- 1Novel Bacteria and Drug Discovery Research Group, School of Pharmacy, Monash University Malaysia, Bandar Sunway, Malaysia
- 2Division of Physiology, School of Medical Sciences, University of Phayao, Phayao, Thailand
- 3Center of Health Outcomes Research and Therapeutic Safety, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
- 4Faculty of Pharmaceutical Sciences, Pharmaceutical Outcomes Research Center, Naresuan University, Phitsanulok, Thailand
- 5Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 6Department of Pharmacy, Absyn University Peshawar, Peshawar, Pakistan
- 7UKM Medical Molecular Biology Institute, UKM Medical Centre, University Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 8Division of Genetics and Molecular Biology, Faculty of Science, Institute of Biological Sciences, University of Malaya, Kuala Lumpur, Malaysia
Streptomyces colonosanans MUSC 93JT, a novel strain isolated from mangrove forest soil located at Sarawak, Malaysia. The bacterium was noted to be Gram-positive and to form light yellow aerial and vivid yellow substrate mycelium on ISP 2 agar. The polyphasic approach was used to determine the taxonomy of strain MUSC 93JT and the strain showed a range of phylogenetic and chemotaxonomic properties consistent with those of the members of the genus Streptomyces. Phylogenetic and 16S rRNA gene sequence analysis indicated that closely related strains include Streptomyces malachitofuscus NBRC 13059T (99.2% sequence similarity), Streptomyces misionensis NBRC 13063T (99.1%), and Streptomyces phaeoluteichromatogenes NRRL 5799T (99.1%). The DNA–DNA relatedness values between MUSC 93JT and closely related type strains ranged from 14.4 ± 0.1 to 46.2 ± 0.4%. The comparison of BOX-PCR fingerprints indicated MUSC 93JT exhibits a unique DNA profile. The genome of MUSC 93JT consists of 7,015,076 bp. The DNA G + C content was determined to be 69.90 mol%. The extract of strain MUSC 93JT was demonstrated to exhibit potent antioxidant activity via ABTS, metal chelating, and SOD assays. This extract also exhibited anticancer activity against human colon cancer cell lines without significant cytotoxic effect against human normal colon cells. Furthermore, the chemical analysis of the extract further emphasizes the strain is producing chemo-preventive related metabolites. Based on this polyphasic study of MUSC 93JT, it is concluded that this strain represents a novel species, for which the name Streptomyces colonosanans sp. nov. is proposed. The type strain is MUSC 93JT (= DSM 102042T = MCCC 1K02298T).
Introduction
The discovery of new and useful compounds is constantly in need for the prevention and/or treatment of diseases. Nature has been an interesting source of many useful compounds that have important applications in various fields such as pharmacy, medicine, and biochemistry (Burja et al., 2001; Karikas, 2010). Researchers have been exploring natural sources such as plants and microorganisms for the discovery of novel drugs. Microorganisms have gained increasing attention in drug discovery and many studies revealed that microorganisms from different ecosystems have shown some potentials for human use as many interesting compounds have been derived from them (Burja et al., 2001; Chin et al., 2006).
In the field of microbial drug discovery, Actinobacteria strains have been greatly explored due to their ability to produce diverse bioactive secondary metabolites; accounting for 45% of all discovered bioactive microbial metabolites (Sharma and Shah, 2014). Particularly, the dominant genus of this phylum which is Streptomyces have a significant contribution to mankind (Azman et al., 2015). The genus Streptomyces is proposed by Waksman and Henrici (1943) and it is a group of Gram positive bacteria comprised ~780 species with validly published names (http://www.bacterio.cict.fr/). Members of this genus are producers of more than 75% of the naturally occurring antibiotics (Kinkel et al., 2014; Lee et al., 2014e; Ser et al., 2015a). Other than antibiotics, Streptomyces bacteria are prolific producers of various compounds with important biological activities such as antifungal, anticancer, antioxidant, and immunosuppressive activities (Kino et al., 1987; Rashad et al., 2015; Ser et al., 2016a; Law et al., 2017). It is known that exploring new taxa is one of the successful strategies that can lead to the discovery of therapeutic agents (Williams, 2009; Ser et al., 2016c). In previous drug screening programs, it is unfortunate that the screening of novel Actinobacteria from terrestrial source have resulted in inefficient rediscovery of known bioactive compounds (Ser et al., 2016c). Therefore, this highlighted the need to discover novel Actinobacteria from new or under explored area such as the mangrove environments.
Mangrove environments consists of special woody plant area mainly located in intertidal zones of estuaries, deltas, lagoons, backwaters, creeks, marshes, tropical, and subtropical coastal regions (Mangamuri et al., 2012; Ser et al., 2015a). Mangrove is one of the world's most dynamic environments which occupies millions of hectors across the world coastal areas and it has been a habitat to various flora and fauna of terrestrial, freshwater, and marine species (Mangamuri et al., 2012; Lee et al., 2014e). According to the report by Giri et al. (2011), the largest extent of mangroves is found in Asia; and Malaysia is one of the most mangrove-rich countries in Asia. Additionally, one of the least disturbed mangrove areas in Malaysia is situated at the state of Sarawak, in which most of its mangrove forests are still in pristine condition (Ashton and Macintosh, 2002). Hence, this provides a great opportunity to explore the actinobacterial population present in these mangrove forests.
Owing to the presence of various microbial enzymatic and metabolic activities, the mangrove ecosystem is highly rich in nutrient and organic matter that in turn facilitates the rapid development of species diversity in response to environmental variation (Satheeja and Jebakumar, 2011; Mangamuri et al., 2012). Furthermore, this ecosystem experiences constant fluctuations in salinity and tidal gradient that could trigger metabolic pathway adaptations and possibly lead to the production of pharmaceutically important metabolites. Hence, there are growing interests in the utilization of mangrove microorganism resources and this have subsequently led to the discovery of novel Streptomyces (Hong et al., 2009; Lee et al., 2014d,e).
In recent studies, researchers have successfully identified a number of novel Streptomyces from mangrove environments in different countries. For examples, Streptomyces avicenniae (Xiao et al., 2009), Streptomyces xiamenensis (Xu et al., 2009), Streptomyces sanyensis (Sui et al., 2011), and Streptomyces qinglanensis (Hu et al., 2012) from mangrove environments in China, Streptomyces sundarbansensis (Arumugam et al., 2011) from mangrove environments in India, and Streptomyces pluripotens (Lee et al., 2014d), Streptomyces mangrovisoli (Ser et al., 2015b), Streptomyces humi (Zainal et al., 2016), Streptomyces antioxidans (Ser et al., 2016c), and Streptomyces malaysiense (Ser et al., 2016b) from mangrove environments in Malaysia. In addition, several studies also reported that mangrove Streptomyces are capable of producing antioxidant and anticancer agents (Ser et al., 2015a, 2016b,c; Tan et al., 2015). Thus, this prompted the investigation of Streptomyces from underexplored mangrove forest in Sarawak.
As a matter of fact, the current global burden of cancer is increasing continuously and this is mainly due to increasing urbanization, followed by the changes in environmental conditions and lifestyle (Karikas, 2010; Ser et al., 2016b; Siegel et al., 2016). Over the years, natural compounds play a relevant role in cancer therapy and prevention (Nobili et al., 2009). Several anticancer agents that have been successfully derived from Streptomyces include aclarubicin, bleomycin, doxorubicin, mitomycin C, and pentostatin (Tan et al., 2006, 2015). Besides, the knowledge acquired throughout years of research conducted in cancer biology has emphasized the cancer initiation and progression is mainly associated with oxidative stress- a condition characterized by elevated amounts of free radicals (Reuter et al., 2010; Ser et al., 2016b). Oxidative stress is known to cause modification or damage to important cellular macromolecules including DNA which could dramatically increase the risk of cancer (Reuter et al., 2010; Ser et al., 2015a, 2016b; Tan et al., 2015). Meanwhile, antioxidant is acknowledged to play important role in biological system. It exerts its scavenging ability to neutralize the free radicals and thus preventing deleterious effects of excessive free radicals during occurrence of oxidative stress (Ser et al., 2015a, 2016b). In view of the importance of antioxidant, efforts have been made to search for effective natural antioxidants. A number of antioxidants have been derived from Streptomyces such as carazostatin (Kato et al., 1989), antiostatins A1 to A4 and B2 to B5 (Mo et al., 1990), carbazoquinocins A to F (Tanaka et al., 1995), and benthocyanins A, B, and C (Shin-ya et al., 1991; Shinya et al., 1993).
This study explores novel Streptomyces strains present in soil samples collected from the mangrove forest located at Kuching, Sarawak. A novel strain, MUSC 93JT was discovered and polyphasic approach demonstrated that MUSC 93JT represents a novel species of the Streptomyces genus, for which the name Streptomyces colonosanans sp. nov. is proposed. With the advancement of next generation sequencing (NGS) technology, the genome of MUSC 93JT was analyzed in this study. The study also aim to investigate the antioxidant and anticancer properties of MUSC 93JT. Furthermore, gas chromatography-mass spectrometry (GC-MS) was conducted for chemical analysis of MUSC 93JT extract in order to reveal the active compounds present in the extract. To the best of our knowledge, there is no literature reported so far in regards to the exploration of biological properties of Streptomyces isolated from Sarawak mangrove environments. Therefore, the outcome of this study provides a further in depth understanding on bioprospecting potential of Streptomyces from under-explored region of Malaysia and at the same time granting the solid foundation to support for a further in depth molecular studies on chemopreventive property possessed by Streptomyces colonosanans sp. nov.
Materials and Methods
Isolation and Maintenance of Strain
Strain MUSC 93JT was isolated from a soil sample collected at site KTTAS 1 (1°41′48.57′N 110°11′15.30″E), situated in the mangrove forest of Kuching, state of Sarawak, Malaysia, in June 2015. Samples of the upper 20 cm topsoil layer (after the top 2–3 cm of soils removed) were collected using an aseptic metal trowel, placed in sterile Eppendorf tube and stored in −20°C. Air-dried soil samples were ground with a mortar and pestle. Selective pretreatment of soil samples was performed using wet heat in sterilized water (15 min at 50°C; Takahashi et al., 1996). One gram of air-dried soil was mixed with 9 mL sterilized water and then the suspension was spread onto an isolation medium ISP 2 (Shirling and Gottlieb, 1966) supplemented with cycloheximide (50 mg/L) and nalidixic acid (20 mg/L), and incubated at 28°C for 14 days. Pure cultures of strain MUSC 93JT were obtained and maintained on ISP 2 agar slants at 28°C and stocked in glycerol suspensions (20%, v/v) at −20°C.
