- 1Department of Research and Development, MiZ Company Limited, Kamakura, Japan
- 2MiZ Inc., Newark, CA, United States
- 3Professor Emeritus, Keio University, Tokyo, Japan
- 4Faculty of Data Science, Musashino University, Tokyo, Japan
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a disorder that is characterized by fatigue that persists for more than 6 months, weakness, sleep disturbances, and cognitive dysfunction. There are multiple possible etiologies for ME/CFS, among which mitochondrial dysfunction plays a major role in abnormal energy metabolism. The potential of many substances for the treatment of ME/CFS has been examined; however, satisfactory outcomes have not yet been achieved. The development of new substances for curative, not symptomatic, treatments is desired. Molecular hydrogen (H2) ameliorates mitochondrial dysfunction by scavenging hydroxyl radicals, the most potent oxidant among reactive oxygen species. Animal experiments and clinical trials reported that H2 exerted ameliorative effects on acute and chronic fatigue. Therefore, we conducted a literature review on the mechanism by which H2 improves acute and chronic fatigue in animals and healthy people and showed that the attenuation of mitochondrial dysfunction by H2 may be involved in the ameliorative effects. Although further clinical trials are needed to determine the efficacy and mechanism of H2 gas in ME/CFS, our literature review suggested that H2 gas may be an effective medical gas for the treatment of ME/CFS.
Introduction
The core symptoms of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are severe fatigue that persists for more than 6 months, extreme exhaustion after exertion, memory impairment, difficulty concentrating, and sleep disturbances (1, 2). It is also an intractable disease that is sometimes accompanied by headache, arthralgia, myalgia, gastrointestinal symptoms, immune system abnormalities, and hypersensitivity to light, sound, smell, and chemicals (1, 2). Symptoms vary in appearance and severity from patient to patient, but decrease overall quality of life as well as social, occupational, and personal activities, and some patients may even become bedridden (1, 2). Although the objective abnormality of ME/CFS has been questioned, recent developments in neuroimaging as well as analytical techniques for blood markers, energy metabolism, and mitochondrial functions have provided evidence for biological abnormalities in this disease (3, 4). Many studies have implicated disorders in the structure and functions of mitochondria, which are responsible for abnormal energy metabolism, in the pathogenesis of ME/CFS (5–25). The prevalence of ME/CFS is estimated to be 0.1–0.5% of the population (26, 27). The number of patients in the United States (US) is estimated to be between 836,000 and 2.5 million (4). The direct and indirect economic cost of ME/CFS in the US has been reported to be as high as 17–24 billion US dollars per year (4).
Molecular hydrogen (H2) is a flammable, colorless, odorless, tasteless, and non-toxic gaseous molecule. It functions as an antioxidant that selectively scavenges reactive oxygen species (ROS) and reactive nitrogen species with very high oxidative capacity, namely, hydroxyl radicals (·OH) and peroxynitrite, respectively (28). H2 has been shown to exert therapeutic effects on various diseases, such as cancer (29–32), cardiovascular disease (33, 34), neurological disease (35, 36), respiratory disease (37, 38), and metabolic syndrome (39, 40). The effects of H2 are not limited to antioxidant activity, they also include anti-inflammatory, anti-apoptotic, and anti-allergic activities, improvements in lipid metabolism, and the regulation of gene expression and signal transduction (41, 42). In mammalian cells, H2 is an inactive molecule that has no metabolic system and does not react with biological substances; however, it reacts with ·OH, which is abundant in mitochondria (43). H2 easily passes through the blood-brain barrier. Due to its excellent diffusivity, H2 easily crosses biological membranes to reach the inside of mitochondria and protect cells from cellular damage caused by ·OH (41, 42). In our recent reviews, we reported that the protective effects of H2 on mitochondria may lead to preventive and therapeutic effects for chronic inflammatory diseases (44, 45).
Previous studies reported that drinking H2 water or inhaling H2 gas was effective in acute or chronic experiments on animals and humans. The administration of H2 to mice, rats, and racehorses subjected to acute or chronic exercise stress was found to exert anti-fatigue effects (46–49). Similar findings were obtained in healthy subjects who drank hydrogen-rich water (HRW) or inhaled H2 gas before or after exercise (50–57). The efficacy of HRW in patients with ME/CFS was suggested by Morris et al. (58) and Lucas et al. in their reviews (59). However, to the best of our knowledge, the efficacy of HRW or H2 gas inhalation remains unknown in patients with ME/CFS. Therefore, we herein reviewed the literature on the effects of H2 for acute or chronic fatigue and discussed the possible efficacy of H2 for ME/CFS.
