΨ works in detail. Our results suggest an electrogenic mechanism as a response to anoxia. Apparently, the process is not electron-donor dependent, as it was independent of succinate availability.
A boosted membrane potential can give the ATP regeneration of D. shibae a flying start, once oxygen becomes available again. The membrane potential is known to be the more potent component of the proton-motive force for bacteria that live in pH-neutral environments (Krulwich et al., 2011). The bioenergetic kickstart is beneficial for D. shibae with respect to its natural environment. Diurnal alterations in oxygen availability are very common in phototrophic biofilms (Steunou et al., 2008). D. shibae is abundant in this habitat as a dinoflagellate epibiont (Biebl et al., 2005; Parsons and Preskitt, 2007) and being able to adapt to these alteration is advantageous for its survival (Wagner-Döbler et al., 2010).
However, ΔΨ cannot be solely responsible for the observed ATP regeneration capacity. It is build up by a remarkably low amount of charges per cell and has about -135 mV in its normal state (Krulwich et al., 2011). Assuming the cell size described by Biebl et al. (2005) and the standard membrane capacity (1 μF/cm2, Adrian, 1980), about 33 103 charges would be required to increase ΔΨ by -180 mV. With an H+/ATP ratio of 4 (Elston et al., 1997) the cell could regenerate about 48 μM of intracellular ATP. In order to regenerate 12 mM of ATP (Holert et al., 2011), the process must be quickly supported by respiration- or light-driven proton translocation.
The increased ΔΨ during anoxia cannot possibly be caused by the ΔpH. A ΔpH value of 0.5 could at maximum builds up 30 mV membrane potential. We observed the ΔΨ increase at favorable and unfavorable ΔpH values above and below the intracellular pH. This demonstrates that the documented effect is significantly larger than 30 mV and based on a different mechanism.
Author Contributions
CK concept, experiments, data analysis, and wrote manuscript. HC concept and wrote manuscript.
Funding
This study has been funded by the German Science Foundation within the Collaborative Research Centre SFB-TRR51 (Roseobacter).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Robert Strodel and Minh Dang Nguyen for support during the lab work, as well as Johannes Holert and Elisabeth Härtig for comments on the manuscript. We are also grateful for the technical assistance by Frank Meyerjürgens and Jana Schmidt.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.00695/full#supplementary-material
References
Adrian, R. H. (1980). “The capacity of cell membranes: gating current measurements,” in Developments in Biophysical Research, eds A. Borsellino, P. Omodeo, R. Strom, A. Vecli, and E. Wanke (New York, NY: Springer), 49–55. doi: 10.1007/978-1-4684-1077-8_6
Alakomi, H. L., Paananen, A., Suihko, M. L., Helander, I. M., and Saarela, M. (2006). Weakening effect of cell permeabilizers on gram negative bacteria causing biodeterioration. Appl. Environ. Microbiol. 72, 4695–4703. doi: 10.1128/AEM.00142-06
Batzke, A., Engelen, B., Sass, H., and Cypionka, H. (2007). Phylogenetic and physiological diversity of cultured deep-biosphere bacteria from equatorial pacific ocean and Peru margin sediments. Geomicrobiol. J. 24, 261–273. doi: 10.1080/01490450701456453
Béjà, O., Suzuki, M. T., Heidelberg, J. F., Nelson, W. C., Preston, C. M., Hamada, T., et al. (2002). Unsuspected diversity among marine aerobic anoxygenic phototrophs. Nature 415, 630–633. doi: 10.1038/415630a
Biebl, H., Allgaier, M., Tindall, B. J., Koblizek, M., Lünsdorf, H., Pukall, R., et al. (2005). Dinoroseobacter shibae gen. nov., sp. nov., a new aerobic phototrophic bacterium isolated from dinoflagellates. Int. J. Syst. Evol. Microbiol. 55, 1089–1096. doi: 10.1099/ijs.0.63511-0
Cypionka, H. (2005). Mpwin Software. Vers. 2008.08.25. Available at: www.pmbio.icbm.de/downlist.htm
Cypionka, H. (2011). CountThem Software. Vers. 2011.08.18. Available at: www.pmbio.icbm.de/downlist.htm
Cypionka, H., Völcker, E., and Rohde, M. (2016). Erzeugung virtueller 3D-Bilder mit jedem Lichtmikroskop oder REM. BIOspektrum 22, 143–145. doi: 10.1007/s12268-016-0668-1