Genomic and Phylogenetic Analyses
Genomic DNA extraction for PCR was performed as described by Hong et al. (2009). PCR amplification of the 16S rRNA gene was conducted according to the protocol described by Lee et al. (2014d). The 16S rRNA gene sequence of strain MUSC 93JT was aligned with representative sequences of related type strains in the genus Streptomyces retrieved from the GenBank/EMBL/DDBJ databases using CLUSTAL-X software (Thompson et al., 1997). The alignment was first verified manually and adjusted, followed by construction of phylogenetic trees with neighbor-joining (Saitou and Nei, 1987; Figure 1) and maximum-likelihood algorithms (Felsenstein, 1981; Figure S1), utilizing the MEGA version 6.0 (Tamura et al., 2013). For neighbor-joining algorithm, the evolutionary distances were computed using the Kimura's two-parameter model (Kimura, 1980). The calculations of level of sequence similarity were performed by EzTaxon-e server (http://eztaxon-e.ezbiocloud.net/; Kim et al., 2012). Bootstrap based on 1,000 resampling method of Felsenstein (1985) was used to analyze the stability of the resultant tree topologies.
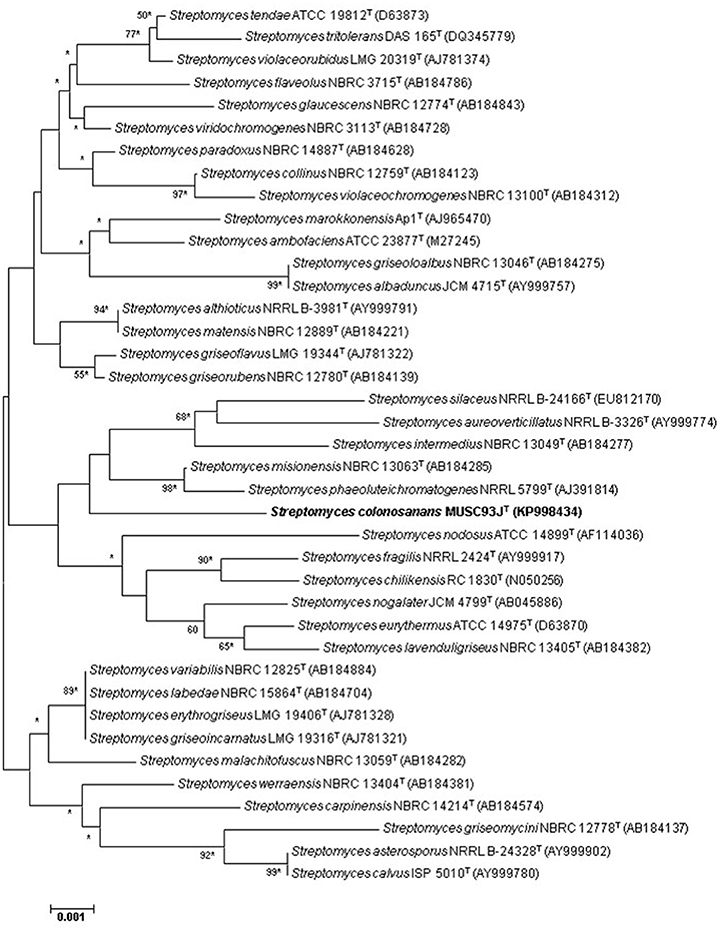
Figure 1. Neighbor-joining phylogenetic tree based on almost complete 16S rRNA sequences (1,490 nucleotides) showing the relationship between strain MUSC 93JT and representatives of some other related taxa. Numbers at nodes indicate percentages of 1,000 bootstrap re-samplings, only values above 50% are shown. Bar, 0.002 substitutions per site. Asterisks indicate that the corresponding nodes were also recovered using the maximum-likelihood tree-making algorithm.
For DNA-DNA hybridization, the extraction of genomic DNA of strain MUSC 93JT, Streptomyces malachitofuscus JCM 4493T, Streptomyces misionensis NBRC 13063T and Streptomyces phaeoluteichromatogenes DSM 41898T were conducted according to the protocol described by Cashion et al. (1977). DNA-DNA hybridization was performed by the Identification Service of the DSMZ, Braunschweig, Germany based on the procedure as described by De Ley et al. (1970) with slight modifications according to Huss et al. (1983). The G + C content of strain MUSC 93JT was determined by HPLC (Mesbah et al., 1989).
BOX-PCR fingerprint analysis was performed for the characterization of strain MUSC 93JT and the closely related strains with the use of primer BOX-A1R (5′-CTACGGCAAGGCGACGCTGACG-3′; Versalovic et al., 1991; Lee et al., 2014b). The BOX-PCR cycling parameters were performed as described by Lee et al. (2014a) and the PCR products were visualized using 2% agarose gel electrophoresis.
Chemotaxonomic Characteristics
Biomass and freeze-dried cells for chemotaxonomic studies were obtained after growing in TSB at 28°C for 5 days on a rotary shaker. The analyses of peptidoglycan amino acid composition and sugars of strain MUSC 93JT were performed by the Identification Service of the DSMZ using published protocols (Schumann, 2011). Analysis of fatty acids (Sasser, 1990), polar lipids (Kates, 1986), and respiratory quinones were performed by the Identification Service of the DSMZ. Major diagnostic whole cell sugars of strain MUSC 93JT were obtained according to the description by Whiton et al. (1985) and analyzed by TLC on cellulose plates (Staneck and Roberts, 1974).
Phenotypic Characteristics
The cultural characteristics of strain MUSC 93JT was determined following growth on ISP 2, ISP 3, ISP 4, ISP 5, ISP 6, and ISP 7 agar (Shirling and Gottlieb, 1966), actinomycetes isolation agar (AIA; Atlas, 1993), starch casein agar (SCA; Küster and Williams, 1964), Streptomyces agar (SA; Atlas, 1993), and nutrient agar (Macfaddin, 2000) at 28°C for 14 days. The morphology of strain MUSC 93JT was observed after incubation on ISP 2 agar plate at 28°C for 7–14 days (Figure 2), using Light microscopy (80i, Nikon) and scanning electron microscopy (JEOL-JSM 6400). The designations of colony colors were made according to the ISCC-NBS color charts. Gram staining was carried out by standard Gram reaction and confirmed by using KOH lysis (Cerny, 1978). The pH range for growth and NaCl tolerance were evaluated using tryptic soy broth (TSB). The pH range tested was between pH 4.0 and 10.0 at an interval of 1 pH unit. The concentration of NaCl was tested at a range of 0–10% (w/v) at intervals of 2%. The effects of temperatures on growth was examined on ISP 2 agar. The temperature range tested for growth was between 4 and 44°C at intervals of 4°C. The growth responses to pH, NaCl, and temperature were observed for 14 days. The production of melanoid pigments was examined using ISP 7 medium following protocol described by Lee et al. (2014c). Hemolytic activity was examined on blood agar medium containing 5% (w/v) peptone, 3% (w/v) yeast extract, 5% (w/v) NaCl, and 5% (v/v) horse blood (Carrillo et al., 1996). Plates were examined for hemolysis after incubation at 32°C for 7–14 days. Amylolytic, lipase, cellulase, chitinase, catalase, protease, and xylanase activities were determined by growing cells on ISP 2 medium following protocol as described by Lee et al. (2014c). The presence of clear zones around colonies indicates the potential of isolates for surfactant production. The carbon-source utilization and chemical sensitivity assays were determined using Biolog GenIII MicroPlates (Biolog, USA) according to the manufacturer's instructions.
All of the phenotypic assays mentioned were performed concurrently for strain MUSC 93JT, Streptomyces malachitofuscus JCM 4493T, Streptomyces misionensis NBRC 13063T, and Streptomyces phaeoluteichromatogenes DSM 41898T.
Extract Preparation of MUSC 93JT
Before fermentation process, strain MUSC 93JT was grown in TSB (Biomerge, Malaysia) as seed medium for 14 days. The fermentation medium, Han's Fermentation Media 1 (HFM1) was autoclaved at 121°C for 15 min prior to experiment (Hong et al., 2009; Lee et al., 2012). The fermentation was conducted in 500 mL Erlenmeyer flask containing 200 mL HFM1 (Biomerge, Malaysia) with 200 μL seed media added into it and shaking at 200 rpm for 7–10 days at 28°C. The resulting Han's Fermentation medium was recovered by centrifugation at 12000 g for 15 min. The supernatant was filtered and collected, then subjected to freeze drying process. The freeze-dried sample was extracted with methanol for 72 h. The methanol-containing extract was filtered and collected, then subjected to re-extraction under same condition for twice at 24 h interval. The collected extract was concentrated with extracting solvent evaporated by rotary vacuum evaporator at 40°C. The final concentrate extract of MUSC 93JT was collected and suspended in dimethyl sulfoxide (DMSO) as vehicle reagent prior to bioactivity screening assays.
Determination of Antioxidant Activity of MUSC 93JT Extract Using Different Assays
The 2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Assay
The 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay was conducted according to the protocol described by Miser-Salihoglu et al. (2013) with some modifications. ABTS radical cation (ABTS·) was produced via reacting ABTS stock solution (7 mM) and potassium persulfate (2.45 mM) for 24 h before the assay. The absorbance was measured at 743 nm and the change in radical amount was indicated by the reduction in absorbance value.
Superoxide Anion Scavenging/Superoxide Dismutase (SOD)
Superoxide anion scavenging /superoxide dismutase (SOD) activity was determined using SOD assay Kit—WST (Sigma-Aldrich), a commercially available colorimetric microtiter plate method, according to the protocol given by the manufacturer. SOD activity of the extract was determined colorimetrically at 450 nm as the reduction of the Dojindo's highly water-soluble tetrazolium salt, WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) by superoxide anion, . Sample extract solution (20 μL) at different concentrations were added to the 96-well-plate, respectively. The reaction solutions were added as described in the protocol and then the plate was incubated at 37°C for 20 min prior to measurement of absorbance at 450 nm using a microplate reader. The percentage of SOD activity (percentage of WST-1 reduction) was calculated as follows (Tan et al., 2015):
Metal Chelating
Metal Chelating activity was examined by measuring the formation of Fe2+-ferrozine complex as described in previous study conducted by Manivasagan et al. (2013) with slight modification. FeSO4 (2 mM) was added into the extract followed by the addition of ferrozine (5 mM) to initiate the reaction prior to measurement of absorbance at 562 nm using spectrophotometer.