Criteria, Pathogenesis, and Etiology of ME/CFS
Since there is no specific diagnostic test for ME/CFS, a patient is initially examined for several other possible clinical diagnoses. If all are ruled out, the patient is then diagnosed according to the ME/CFS criteria. The main ME/CFS criteria used are the 1994 Fukuda Criteria (FC) (2), the 2003 Canadian Consensus Criteria (CCC) (60), the 2011 International Consensus Criteria (ICC) (1), and the 2015 Institute of Medicine Criteria (IOMC) (61). Of these, FC continues to be the most frequently used, although some argue that they are too broad in scope and overlap with other conditions to make an appropriate diagnosis; CCC, ICC, and IOMC include more ME/CFS-specific symptoms, such as post-exertional malaise. A major symptom common to all four criteria is the presence of persistent fatigue that does not improve with rest.
As described in the previous chapter, recent studies that conducted analyses of neuroimages and blood markers as well as energy metabolism and mitochondria revealed the presence of a number of objective biological abnormalities in ME/CFS (8). ME/CFS may be triggered by the activation of the immune system inside and outside the brain, resulting in the release of inflammatory cytokines (62, 63). These findings suggest that ME/CFS is associated with abnormalities related to the central and autonomic nervous systems, abnormalities in systemic energy metabolism, abnormalities in the immune system, and the involvement of oxidative and nitrosative stress (3). Systemic energy metabolism abnormalities have been explained by alterations in the structure and functions of mitochondria in the muscles and leukocytes of patients with ME/CFS, suggesting that mitochondrial dysfunction is involved in energy metabolism abnormalities in this disease (5–18). Recent studies reported that some of the “sequelae” of patients affected by coronavirus disease 2019 (COVID-19), which is raging worldwide, include ME/CFS-like diseases (64, 65).
ME/CFS is difficult to diagnose in general practice, and it may take many years before a diagnosis is confirmed. Furthermore, since there is no effective treatment, many patients are forced to stay at home and subsequently deteriorate. A double-blind, randomized, controlled trial on the antibody drug rituximab was recently conducted on patients with ME/CFS; however, its efficacy was not confirmed (66). Patients with ME/CFS may have malfunctioning mitochondria and metabolic pathways, resulting in various deficiencies, such as in the metabolism of fatty acids and amino acids, as well as inefficient ATP synthesis (67). Therefore, the use of supplements with protective effects against mitochondrial dysfunction has been attempted as part of treatment. The efficacies of supplements, such as nicotinamide adenine dinucleotide hydrogen (NADH), coenzyme Q10 (CoQ10), and acetyl L-carnitine (ALC), have been investigated (68–70). Although these substances exhibited some efficacy, their effects were limited (68–70). Therefore, the development of new therapeutic substances and treatments that are curative rather than symptomatic is desired.
Effects of H2 on Mitochondrial Dysfunction
In mitochondria, the electron transfer system generates an electrochemical potential on the inner membrane side, and this electrochemical energy is converted into chemical energy for ATP. Although the mitochondrial inner membrane is a good insulator, electrons leak out at a certain frequency. Leaked electrons react with oxygen in the mitochondria to produce superoxide anions () (41, 42, 71). is also produced at a certain frequency in the citric acid circuit in the mitochondria by α-ketoglutarate dehydrogenase. The ROS scavenging system is well developed in vivo, and is converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD), which is further converted to water by glutathione peroxidase or catalase (41, 42, 71). reduces transition metal ions, such as Fe3+ and Cu2+, which react with H2O2 to produce ·OH (Fenton reaction) (41, 42, 71). ·OH is produced by the reaction of and H2O2 catalyzed by transition metal ions (Heber-Weiss reaction). It is the most potent oxidizing ROS and indiscriminately reacts with nucleic acids, lipids, and proteins. Since H2 is a substance with excellent permeability to mitochondria, it may react with ·OH produced in mitochondria and convert it to water for detoxification (·OH + H2 → H· + H2O) (41–44, 71).
Oxidative stress in mitochondria induces chronic inflammation, which may contribute to ME/CFS, including acute and chronic fatigue. Inflammation is induced by the release of inflammatory cytokines, such as interleukin (IL)-1β and IL-18 by macrophages and dendritic cells in direct response to inflammatory triggering stimuli (44, 72). The production of these cytokines is transient in nature; however, when they are continuously produced due to some disturbance, acute inflammation is delayed, and chronic inflammation is triggered. Inflammasomes, an intracellular protein complex, play an important role in the production of IL-1β and IL-18 (44, 72–75). Among them, nucleotide-binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasomes play an important role in the production of IL-1β and IL-18 (44, 72–75). They are activated not only by pathogen-associated molecular patterns from silica, asbestos, and low osmolarity, but also by damage-associated molecular patterns as diverse stimuli from extrinsic factors, such as silica, asbestos, and low osmolarity to intrinsic factors, including ATP and urate crystals (44, 72–75).