Dumas, E., Gao, C., Suffern, D., Bradforth, S., Dimitrijevic, N., and Nadeau, J. (2010). Interfacial charge transfer between CdTe quantum dots and Gram negative vs Gram positive bacteria. Environ. Sci. Technol. 44, 1464–1470. doi: 10.1021/es902898d
Elston, T., Wang, H., and Oster, G. (1997). Energy transduction in ATP synthase. Nature 391, 510–513. doi: 10.1038/35185
Holert, J., Hahnke, S., and Cypionka, H. (2011). Influence of light and anoxia on chemiosmotic energy conservation in Dinoroseobacter shibae. Environ. Microbiol. Rep. 3, 136–141. doi: 10.1111/j.1758-2229.2010.00199.x
Jiao, N., Yang, Y., and Luo, T. (2004). Membrane potential based characterization by flow cytometry of physiological states in an aerobic anoxygenic phototrophic bacterium. Aquat. Microb. Ecol. 37, 149–158. doi: 10.3354/ame037149
Kolber, Z. S., Plumley, F. G., Lang, A. S., Beatty, J. T., Blankenship, R. E., VanDover, C. L., et al. (2001). Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292, 2492–2495. doi: 10.1126/science.1059707
Krulwich, A., Sachs, G., and Padan, E. (2011). Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 9, 330–343. doi: 10.1038/nrmicro2549
Laass, S., Kleist, S., Bill, N., Druppel, K., Kossmehl, S., Wohlbrand, L., et al. (2014). Gene regulatory and metabolic adaptation processes of Dinoroseobacter shibae DFL12T during oxygen depletion. J. Biol. Chem. 289, 13219–13231. doi: 10.1074/jbc.M113.545004
Novo, D., Perlmutter, N., Hunt, R., and Shapiro, H. (1999). Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 35, 55–63. doi: 10.1002/(SICI)1097-0320(19990101)35:1<55::AID-CYTO8>3.0.CO;2-2
Parsons, M. L., and Preskitt, L. B. (2007). A survey of epiphytic dinoflagellates from the coastal waters of the island of Hawai’i. Harmful Algae 6, 658–669. doi: 10.1016/j.hal.2007.01.001
Piekarski, T., Buchholz, I., Drepper, T., Schobert, M., Wagner-Döbler, I., Tielen, P., et al. (2009). Genetic tools for the investigation of Roseobacter clade bacteria. BMC Microbiol. 9:265. doi: 10.1186/1471-2180-9-265
Scholes, P., and Mitchell, P. (1970). Acid-base titration across the plasma membrane of Micrococcus denitrificans: factors affecting the effective proton conductance and the respiratory rate. J. Bioernerg. 1, 61–72. doi: 10.1007/BF01516089
Soora, M., and Cypionka, H. (2013). Light Enhances Survival of Dinoroseobacter shibae during long-term starvation. PLoS ONE 8:e57487. doi: 10.1371/journal.pone.0057487
Steunou, A. S., Jensen, S. I., Brecht, E., Becraft, E. D., Bateson, M. M., Kilian, O., et al. (2008). Regulation of nif gene expression and the energetics of N2 fixation over the diel cycle in a hot spring microbial mat. ISME J. 2, 364–378. doi: 10.1038/ismej.2007.117
Wagner-Döbler, I., Ballhausen, B., Berger, M., Brinkhoff, T., Buchholz, I., Bunk, B., et al. (2010). The complete genome sequence of the algal symbiont Dinoroseobacter shibae: a hitchhiker’s guide to life in the sea. ISME J. 4, 61–77. doi: 10.1038/ismej.2009.94
Wilks, J., and Slonczewski, L. (2007). pH of the cytoplasm and periplasm of Escherichia coli: rapid measurement by green fluorescent protein fluorimetry. J. Bacteriol. 189, 5601–5607. doi: 10.1128/JB.00615-0
Woodward, A. M., and Kell, D. B. (1991). On the relationship between the nonlinear dielectric properties and respiratory activity of the obligately aerobic bacterium Micrococcus luteus. J. Electroanal. Chem. Interfacial Electrochem. 321.3, 423–439.
Keywords: proton-motive force, Roseobacter clade, Micrococcus luteus, short-term anoxia, intracellular pH, fluorescence microscopy, DiOC2(3), JC-10
Citation: Kirchhoff C and Cypionka H (2017) Boosted Membrane Potential as Bioenergetic Response to Anoxia in Dinoroseobacter shibae. Front. Microbiol. 8:695. doi: 10.3389/fmicb.2017.00695
Received: 17 August 2016; Accepted: 04 April 2017;
Published: 20 April 2017.
Edited by:
Inês A. Cardoso Pereira, Instituto de Tecnologia Química e Biológica (ITQB-NOVA), PortugalReviewed by:
Pablo Ivan Nikel, The Novo Nordisk Foundation Center for Biosustainability (DTU Biosustain), DenmarkWolfgang Nitschke, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2017 Kirchhoff and Cypionka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heribert Cypionka, heribert.cypionka@icbm.de
 Christian Kirchhoff
Christian Kirchhoff Heribert Cypionka
Heribert Cypionka