Maintenance and Growth Condition of Human Cell Lines
The human normal colon CCD-18Co cells were maintained in DMEM media supplemented with 10% fetal bovine serum in a humidified incubator with 5% CO2 in air at 37°C (Ser et al., 2016c). All of the human derived cancer cell lines evaluated in this study were maintained in RPMI (Roswell Park Memorial Institute)-1640 (Gibco) supplemented with 10% fetal bovine serum and 1x antibiotic-antimycotic (Gibco) in a humidified incubator with 5% CO2 in air at 37°C (Tan et al., 2015).
Investigation of Cytotoxicity Activity of MUSC 93JT Using 3-(4,5-Dimethylthazol-2yl)-2,5-Diphenyl Tetrazolium-Bromide (MTT) Assay
For evaluation of cytotoxicity, the human normal colon CCD-18Co cells were included in this study, while the human derived cancer cell lines included were colon cancer cell lines: HCT-116, HT-29, Caco-2, and SW480. The cytotoxic activity of MUSC 93JT extract was examined using MTT assay following the protocol previously described by Williams (1989). The cell viability was determined spectrophotometrically at 570 nm (with 650 nm as reference wavelength) using a microplate reader. The percentage of cell viability was calculated as follows:
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC-MS analysis was performed according to previously developed method with slight modification (Supriady et al., 2015). The analysis was conducted using Agilent Technologies 6980N (GC) equipped with 5979 Mass Selective Detector (MS), with HP-5MS (5% phenyl methyl siloxane) capillary column of dimensions 30.0 m × 250 μm × 0.25 μm and helium as carrier gas at 1 mL/min. The column temperature was programmed initially at 40°C for 10 min, followed by an increase of 3°C/min to 250°C and was kept isothermally for 5 min. The MS was operating at 70 eV. The constituents were identified by comparison of their mass spectral data with those from NIST 05 Spectral Library.
Genome Sequencing and Bioinformatics Analysis of MUSC 93JT
Genomic DNA of MUSC 93JT was extracted using Masterpure™ DNA purification kit (Epicentre, Illumina Inc., Madison, WI, USA) followed by RNase (Qiagen, USA) treatment (Ser et al., 2015c, 2016d) Subsequently, the DNA quality was examined using NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA) and a Qubit version 2.0 fluorometer (Life Technologies, Carlsbad, CA, USA). DNA library construction was performed using Nextera™ DNA Sample Preparation kit (Nextera, USA) and the library quality was validated by Bioanalyzer 2100 high sensitivity DNA kit (Agilent Technologies, Palo Alto, CA). Paired-end sequencing was carried out on MiSeq platform with MiSeq Reagent Kit 2 (2 × 250 bp; Illumina Inc., Madison, WI, USA). The paired-end reads were then trimmed and de novo assembled with CLC Genomics Workbench version 7 (CLC bio, Denmark). Gene prediction was carried out using Prodigal version 2.6, whereas rRNA and tRNA were predicted using RNAmmer and tRNAscan SE version 1.21 (Lowe and Eddy, 1997; Lagesen et al., 2007; Hyatt et al., 2010). The assembly was annotated using Rapid Annotation using Subsystem Technology (RAST) and by the NCBI Prokaryotic Genomes Annotation Pipeline (Angiuoli et al., 2008; Aziz et al., 2008). Results of genome sequencing and bioinformatics analysis were presented in the description of Streptomyces colonosanans sp. nov.
Statistical Analysis
Experiments involved the investigation of antioxidant and cytotoxic activities were done in quadruplicate. Data analysis was performed with SPSS statistical analysis software and the results were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by appropriate post-hoc test (Tukey) was performed to determine the significant differences between groups. A difference was considered statistically significant when p ≤ 0.05.
Results and Discussion
Genomic and Phylogenetic Analyses of Strain MUSC 93JT
The nearly complete 16S rRNA gene sequence was determined for strain MUSC 93JT (1490 bp; GenBank/EMBL/DDBJ accession number KP998434) and manual alignment of the sequence was performed with the corresponding partial 16S rRNA gene sequences of the type strains of representative members of the genus Streptomyces retrieved from GenBank/EMBL/DDBJ databases. Phylogenetic trees were constructed based on the 16S rRNA gene sequences to determine the phylogenetic position of this strain (Figure 1 and Figure S1). Phylogenetic analysis exhibited that closely related strains include Streptomyces misionensis NBRC 13063T (99.1% sequence similarity) and Streptomyces phaeoluteichromatogenes NRRL 5799T (99.1% sequence similarity), as they formed a distinct clade (Figure 1). The analysis of 16S rRNA gene sequence for strain MUSC 93JT exhibited highest sequence similarity to strain Streptomyces malachitofuscus NBRC 13059T (99.2%), Streptomyces misionensis NBRC 13063T (99.1%) and Streptomyces phaeoluteichromatogenes NRRL 5799T (99.1%); sequences similarities of <99.0% were obtained with the type strains of other species of the genus Streptomyces.
The DNA–DNA relatedness values between strain MUSC 93JT and Streptomyces malachitofuscus JCM 4493T (14.4 ± 0.1%), Streptomyces misionensis NBRC 13063T (46.2 ± 0.4%) and Streptomyces phaeoluteichromatogenes DSM 41898T (20.7 ± 1.0%) were significantly below 70%, the threshold value for the delineation of bacterial species (Wayne et al., 1987).
The BOX-PCR results indicated that strain MUSC 93JT exhibited a unique BOX-PCR fingerprint compared with closely related type strains: Streptomyces malachitofuscus JCM 4493T, Streptomyces misionensis NBRC 13063T, and Streptomyces phaeoluteichromatogenes DSM 41898T (Refer to Figure S2). These results are in line with results of phylogenetic analysis and DNA-DNA hybridizations, which demonstrate that strain MUSC 93JT represents a novel species in the genus Streptomyces.
Chemotaxonomic Analyses of Strain MUSC 93JT
The fatty acids profiles of strain MUSC 93JT and closely related type strains are presented in Table 1. The major cellular fatty acids in MUSC 93JT were identified as anteiso-C15:0 (23.1%), C16:0 (18.6%), and iso-C16:0 (15.1%). The fatty acids profile of MUSC 93JT is consistent with those of closely related phylogenetic neighbors such as Streptomyces malachitofuscus JCM 4493T, Streptomyces misionensis NBRC 13063T, and Streptomyces phaeoluteichromatogenes DSM 41898T, which contain anteiso-C15:0 (12.6–40.1%) and iso-C16:0 (14.4–18.3%) as major fatty acids (Table 1). However, the fatty acid profile of MUSC 93JT was quantitatively different from those of these type strains; for instance, the anteiso-C15:0 (23.1%) was found to be predominant in strain MUSC 93JT, but the amount of anteiso-C15:0 was much lesser (12.6%) in Streptomyces malachitofuscus JCM 4493T (Table 1).
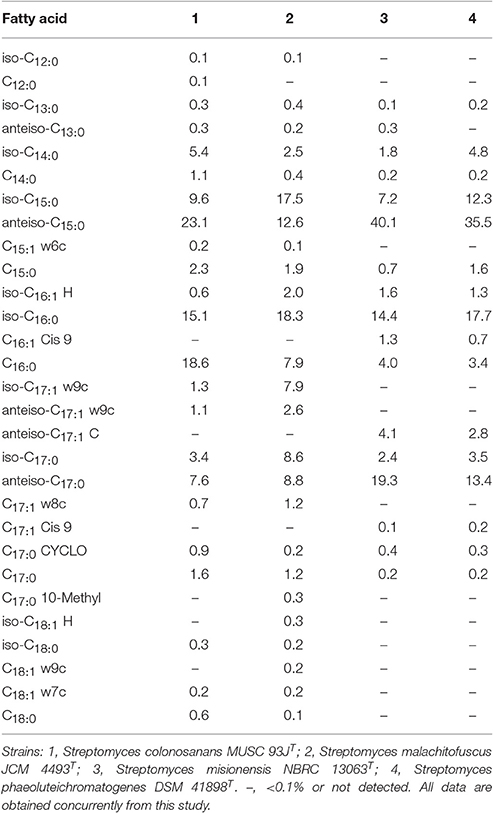
Table 1. Cellular fatty acid composition of strain MUSC 93JT and its closely related Streptomyces species.
Based on the results of the polar lipids analysis, strain MUSC 93JT showed the presence of aminolipid, diphosphatidylglycerol, lipid, phospholipid, phosphatidylinositol, phosphatidylethanolamine, and phosphoglycolipid. The differences in polar lipid profiles showed that MUSC 93JT is different from related type strains; for example, strain MUSC 93JT contain two aminolipids (Figure S3A), while Streptomyces malachitofuscus JCM 4493T only contain one aminolipids (Figure S3B).
The chemotaxonomic analyses also demonstrated that the cell wall of strain MUSC 93JT is of cell-wall type I as it contains LL-diaminopimelic (Lechevalier and Lechevalier, 1970). Many other species of the genus Streptomyces were also found to have LL-diaminopimelic (Lee et al., 2005, 2014d; Xu et al., 2009; Hu et al., 2012; Ser et al., 2015b,d). The predominant menaquinones of strain MUSC 93JT were identified as MK-9(H8) (42%) and MK-9(H6) (35%). This finding is in agreement with the report of the study conducted by Kim et al. (2003), in which the predominant menaquinones of Streptomyces are MK-9(H8) and MK-9(H6). The cell wall peptidoglycan was determined to contain LL-diaminopimelic acid. The whole cell sugars were found to be glucose, mannose, and ribose. The G + C content of strain MUSC 93JT was found to be 69.9 mol%; this is within the range of 67.0–78.0 mol% described for species of the genus Streptomyces (Kim et al., 2003).
Phenotypic Analyses of Strain MUSC 93JT
Strain MUSC 93JT exhibited good growth on ISP 2 agar, ISP 6 agar, and Streptomyces agar after 7 days at 28°C, moderate growth on starch casein agar, weak growth on nutrient agar, and no growth on actinomycetes isolation agar, ISP 3, ISP 4, ISP 5, and ISP 7 agar. The 15-day-old culture of strain MUSC 93JT formed light yellow aerial and vivid yellow substrate mycelium on ISP 2 agar (Table 2). These morphological characteristics are consistent with grouping of the strain to the genus Streptomyces (Williams, 1989). The NaCl tolerance, temperature ranges, and pH for growth of strain MUSC 93JT occurred at 0–4% (optimum 0–2%), 24–36°C (optimum 0–2%), and pH 6.0–7.0 (optimum pH 6.0), respectively. The cells of MUSC 93JT were positive for catalase and haemolytic activities. Hydrolysis of soluble starch, casein and tributyrin (lipase) were positive; but negative for hydrolysis of chitin, carboxymethylcellulose, and xylan. Based on a range of phenotypic properties, strain MUSC 93JT can be differentiated from closely related members of the genus Streptomyces (Table 2). In chemical sensitivity assays, cells are resistant to 1% sodium lactate, D-serine, rifamycin RV, minocycline, lincomycin, niaproof 4, tetrazolium violet, tetrazolium blue, nalidixic acid, potassium tellurite, aztreonam, sodium butyrate, and sodium bromate.