Recent studies demonstrated that mitochondria-derived ROS significantly contributed to the activation of NLRP3 inflammasomes. ROS produced by poorly functioning mitochondria (mtROS) oxidize mitochondrial DNA (mtDNA), which then binds directly to NLRP3 to promote the formation of inflammasomes (44, 76). Caspase-1, which is activated in inflammasomes, processes the precursor forms of IL-1β and IL-18 into their mature forms, which are released into the extracellular space and induce inflammation (44). On the other hand, the activation of NLRP3 inflammasomes requires a preceding “priming” stimulus, typically lipopolysaccharide, which induces the expression of genes encoding the precursors IL-1β and NLRP3 via its receptor, toll-like receptor 4 (44, 77, 78). In our recent review, we demonstrated that among mtROS, ·OH may mainly promote the oxidation of mtDNA (44). As a mechanism for the amelioration of chronic inflammation by H2, we showed that the scavenging of ·OH in mitochondria may be involved in the suppression of the cascade from NLRP3 inflammasome activation to the release of inflammatory cytokines (44).
Anti-Fatigue Effects of H2 and Underlying Mechanisms
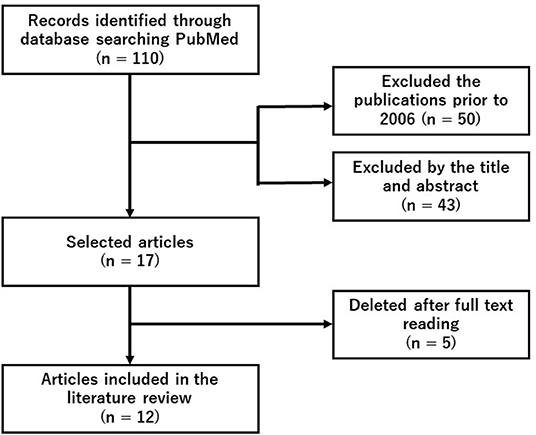
We did a systematic search of PubMed using the search terms (“hydrogen” and “fatigue” and “exercise”) on November 24, 2021. Since this search detected 12 original articles, we conducted a following literature review (Figure 1 and Table 1).
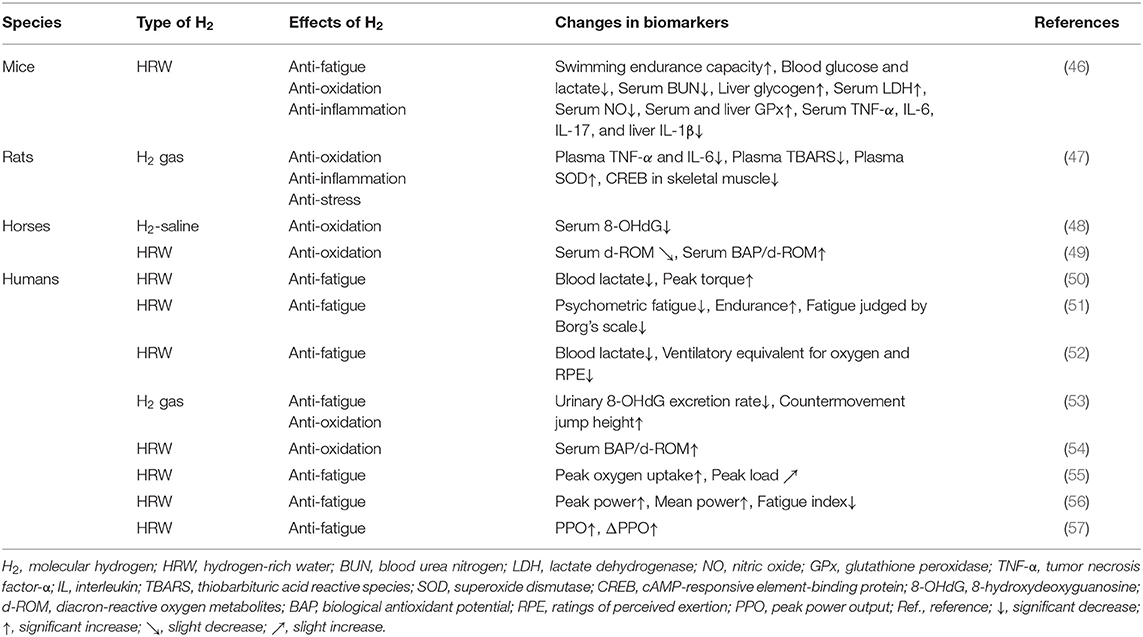
Table 1. Summary of anti-fatigue effects of molecular hydrogen (H2) in animal models and human clinical trials.