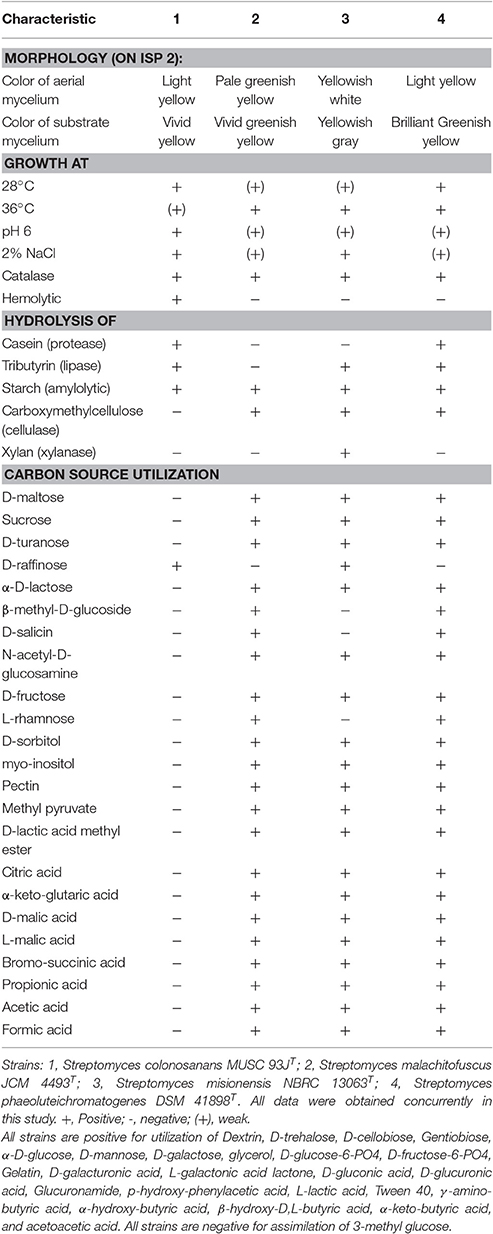
Table 2. Differentiation characteristics of strain MUSC 93JT and type strains of phylogenetically closely related species of the genus Streptomyces.
According to the outcomes of genomic, phylogenetic, chemotaxonomic and phenotypic analyses, strain MUSC 93JT merits assignment to a novel species in the genus Streptomyces, for which the name Streptomyces colonosanans sp. nov. is proposed.
Antioxidant Activity of Strain MUSC 93JT Extract
Oxygen free radicals, also known as reactive oxygen species (ROS) are products of a normal cellular metabolism process in an organism (Valko et al., 2006). Oxidative stress occurs when there is an overproduction free radicals and deficiency of antioxidants, resulting in the accumulation of free radicals (Valko et al., 2006; Reuter et al., 2010). This condition may cause damage to DNA, proteins, and lipids which has been associated with the development of age-related diseases such as cancer, arthritis, and neurodegenerative disorders in living organisms (Valko et al., 2006; Tan et al., 2015). For that reason, antioxidants are required as they may play an important role in preventing the deleterious effects of free radicals and thus they are regard as potential bioactive agents against cancers in human (Kawanishi et al., 2005; Reuter et al., 2010; Ser et al., 2016b). For example, a trial conducted by Blot et al. (1993) in China suggested that combination of antioxidants of beta carotene, vitamin E, and selenium may decrease the risk of gastric cancer. Besides, several studies and meta-analysis of the epidemiological literature have shown that increased intake of lycopene, a potent antioxidant present in tomatoes, is associated with reduced risk of prostate cancer (Gann et al., 1999; Giovannucci et al., 2002; Etminan et al., 2004) and gastric cancer (Yang et al., 2013).
In the early 1980s, the scientific community started to focus on the exploration of microbial antioxidants. Since then, researchers have discovered and characterized a variety of antioxidant compounds from microorganisms in hope to be developed into novel therapeutic agents (Hall, 2001). Due to the pathophysiological complexity of the human diseases, the bioprospecting activities in search for more effective and specific antioxidants from natural resources is still required. In this context, Streptomyces bacteria emerges as one of the good sources of natural compounds since they are prolific producers of bioactive secondary metabolites. Furthermore, several studies have reported the detection of compounds with antioxidant property extracted from Streptomyces spp., for instance, thiazostatin A and thiazostatin B (Shindo et al., 1989), diphenazithionin (Hosoya et al., 1996), dihydroherbimycin A (Chang and Kim, 2007) as well as 5-(2,4-dimethylbenzyl)pyrrolidin-2-one (Saurav and Kannabiran, 2012).
The present study showed that MUSC 93JT merits assignment to a novel species in the genus Streptomyces based on the polyphasic approach analyses. Since the strain MUSC 93JT is a novel Streptomyces species, it would be interesting to investigate the antioxidant potential of this strain. Hence, the strain was further examined for its antioxidant potential using ABTS, metal chelating, and SOD activity assays. According to the results of antioxidant assays, it can be observed that MUSC 93JT extract exhibited significant free radical scavenging activity (Table 3). In ABTS assay, ABTS radical cation was produced by the reaction between a strong oxidizing agent potassium persulfate with ABTS salt and the ability of antioxidant to scavenge the ABTS radical generated in the aqueous phase will be measured (Shalaby and Shanab, 2013). The results showed that MUSC 93JT extract was capable of scavenging 11.80 ± 3.75% of ABTS radicals at the highest test concentration of 2 mg/mL. Besides, MUSC 93JT extract exhibited metal chelating activity of 50.06 ± 1.95% at 2 mg/mL concentration. This indicated the antioxidative potential of MUSC 93JT extract through prevention of transition metals from enhancing the production of ROS (Ser et al., 2016b). In addition, the SOD assay also confirmed the antioxidant potential of MUSC 93JT extract. In SOD assay, the superoxide anion scavenging activity of this extract was determined by the 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST) reduction method. The superoxide anion radical produced from hypoxanthine-xanthine oxidase reaction reduces WST-1 to produce yellow formazan (Dudonné et al., 2009; Tan et al., 2015). MUSC 93JT extract exhibited superoxide dismutase (SOD)-like activity which may subsequently prevent the formation of yellow WST-1 formazan. The SOD-like activity of this extract was 83.32 ± 2.62% at the highest tested concentration of 2 mg/mL. All of these assays revealed significant antioxidant potential of MUSC 93JT extract and thus suggested the presence of antioxidant(s) in it.
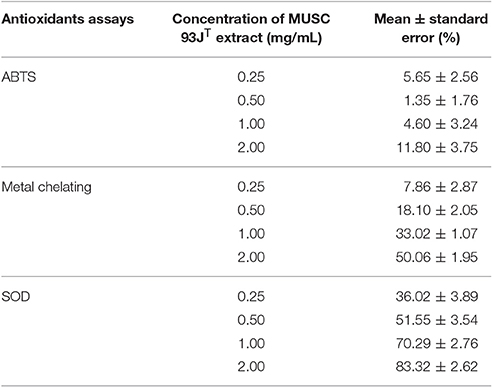
Table 3. Radical scavenging activity of MUSC 93JT evaluated using ABTS, metal chelating, and SOD assays.
Cytotoxic Activity of Strain MUSC 93JT Extract
In present study, cytotoxic potential of MUSC 93JT extract was examined on human colon cancer cell lines namely HCT-116, HT-29, Caco-2, and SW480 by using the MTT assay. MTT assay is a tetrazolium-based colorimetric assay which operates by measuring the mitochondrial activity in living cells only. The activity of mitochondrial dehydrogenase enzyme of viable cells will transform the MTT tetrazolium salt (yellow) to MTT formazan crystal (purple) (Gerlier and Thomasset, 1986; Ser et al., 2015a; Tan et al., 2015). The use of different type of human colon cancer cell lines with different molecular characteristics as the panels for this experimentation is to assess varying efficacy of cytotoxic activity toward different genetic makeup of cancer cell lines (Tan et al., 2015). The tested results of MUSC 93JT extract against tested colon cancer cell lines were shown in (Figure 3).
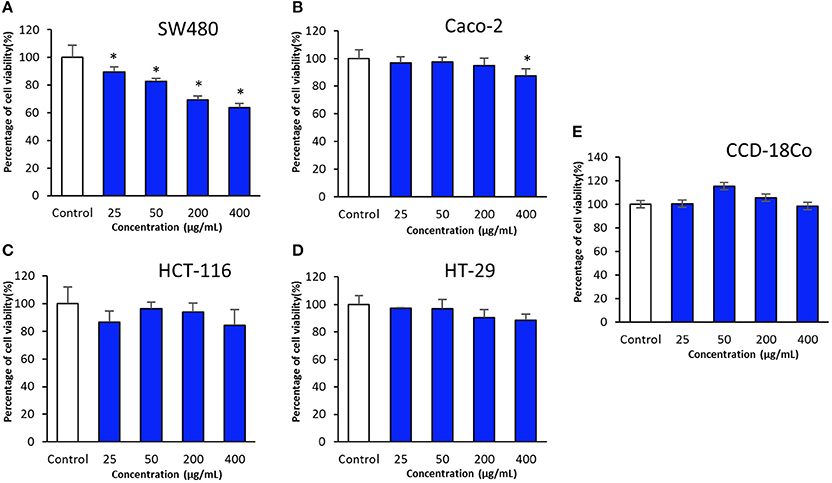
Figure 3. Cytotoxic activity of MUSC 93JT extract against human colon cancer and normal cell lines. The measurement of cell viability was done using MTT assay. The graphs show cytotoxicity effect of MUSC 93JT extract against (A) SW480, (B) Caco-2, (C) HCT-116, (D) HT-29, and (E) CCD-18Co. All data are expressed as mean ± standard deviation and significance level are set as 0.05. Symbol (*) indicates p < 0.05 significant difference between the cells treated with MUSC 93JT extract and control (without MUSC 93JT extract).
The results revealed that MUSC 93JT extract showed varying levels of cytotoxicity against HCT-116, HT-29, Caco-2, and SW480. It was found that the extract exhibited highest cytotoxic effect on SW480 with cell viability recorded at 63.6 ± 3.0% when tested with highest concentration of 400 μg/mL, followed by HCT-116 with cell viability 84.3 ± 11.5% (at concentration 400 μg/mL), then Caco-2 with cell viability 87.5 ± 5.3% (at concentration 400 μg/mL), and lastly the lowest cytotoxic effect on HT-29 with cell viability 88.4 ± 4.4% (at concentration 400 μg/mL; Figure 3). Furthermore, it can be observed that there was a dose dependent effect when the extract was tested against SW480 cells. SW480 cells were significantly inhibited (p < 0.05) by increased concentration of the extract. As for the human normal colon CCD-18Co cells, no significant cytotoxic effect was exerted by MUSC 93JT extract against these cells (Figure 3). Overall, the results suggested that MUSC 93JT extract is more cytotoxic toward the colon cancer cells lines than the normal colon cells with particularly stronger cytotoxic activity against colon cancer cell line SW480.
GC-MS Analysis of Strain MUSC 93JT Extract
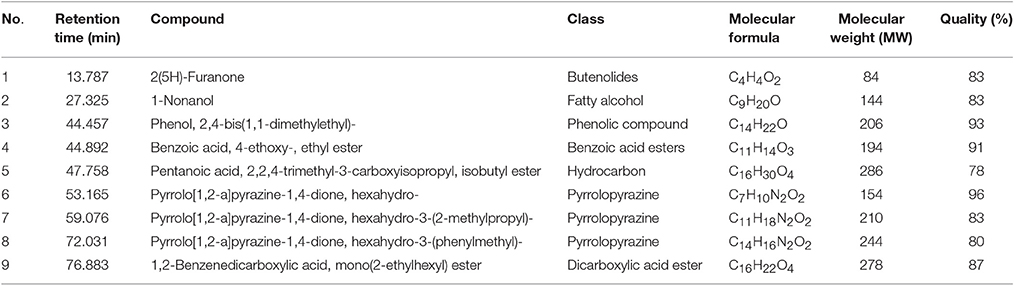
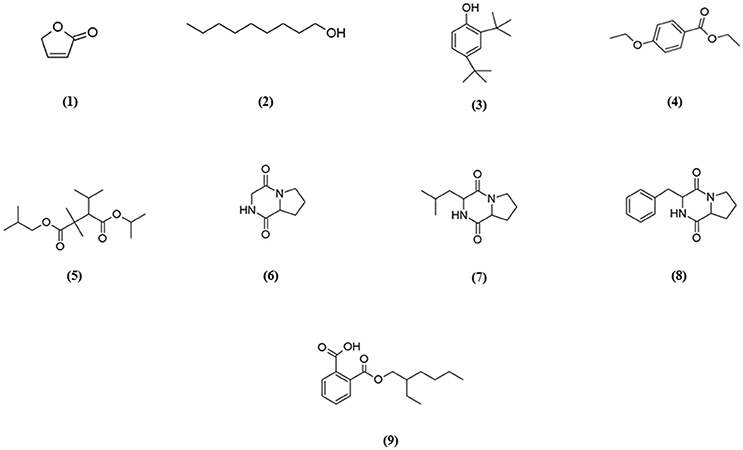
GC-MS analysis was conducted to aid in chemical profiling and to identify compounds that present in the extract. The results of GC-MS analysis revealed that strain MUSC 93JT extract contains nine compounds (Table 4): 2(5H)-Furanone (1), 1-Nonanol (2), Phenol, 2,4-bis (1,1-dimethylethyl)-(3), Benzoic acid, 4-ethoxy-, ethyl ester (4), Pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl, isobutyl ester (5), Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- (6), Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- (7), Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- (8), 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester (9) with chemical structures as shown in Figure 4. From this analysis, butenolides, fatty alcohol, phenolic, benzoic acid esters, hydrocarbon, pyrrolopyrazine, and dicarboxylic acid ester were the main classes of compounds present in strain MUSC 93JT extract.
Pyrrolopyrazines are capable of exerting a variety of bioactivities such as antioxidant, antitumor, anti-angiogenesis, and antimicrobial (Ser et al., 2015a,b). According to the GC-MS analysis, three pyrrolopyrazine compounds were detected in the MUSC 93JT extract, including Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- (6), Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- (7), and Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- (8). In previous studies, pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- (6) was reported to be present in Streptomyces mangrovisoli, a novel Streptomyces species isolated from mangrove forest in Malaysia by Ser et al. (2015b) and it was suggested that this compound may be responsible for the antioxidant activity of this species. In addition, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- (6) was also found in Streptomyces pluripotens and Bacillus sp. with the capability to reduce oxidative damages by free radicals (Gopi et al., 2014; Ser et al., 2015a). Moreover, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- (7) was detected in Streptomyces sp. VITMK1 isolated from India mangrove soil by Manimaran et al. (2015). Mithun and Rao (2012) also reported the detection of Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- (7) in Micrococcus luteus with promising anticancer activity on HCT15. Also, all three pyrrolopyrazine compounds: Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- (6), Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- (7), and Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- (8) were detected in Streptomyces sp. MUM 256 isolated from mangrove forest in Malaysia by Tan et al. (2015) which exhibited antioxidant and anticancer activities that could be due to the presence of these pyrrolopyrazines. Hence, the antioxidant and cytotoxic activities exhibited by MUSC 93JT extract could be mainly due to the presence of these pyrrolopyrazine compounds.
Besides, phenolic compounds have been regard as important antioxidant agents responsible in scavenging ROS (Narendhran et al., 2014). Phenol, 2,4-bis(1,1-dimethylethyl)- (3) was the phenolic compound detected in strain MUSC 93JT extract. Some of the recent studies have demonstrated the presence of phenol, 2,4-bis(1,1-dimethylethyl)- in Streptomyces bacteria through GC-MS analysis. For instance, Narendhran et al. (2014) successfully detected phenol, 2,4-bis(1,1-dimethylethyl)- in Streptomyces cavouresis KUV39 isolated from vermicompost samples collected in India. The study also presented that this compound could be responsible for the antioxidant and cytotoxic properties of Streptomyces cavouresis KUV39. Besides, phenol, 2,4-bis(1,1-dimethylethyl)- was also detected in Streptomyces sp. MUM256 with potential antioxidant activity in the study conducted by Tan et al. (2015). A recently discovered novel Streptomyces antioxidans by Ser et al. (2016c) reported the detection of phenol, 2,4-bis(1,1-dimethylethyl)- was detected in the extract, which may had contributed to the strain's free radical scavenging activities.
The compound 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester (9) detected in MUSC 93JT extract has been previously reported to possess potential antibacterial, antifungal, and cytotoxic activities (Saxena et al., 2015; Tan et al., 2015). This compound was also detected in other Streptomyces sp., with cytotoxic activity against several cancer cell lines such as liver cancer cell line HepG2 and breast cancer cell line MCF7 (Krishnan et al., 2014).
Lastly, other detected compounds in MUSC 93JT including 2(5H)-Furanone (1), 1-Nonanol (2), Benzoic acid, 4-ethoxy-, ethyl ester (4), and Pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl, isobutyl ester (5) were also previously found to be present in microbes. For instance, 2(5H)-Furanone was detected in Lactobacillus helveticus (Ndagijimana et al., 2006), 1-Nonanol was detected in Streptomyces albidoflavus (Sunesson et al., 1997), Benzoic acid, 4-ethoxy-, ethyl ester was detected in Bacillus sp. (Mishra and Thakur, 2016), and Pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl, isobutyl ester was detected in Aspergillus carbonarius ITEM 5010 (Sinha et al., 2015).
Overall, most of the chemical compounds identified by GC-MS are well-known for their antioxidant and anticancer activities. Therefore, we propose that these compounds, either alone or in combination, might be the main contributing factors for the antioxidant and cytotoxic properties present in MUSC 93JT extract.
Description of Streptomyces colonosanans sp. nov.
Streptomyces colonosanans (co.lo.no.sa'nans. Gr. n. kolon, intestine, colon; L. part. adj. sanans, healing; N.L. part. adj. colonosanans, colon-healing).
Cells stain Gram-positive, forming light yellow aerial and vivid yellow substrate mycelium on ISP 2 agar. The colors of the aerial and substrate mycelium are media-dependent (Table S1). Cells grow well on ISP 2 agar, ISP 6 agar, and Streptomyces agar after 7 days at 28°C, cells grow moderately on starch casein agar, and cells grow weakly on nutrient agar and did not grow on actinomycetes isolation agar, ISP 3, ISP 4, ISP 5, and ISP 7 agar. Cells grow at 0–4% NaCl tolerance (optimum 0–2%), 24–36°C (optimum 28–32°C), at pH 6.0–7.0 (optimum pH 6.0). Cells are positive for catalase and hemolytic activities. Hydrolysis of soluble starch, casein and tributyrin (lipase) are positive; but negative for hydrolysis of chitin carboxymethylcellulose and xylan.
The following compounds are utilized as sole carbon sources: α-D-glucose, α-hydroxy-butyric acid, β-hydroxyl-D,L-butyric acid, D-cellobiose, dextrin, D-fructose-6-phosphate, L-fucose, D-galactose, D-galacturonic acid, D-glucose-6-phosphate, D-gluconic acid, D-glucuronic acid, D-mannose, D-melibiose, D-raffinose, D-trehalose, gelatin, gentiobiose, glucuronamide, glycerol, L-galactonic acid lactone, L-lactic acid, p-hydroxy-phenylacetic acid, Tween 40, γ-amino-butyric acid, acetoacetic acid, and α-keto-butyric acid. The following compounds are not utilized as sole carbon sources: acetic acid, α-D-lactose, α-keto-glutaric acid, β-methyl-D-glucoside, bromo-succinic acid, citric acid, D-arabitol, D-aspartic acid, D-fructose, D-fucose, D-lactic acid methyl ester, D-malic acid, D-maltose, D-mannitol, D-saccharic acid, D-salicin, D-serine, D-sorbitol, D-turanose, formic acid, glycyl-L-proline, inosine, L-malic acid, L-rhamnose, methyl pyruvate, mucic acid, N-acetyl-β-D-mannosamine, N-acetyl-D-galactosamine, N-acetyl-D-glucosamine, N-acetyl-neuraminic acid, pectin, propionic acid, quinic acid, stachyose, sucrose, myo-inositol, and 3-methyl glucose. The following compounds are not utilized as sole nitrogen sources: L-alanine, L-arginine, L-aspartic acid, L-glutamic acid, L-histidine, L-pyroglutamic acid, and L-serine.
The cell wall peptidoglycan contains LL-diaminopimelic acid. The predominant menaquinones were identified as MK-9(H8) and MK-9(H6). The whole cell sugars are glucose, mannose and ribose. The polar lipids consist of aminolipid, diphosphatidylglycerol, lipid, phospholipid, phosphatidylinositol, phosphatidylethanolamine, and phosphoglycolipid. The predominant cellular fatty acids (>10.0%) are anteiso-C15:0, C16:0, and iso-C16:0.
The type strain is MUSC 93JT (= DSM 102042T = MCCC 1K02298T), isolated from mangrove soil collected from mangrove forest located in Kuching, state of Sarawak, Malaysia. The 16S rRNA gene sequence of strain MUSC 93JT has been deposited in GenBank/EMBL/DDBJ under the accession number KP998434. The genome of MUSC 93JT consists of 7,015,076 bp with average coverage of 53.0-fold and the G + C content of the genomic DNA of the type strain is 69.90 mol%. The whole project of MUSC 93JT has been deposited at DDBJ/EMBL/GenBank under accession number MLYP00000000. A total of 5,859 coding genes was predicted and assigned to 402 subsystems, along with 66 tRNA and 5 RNA genes (Table 5). Based on RAST annotation, most of the genes were involved in amino acids and derivatives metabolism (9.18%), followed by carbohydrates metabolism (6.21%) and protein metabolism subsystems (4.91%).
Conclusion
This study has revealed that strain MUSC 93JT is a novel species of the genus Streptomyces, isolated from the soil of mangrove forest located in Kuching, Sarawak. The name Streptomyces colonosanans sp. nov. is proposed and the type strain is MUSC 93JT (= DSM 102042T = MCCC 1K02298T). This study revealed that the extract of strain MUSC 93JT has significant antioxidant potential with radical scavenging activity up to 83.32 ± 2.62% via SOD assay. Additionally, this strain exhibited cytotoxic activity against several human colon cancer cell lines, with highest cytotoxic effect on SW480 with cell viability of 63.6 ± 3.0%. Chemical analysis via GC-MS further confirms that the strain is capable of producing chemo-preventive related metabolites. Hence, this study demonstrated the biopharmaceutical potential of novel strain Streptomyces colonosanans MUSC 93JT with capability to produce bioactive compounds responsible for the antioxidant and cytotoxic activities. Strain MUSC 93JT serves as a potentially high quality resource for drug discovery and further studies pertaining the development of chemo-preventive drugs from this strain are highly valuable.
Author Contributions
The experiments, data analysis, and manuscript writing were performed by JL and HS, while AD, SS, SIB, TK, NM, K-GC, BG, and LL provided vital guidance, insight and technical support for the completion of the project. L-HL and BG founded the research project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by MOSTI ScienceFund Grant (Project No. 06-02-10-SF0300) and External Industry Grants from Biotek Abadi Sdn Bhd (vote no. GBA-808138 and GBA-808813) awarded to L-HL, University of Malaya for High Impact Research Grant (UM-MOHE HIR Nature Microbiome Grant No. H-50001-A000027 and No. A000001-50001) awarded to K-GC, and supported by a grant from “Research Center of the Female Scientific and Medical Colleges,” Deanship of Scientific Research, King Saud University awarded to SIB. The authors are thankful to Professor Bernhard Schink for the support in the Latin etymology of the new species name.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.00877/full#supplementary-material
References
Angiuoli, S. V., Gussman, A., Klimke, W., Cochrane, G., Field, D., Garrity, G. M., et al. (2008). Toward an online repository of Standard Operating Procedures (SOPs) for (meta) genomic annotation. OMICS 12, 137–141. doi: 10.1089/omi.2008.0017
Arumugam, M., Mitra, A., Pramanik, A., Saha, M., Gachhui, R., and Mukherjee, J. (2011). Streptomyces sundarbansensis sp. nov., an actinomycete that produces 2-allyloxyphenol. Int. J. Syst. Evol. Microbiol. 61, 2664–2669. doi: 10.1099/ijs.0.028258-0
Ashton, E. C., and Macintosh, D. J. (2002). Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. For. Ecol. Manage. 166, 111–129. doi: 10.1016/S0378-1127(01)00673-9
Aziz, R. K., Bartels, D., Best, A. A., Dejongh, M., Disz, T., Edwards, R. A., et al. (2008). The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75
Azman, A.-S., Othman, I. S., Velu, S., Chan, K.-G., and Lee, L.-H. (2015). Mangrove rare actinobacteria: taxonomy, natural compound, and discovery of bioactivity. Front. Microbiol. 6:856. doi: 10.3389/fmicb.2015.00856
Blot, W. J., Li, J.-Y., Taylor, P. R., Guo, W., Dawsey, S., Wang, G.-Q., et al. (1993). Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Nat. Cancer Inst. 85, 1483–1491. doi: 10.1093/jnci/85.18.1483
Burja, A. M., Banaigs, B., Abou-Mansour, E., Burgess, J. G., and Wright, P. C. (2001). Marine cyanobacteria—a prolific source of natural products. Tetrahedron 57, 9347–9377. doi: 10.1016/S0040-4020(01)00931-0
Carrillo, P., Mardaraz, C., Pitta-Alvarez, S., and Giulietti, A. (1996). Isolation and selection of biosurfactant-producing bacteria. World J. Microbiol. Biotechnol. 12, 82–84. doi: 10.1007/BF00327807
Cashion, P., Holder-Franklin, M., Mccully, J., and Franklin, M. (1977). A rapid method for the base ratio determination of bacterial DNA. Anal. Biochem. 81, 461–466. doi: 10.1016/0003-2697(77)90720-5
Cerny, G. (1978). Studies on the aminopeptidase test for the distinction of gram-negative from gram-positive bacteria. Euro. J. Appl. Microbiol. Biotechnol. 5, 113–122. doi: 10.1007/BF00498805
Chang, H. B., and Kim, J.-H. (2007). Antioxidant properties of dihydroherbimycin A from a newly isolated Streptomyces sp. Biotechnol. Lett. 29, 599–603. doi: 10.1007/s10529-006-9288-z
Chin, Y.-W., Balunas, M. J., Chai, H. B., and Kinghorn, A. D. (2006). Drug discovery from natural sources. AAPS J. 8, E239–E253. doi: 10.1007/BF02854894
De Ley, J., Cattoir, H., and Reynaerts, A. (1970). The quantitative measurement of DNA hybridization from renaturation rates. Euro. J. Biochem. 12, 133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x
Dudonné, S., Vitrac, X., Coutiere, P., Woillez, M., and MéRillon, J.-M. (2009). Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 57, 1768–1774. doi: 10.1021/jf803011r
Etminan, M., Takkouche, B., and Caamaño-Isorna, F. (2004). The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol. Biomarkers Prev. 13, 340–345.
Felsenstein, J. (1981). Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17, 368–376. doi: 10.1007/BF01734359
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791. doi: 10.2307/2408678
Gann, P. H., Ma, J., Giovannucci, E., Willett, W., Sacks, F. M., Hennekens, C. H., et al. (1999). Lower prostate cancer risk in men with elevated plasma lycopene levels. Cancer Res. 59, 1225–1230.
Gerlier, D., and Thomasset, N. (1986). Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 94, 57–63. doi: 10.1016/0022-1759(86)90215-2
Giovannucci, E., Rimm, E. B., Liu, Y., Stampfer, M. J., and Willett, W. C. (2002). A prospective study of tomato products, lycopene, and prostate cancer risk. J. Nat. Cancer Inst. 94, 391–398. doi: 10.1093/jnci/94.5.391
Giri, C., Ochieng, E., Tieszen, L. L., Zhu, Z., Singh, A., Loveland, T., et al. (2011). Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecol. Biogeogr. 20, 154–159. doi: 10.1111/j.1466-8238.2010.00584.x
Gopi, M., Dhayanithi, N. B., Devi, K. N., and Kumar, T. T. A. (2014). Marine natural product, Pyrrolo [1,2-a] pyrazine-1,4-dione, hexahydro-(C7H10N2O2) of antioxidant properties from Bacillus species at Lakshadweep archipelago. J. Coast. Life Med. 2, 632–637. doi: 10.12980/JCLM.2.201414J40
Hall, C. (2001). “Sources of natural antioxidants: oilseeds, nuts, cereals, legumes, animal products and microbial sources,” in Antioxidants Food, eds J. Pokorný, N. Yanishlieva, and M. Gordon (Cambridge: Woodhead Publishing Ltd.), 180–189. doi: 10.1201/9781439823057.ch9
Hong, K., Gao, A.-H., Xie, Q.-Y., Gao, H. G., Zhuang, L., Lin, H.-P., et al. (2009). Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 7, 24–44. doi: 10.3390/md7010024
Hosoya, Y., Adachi, H., Nakamura, H., Nishimura, Y., Naganawa, H., Okami, Y., et al. (1996). The structure of diphenazithionin, a novel antioxidant from Streptomyces griseus ISP 5236. Tetrahedron Lett. 37, 9227–9228. doi: 10.1016/S0040-4039(96)02190-9
Hu, H., Lin, H.-P., Xie, Q., Li, L., Xie, X.-Q., and Hong, K. (2012). Streptomyces qinglanensis sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 62, 596–600. doi: 10.1099/ijs.0.032201-0
Huss, V. A., Festl, H., and Schleifer, K. H. (1983). Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst. Appl. Microbiol. 4, 184–192. doi: 10.1016/S0723-2020(83)80048-4
Hyatt, D., Chen, G.-L., Locascio, P. F., Land, M. L., Larimer, F. W., and Hauser, L. J. (2010). Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119
Karikas, G. (2010). Anticancer and chemopreventing natural products: some biochemical and therapeutic aspects. J. BUON 15, 627–638.
Kates, M. (1986). “Lipid extraction procedures,” in Techniques of Lipidology (Amsterdam: Elsevier), 106–107.
Kato, S., Kawai, H., Kawasaki, T., Toda, Y., Urata, T., and Hayakawa, Y. (1989). Studies on free radical scavenging substances from microorganisms. I. Carazostatin, a new free radical scavenger produced by Streptomyces chromofuscus DC 118. J. Antibiot. 42, 1879–1881. doi: 10.7164/antibiotics.42.1879
Kawanishi, S., Oikawa, S., and Murata, M. (2005). Evaluation for safety of antioxidant chemopreventive agents. Antioxid. Redox Signal. 7, 1728–1739. doi: 10.1089/ars.2005.7.1728
Kim, O.-S., Cho, Y.-J., Lee, K., Yoon, S.-H., Kim, M., Na, H., et al. (2012). Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 62, 716–721. doi: 10.1099/ijs.0.038075-0
Kim, S. B., Lonsdale, J., Seong, C.-N., and Goodfellow, M. (2003). Streptacidiphilus gen. nov., acidophilic actinomycetes with wall chemotype I and emendation of the family Streptomycetaceae (Waksman and Henrici (1943) AL) emend. Rainey et al. 1997. Antonie van Leeuwenhoek 83, 107–116. doi: 10.1023/A:1023397724023
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120. doi: 10.1007/BF01731581
Kinkel, L. L., Schlatter, D. C., Xiao, K., and Baines, A. D. (2014). Sympatric inhibition and niche differentiation suggest alternative coevolutionary trajectories among Streptomycetes. ISME J. 8, 249–256. doi: 10.1038/ismej.2013.175
Kino, T., Hatanaka, H., Miyata, S., Inamura, N., Nishiyama, M., Yajima, T., et al. (1987). FK-506, a novel immunosuppressant isolated from a Streptomyces. J. Antibiot. 40, 1256–1265. doi: 10.7164/antibiotics.40.1256
Krishnan, K., Mani, A., and Jasmine, S. (2014). Cytotoxic activity of bioactive compound 1, 2-benzene dicarboxylic acid, mono 2-ethylhexyl ester extracted from a marine derived Streptomyces sp. VITSJK8. Int. J. Mol. Cell. Med. 3, 246.
Küster, E., and Williams, S. (1964). Media for the isolation of streptomycetes: starch casein medium. Nature 202, 928–929. doi: 10.1038/202928a0
Lagesen, K., Hallin, P., Rødland, E. A., Stærfeldt, H.-H., Rognes, T., and Ussery, D. W. (2007). RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35, 3100–3108. doi: 10.1093/nar/gkm160
Law, J. W.-F., Ser, H.-L., Khan, T. M., Chuah, L.-H., Pusparajah, P., Chan, K.-G., et al. (2017). The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 8:3. doi: 10.3389/fmicb.2017.00003
Lechevalier, M. P., and Lechevalier, H. (1970). Chemical composition as a criterion in the classification of aerobic actinomycetes. Int. J. Syst. Evol. Microbiol. 20, 435–443. doi: 10.1099/00207713-20-4-435
Lee, J. Y., Lee, J. Y., Jung, H. W., and Hwang, B. K. (2005). Streptomyces koyangensis sp. nov., a novel actinomycete that produces 4-phenyl-3-butenoic acid. Int. J. Syst. Evol. Microbiol. 55, 257–262. doi: 10.1099/ijs.0.63168-0
Lee, L.-H., Azman, A.-S., Zainal, N., Eng, S.-K., Ab Mutalib, N.-S., Yin, W.-F., et al. (2014a). Microbacterium mangrovi sp. nov., an amylolytic actinobacterium isolated from mangrove forest soil. Int. J. Syst. Evol. Microbiol. 64, 3513–3519. doi: 10.1099/ijs.0.062414-0
Lee, L.-H., Azman, A.-S., Zainal, N., Eng, S.-K., Fang, C.-M., Hong, K., et al. (2014b). Novosphingobium malaysiense sp. nov. isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 64, 1194–1201. doi: 10.1099/ijs.0.059014-0
Lee, L.-H., Cheah, Y.-K., Sidik, S. M., Ab Mutalib, N.-S., Tang, Y.-L., Lin, H.-P., et al. (2012). Molecular characterization of Antarctic actinobacteria and screening for antimicrobial metabolite production. World J. Microbiol. Biotechnol. 28, 2125–2137. doi: 10.1007/s11274-012-1018-1
Lee, L.-H., Zainal, N., Azman, A.-S., Ab Mutalib, N.-S., Hong, K., and Chan, K.-G. (2014c). Mumia flava gen. nov., sp. nov., an actinobacterium of the family Nocardioidaceae. Int. J. Syst. Evol. Microbiol. 64, 1461–1467. doi: 10.1099/ijs.0.058701-0
Lee, L.-H., Zainal, N., Azman, A.-S., Eng, S.-K., Ab Mutalib, N.-S., Yin, W.-F., et al. (2014d). Streptomyces pluripotens sp. nov., a bacteriocin-producing streptomycete that inhibits meticillin-resistant Staphylococcus aureus. Int. J. Syst. Evol. Microbiol. 64, 3297–3306. doi: 10.1099/ijs.0.065045-0
Lee, L.-H., Zainal, N., Azman, A.-S., Eng, S.-K., Goh, B.-H., Yin, W.-F., et al. (2014e). Diversity and antimicrobial activities of actinobacteria isolated from tropical mangrove sediments in Malaysia. Sci. World J. 2014:698178. doi: 10.1155/2014/698178
Lowe, T. M., and Eddy, S. R. (1997). tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 955–964. doi: 10.1093/nar/25.5.0955
Macfaddin, J. E. (2000). Biochemical Tests for Identification of Medical Bacteria, eds. J. E. Macfaddin (Philadelphia, PA: Lippincott Williams & Wilkins).
Mangamuri, U. K., Muvva, V., Poda, S., and Kamma, S. (2012). Isolation, Identification and molecular characterization of rare actinomycetes from mangrove ecosystem of nizampatnam. Malays. J. Microbiol. 8, 83–91. doi: 10.21161/mjm.03212
Manimaran, M., Gopal, J. V., and Kannabiran, K. (2015). Antibacterial activity of Streptomyces sp. VITMK1 isolated from mangrove soil of Pichavaram, Tamil Nadu, India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 1–8. doi: 10.1007/s40011-015-0619-5
Manivasagan, P., Venkatesan, J., Sivakumar, K., and Kim, S.-K. (2013). Production, characterization and antioxidant potential of protease from Streptomyces sp. MAB18 using poultry wastes. BioMed. Res. Int. 2013:496586. doi: 10.1155/2013/496586.
Mesbah, M., Premachandran, U., and Whitman, W. B. (1989). Precise measurement of the G+ C content of deoxyribonucleic acid by high-performance liquid chromatography. Int. J. Syst. Evol. Microbiol. 39, 159–167. doi: 10.1099/00207713-39-2-159
Miser-Salihoglu, E., Akaydin, G., Caliskan-Can, E., and Yardim-Akaydin, S. (2013). Evalution of antioxidant activity of various herbal folk medicines. Nutr. Food Sci. 3:222. doi: 10.4172/2155-9600.1000222
Mishra, M., and Thakur, I. S. (2016). 16S r-DNA based denaturing gradient gel electrophoresis method for evaluation of survival of lignin degrading bacteria Bacillus Sp. in soil microcosms. J. Chem. Biol. Phys. Sci. 6, 396.
Mithun, V. S. L., and Rao, C. S. V. (2012). Isolation and molecular characterization of anti-cancerous compound producing marine bacteria by using 16S rRNA sequencing and GC-MS techniques. IJMER 2, 4510–4515.
Mo, C.-J., Shin-Ya, K., Furihata, K., Furihata, K., Shimazu, A., Hayakawa, Y., et al. (1990). Isolation and structural elucidation of antioxidative agents, antiostatins A1 to A4 and B2 to B5. J. Antibiot. 43, 1337–1340. doi: 10.7164/antibiotics.43.1337
Narendhran, S., Rajiv, P., Vanathi, P., and Sivaraj, R. (2014). Spectroscopic analysis of bioactive compounds from Streptomyces cavouresis kuv39: Evaluation of antioxidant and cytotoxicity activity. Int. J. Pharm. Pharm. Sci. 6, 319–322. Available online at: https://innovareacademics.in/journals/index.php/ijpps/article/viewFile/2009/911
Ndagijimana, M., Vallicelli, M., Cocconcelli, P. S., Cappa, F., Patrignani, F., Lanciotti, R., et al. (2006). Two 2 [5H]-furanones as possible signaling molecules in Lactobacillus helveticus. Appl. Environ. Microbiol. 72, 6053–6061. doi: 10.1128/AEM.00363-06
Nobili, S., Lippi, D., Witort, E., Donnini, M., Bausi, L., Mini, E., et al. (2009). Natural compounds for cancer treatment and prevention. Pharmacol. Res. 59, 365–378. doi: 10.1016/j.phrs.2009.01.017
Rashad, F. M., Fathy, H. M., El-Zayat, A. S., and Elghonaimy, A. M. (2015). Isolation and characterization of multifunctional Streptomyces species with antimicrobial, nematicidal and phytohormone activities from marine environments in Egypt. Microbiol. Res. 175, 34–47. doi: 10.1016/j.micres.2015.03.002
Reuter, S., Gupta, S. C., Chaturvedi, M. M., and Aggarwal, B. B. (2010). Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49, 1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006
Saitou, N., and Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425.
Sasser, M. (1990). Identification of bacteria by gas chromatography of cellular fatty acids. MIDI Technical Note 101. (Newark, DE: Microbial ID Inc.). Available online at: http://www.microbialid.com/PDF/TechNote_101.pdf
Satheeja, S. V., and Jebakumar, S. R. (2011). Phylogenetic analysis and antimicrobial activities of Streptomyces isolates from mangrove sediment. J. Basic Microbiol. 51, 71–79. doi: 10.1002/jobm.201000107
Saurav, K., and Kannabiran, K. (2012). Cytotoxicity and antioxidant activity of 5-(2, 4-dimethylbenzyl) pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp. Saudi J. Biol. Sci. 19, 81–86. doi: 10.1016/j.sjbs.2011.07.003
Saxena, S., Meshram, V., and Kapoor, N. (2015). Muscodor tigerii sp. nov.-Volatile antibiotic producing endophytic fungus from the Northeastern Himalayas. Ann. Microbiol. 65, 47–57. doi: 10.1007/s13213-014-0834-y
Schumann, P. (2011). 5- Peptidoglycan Structure. Method. Microbiol. 38, 101–129. doi: 10.1016/B978-0-12-387730-7.00005-X
Ser, H.-L., Ab Mutalib, N.-S., Yin, W.-F., Chan, K.-G., Goh, B.-H., and Lee, L.-H. (2015a). Evaluation of antioxidative and cytotoxic activities of Streptomyces pluripotens MUSC 137 isolated from mangrove soil in Malaysia. Front. Microbiol. 6:1398. doi: 10.3389/fmicb.2015.01398
Ser, H.-L., Law, J. W.-F., Chaiyakunapruk, N., Jacob, S. A., Palanisamy, U. D., Chan, K.-G., et al. (2016a). Fermentation conditions that affect clavulanic acid production in Streptomyces clavuligerus: a systematic review. Front. Microbiol. 7:522. doi: 10.3389/fmicb.2016.00522
Ser, H.-L., Palanisamy, U. D., Yin, W.-F., Chan, K.-G., Goh, B.-H., and Lee, L.-H. (2016b). Streptomyces malaysiense sp. nov.: a novel Malaysian mangrove soil actinobacterium with antioxidative activity and cytotoxic potential against human cancer cell lines. Sci. Rep. 6:24247. doi: 10.1038/srep24247
Ser, H.-L., Palanisamy, U. D., Yin, W.-F., Malek, S. N. A., Chan, K.-G., Goh, B.-H., et al. (2015b). Presence of antioxidative agent, Pyrrolo [1, 2-a] pyrazine-1, 4-dione, hexahydro-in newly isolated Streptomyces mangrovisoli sp. nov. Front. Microbiol. 6:854. doi: 10.3389/fmicb.2015.00854
Ser, H.-L., Tan, L. T.-H., Palanisamy, U. D., Malek, S. N. A., Yin, W.-F., Chan, K.-G., et al. (2016c). Streptomyces antioxidans sp. nov., a novel mangrove soil actinobacterium with antioxidative and neuroprotective potentials. Front. Microbiol. 7:899. doi: 10.3389/fmicb.2016.00899
Ser, H.-L., Tan, W.-S., Ab Mutalib, N.-S., Cheng, H.-J., Yin, W.-F., Chan, K.-G., et al. (2015c). Genome sequence of Streptomyces pluripotens MUSC 135 T exhibiting antibacterial and antioxidant activity. Mar. Gen. 24, 281–283. doi: 10.1016/j.margen.2015.09.010
Ser, H.-L., Tan, W.-S., Ab Mutalib, N.-S., Yin, W.-F., Chan, K.-G., Goh, B.-H., et al. (2016d). Draft genome sequence of mangrove-derived Streptomyces sp. MUSC 125 with antioxidant potential. Front. Microbiol. 7:1470. doi: 10.3389/fmicb.2016.01470
Ser, H.-L., Zainal, N., Palanisamy, U. D., Goh, B.-H., Yin, W.-F., Chan, K.-G., et al. (2015d). Streptomyces gilvigriseus sp. nov., a novel actinobacterium isolated from mangrove forest soil. Antonie van Leeuwenhoek 107, 1369–1378. doi: 10.1007/s10482-015-0431-5
Shalaby, E. A., and Shanab, S. M. (2013). Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 42, 556–564. Available online at: http://nopr.niscair.res.in/bitstream/123456789/24794/1/IJMS%2042(5)%20556-564.pdf
Sharma, S., and Shah, G. (2014). Isolation and screening of actinomycetes for bioactive compounds from the marine coast of South-Gujarat region. Int. J. Res. Sci. Innov. 1, 345–349. Available online at: http://www.rsisinternational.org/Issue7/345-349.pdf
Shindo, K., Takenaka, A., Noguchi, T., Hayakawa, Y., and Seto, H. (1989). Thiazostatin A and thiazostatin B. new antioxidants produced by Streptomyces tolurosus. J. Antibiot. 42, 1526–1529. doi: 10.7164/antibiotics.42.1526
Shin-ya, K., Furihata, K., Hayakawa, Y., Seto, H., Kato, Y., and Clardy, J. (1991). The structure of benthocyanin A. A new free radical scavenger of microbial origin. Tetrahedron Lett. 32, 943–946. doi: 10.1016/S0040-4039(00)92126-9
Shinya, K., Furihata, K., Teshima, Y., Hayakawa, Y., and Seto, H. (1993). Benthocyanins B and C, new free radical scavengers from Streptomyces prunicolor. J. Org. Chem. 58, 4170–4172. doi: 10.1021/jo00067a069
Shirling, E. T., and Gottlieb, D. (1966). Methods for characterization of Streptomyces species. Int. J. Syst. Evol. Microbiol. 16, 313–340. doi: 10.1099/00207713-16-3-313
Siegel, R. L., Miller, K. D., and Jemal, A. (2016). Cancer statistics, 2016. Cancer J. Clin. 66, 7–30. doi: 10.3322/caac.21332
Sinha, M., Sørensen, A., Ahamed, A., and Ahring, B. K. (2015). Production of hydrocarbons by Aspergillus carbonarius ITEM 5010. Fungal Biol. 119, 274–282. doi: 10.1016/j.funbio.2015.01.001
Staneck, J. L., and Roberts, G. D. (1974). Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl. Microbiol. 28, 226–231.
Sui, J.-L., Xu, X.-X., Qu, Z., Wang, H.-L., Lin, H.-P., Xie, Q.-Y., et al. (2011). Streptomyces sanyensis sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 61, 1632–1637. doi: 10.1099/ijs.0.023515-0
Sunesson, A.-L., Nilsson, C.-A., Carlson, R., Blomquist, G., and Andersson, B. (1997). Production of volatile metabolites from Streptomyces albidoflavus cultivated on gypsum board and tryptone glucose extract agar—influence of temperature, oxygen and carbon dioxide levels. Annal. Occup. Hyg. 41, 393–413. doi: 10.1016/S0003-4878(96)00046-4
Supriady, H., Kamarudin, M. N. A., Chan, C. K., Goh, B. H., and Kadir, H. A. (2015). SMEAF attenuates the production of pro-inflammatory mediators through the inactivation of Akt-dependent NF-κB, p38 and ERK1/2 pathways in LPS-stimulated BV-2 microglial cells. J. Funct. Foods 17, 434–448. doi: 10.1016/j.jff.2015.05.042
Takahashi, Y., Matsumoto, A., Seino, A., Iwai, Y., and Omura, S. (1996). Rare actinomycetes isolated from desert soils. Actinomycetologica 10, 91–97. doi: 10.3209/saj.10_91
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Tan, G., Gyllenhaal, C., and Soejarto, D. (2006). Biodiversity as a source of anticancer drugs. Curr. Drug Targets 7, 265–277. doi: 10.2174/138945006776054942
Tan, L. T.-H., Ser, H.-L., Yin, W.-F., Chan, K.-G., Lee, L.-H., and Goh, B.-H. (2015). Investigation of antioxidative and anticancer potentials of Streptomyces sp. MUM256 isolated from Malaysia mangrove soil. Front. Microbiol. 6:1316. doi: 10.3389/fmicb.2015.01316
Tanaka, M., Shin-Ya, K., Furihata, K., and Seto, H. (1995). Isolation and structural elucidation of antioxidative substances, carbazoquinocins A to F. J. Antibiot. 48, 326–328. doi: 10.7164/antibiotics.48.326
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. doi: 10.1093/nar/25.24.4876
Valko, M., Rhodes, C., Moncol, J., Izakovic, M., and Mazur, M. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40. doi: 10.1016/j.cbi.2005.12.009
Versalovic, J., Koeuth, T., and Lupski, R. (1991). Distribution of repetitive DNA sequences in eubacteria and application to finerpriting of bacterial enomes. Nucleic Acids Res. 19, 6823–6831. doi: 10.1093/nar/19.24.6823
Waksman, S. A., and Henrici, A. T. (1943). The nomenclature and classification of the actinomycetes. J. Bacteriol. 46, 337.
Wayne, L., Brenner, D., Colwell, R., Grimont, P., Kandler, O., Krichevsky, M., et al. (1987). Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Evol. Microbiol. 37, 463–464. doi: 10.1099/00207713-37-4-463
Whiton, R., Lau, P., Morgan, S., Gilbart, J., and Fox, A. (1985). Modifications in the alditol acetate method for analysis of muramic acid and other neutral and amino sugars by capillary gas chromatography—mass spectrometry with selected ion monitoring. J. Chromato. A 347, 109–120. doi: 10.1016/S0021-9673(01)95474-3
Williams, P. G. (2009). Panning for chemical gold: marine bacteria as a source of new therapeutics. Trends Biotech. 27, 45–52. doi: 10.1016/j.tibtech.2008.10.005
Williams, S. (1989). “Genus Streptomyces Waksman and Henrici 1943, 339AL,” in Bergey's Manual of Systematic Bacteriology, eds S. T. Williams, M. E. Sharpe, J. G. Holt (Baltimore, MD: Williams & Wilkins), 2452–2492.
Xiao, J., Wang, Y., Luo, Y., Xie, S.-J., Ruan, J.-S., and Xu, J. (2009). Streptomyces avicenniae sp. nov., a novel actinomycete isolated from the rhizosphere of the mangrove plant Avicennia mariana. Int. J. Syst. Evol. Microbiol. 59, 2624–2628. doi: 10.1099/ijs.0.009357-0
Xu, J., Wang, Y., Xie, S.-J., Xu, J., Xiao, J., and Ruan, J.-S. (2009). Streptomyces xiamenensis sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 59, 472–476. doi: 10.1099/ijs.0.000497-0
Yang, T., Yang, X., Wang, X., Wang, Y., and Song, Z. (2013). The role of tomato products and lycopene in the prevention of gastric cancer: a meta-analysis of epidemiologic studies. Med. Hypotheses 80, 383–388. doi: 10.1016/j.mehy.2013.01.005
Keywords: Streptomyces colonosanans, actinobacteria, mangrove, antioxidant, cancer
Citation: Law JW-F, Ser H-L, Duangjai A, Saokaew S, Bukhari SI, Khan TM, Mutalib N-SA, Chan K-G, Goh B-H and Lee L-H (2017) Streptomyces colonosanans sp. nov., A Novel Actinobacterium Isolated from Malaysia Mangrove Soil Exhibiting Antioxidative Activity and Cytotoxic Potential against Human Colon Cancer Cell Lines. Front. Microbiol. 8:877. doi: 10.3389/fmicb.2017.00877
Received: 13 February 2017; Accepted: 01 May 2017;
Published: 16 May 2017.
Edited by:
Dongsheng Zhou, Beijing Institute of Microbiology and Epidemiology, ChinaReviewed by:
Andrei A. Zimin, Institute of Biochemistry and Physiology of Microorganisms (RAS), RussiaAntoine Danchin, Institut de Cardiométabolisme et Nutrition (ICAN), France
Copyright © 2017 Law, Ser, Duangjai, Saokaew, Bukhari, Khan, Ab Mutalib, Chan, Goh and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bey-Hing Goh, goh.bey.hing@monash.edu
Learn-Han Lee, lee.learn.han@monash.edu; leelearnhan@yahoo.com
 Jodi Woan-Fei Law
Jodi Woan-Fei Law Hooi-Leng Ser
Hooi-Leng Ser Acharaporn Duangjai
Acharaporn Duangjai Surasak Saokaew
Surasak Saokaew Sarah I. Bukhari
Sarah I. Bukhari Tahir M. Khan
Tahir M. Khan Nurul-Syakima Ab Mutalib
Nurul-Syakima Ab Mutalib Kok-Gan Chan
Kok-Gan Chan Bey-Hing Goh
Bey-Hing Goh Learn-Han Lee
Learn-Han Lee