H2 exerted anti-fatigue effects in mice, rats, and racehorses subjected to acute or chronic exercise loading (46–49). Similarly, the anti-fatigue effects of H2 on healthy subjects who performed acute or chronic exercise have been investigated (50–57). In this chapter, we provide an overview of the specific anti-fatigue effects of H2 in animal models and human clinical trials, and also discuss the underlying mechanisms (Table 1).
Effects of H2 in Animal Models
Ara et al. investigated the effects of HRW (1.0–1.2 ppm) on fatigue in mice subjected to daily stress-induced swimming for 4 weeks (46). The findings obtained showed higher swimming endurance in the HRW group than in the placebo water (PW) group (46). In addition, blood glucose, lactate, and serum BUN levels were significantly lower, while liver glycogen and serum LDH levels were significantly higher in the HRW group than in the PW group. Moreover, the HRW group showed decreased nitric oxide (NO) and increased glutathione peroxidase (GPx) levels as well as decreased serum tumor necrosis factor-α (TNF-α), IL-6, and IL-17 levels and liver IL-1β levels (46). These findings suggest that HRW exerted its anti-fatigue effects through metabolic regulation, the redox balance, and the inhibition of inflammation.
Nogueira et al. examined the anti-fatigue effects of H2 gas (2%) on rats subjected to acute exercise loading on a treadmill (47). Immediately and 3 h after exercise, rats were euthanized and inflammatory markers in plasma were measured. Skeletal muscle was also collected to examine the phosphorylation status of intracellular signaling proteins. The findings obtained showed that H2 gas suppressed exercise-induced elevations in inflammatory cytokines (TNF-α and IL-6) and thiobarbituric acid reactive species (TBARS), as well as further increases in SOD (47). H2 gas suppressed the phosphorylation of skeletal muscle cAMP-responsive element-binding protein (CREB) (47). Nogueira et al. suggested that H2 plays an important role in the attenuation of exercise-induced inflammation, oxidative stress, and cellular stress (47).
Yamazaki et al. examined the effects of intravenously administered H2-containing saline solution (H2-saline, 0.6 ppm) on oxidative stress in racehorses prior to their participation in a high-intensity simulation race (48). No significant differences were observed in the biological antioxidant potential (BAP) and diacron-reactive oxygen metabolites (d-ROM), between horses administered H2-saline and a placebo. However, H2-saline significantly inhibited 8-hydroxydeoxyguanosine (8-OHdG) at all-time points: immediately after the race, 3 h later, and 24 h later (48). These findings indicate that H2-saline significantly inhibited oxidative stress induced after exhausting races (48).
Tsubone et al. also investigated the effects of HRW (1 ppm) on oxidative stress and the antioxidant capacity in response to treadmill exercise in racehorses (49). HRW and PW were administered orally 30 min before treadmill exercise, and blood samples were collected. In comparisons with PW, HRW slightly reduced d-ROM from the pre-exercise value and significantly increased BAP/d-ROM immediately before exercise, immediately after exercise, and 30 min after exercise (49). Tsubone et al. demonstrated that HRW attenuated oxidative stress induced by treadmill exercise (49). However, we question their results because the difference between the placebo and HRW groups is extremely small.
Effects of H2 in Human Clinical Trials
Intense exercise for a short period of time may induce oxidative stress, which may, in turn, contribute to the development of overtraining symptoms, such as increased fatigue, resulting in muscle microdamage and inflammation. Aoki et al. investigated the effects of HRW on oxidative stress and muscle fatigue during acute exercise (50). Athletes ingested HRW (2.0 ppm) or PW, followed by exercise loading with a cycle ergometer and maximal isometric knee extension. In comparisons with PW, HRW did not induce significant changes in d-ROM, BAP, and creatine kinase after exercise (50). However, it significantly suppressed increases in blood lactate levels (50). It also inhibited the initial decrease in peak torque observed with PW (50). These findings suggest that HRW may reduce blood lactate levels and ameliorate exercise-induced muscle dysfunction.
Mikami et al. examined the effects of HRW on psychological fatigue and endurance in response to exercise loading. In experiment 1, all healthy untrained subjects ingested HRW (0.8 ppm) or PW 30 min before light exercise on a cycle ergometer (51). Psychological fatigue was significantly lower in the HRW group than in the PW group (51). In Experiment 2, trained participants were subjected to moderate exercise with a cycle ergometer 10 min after the ingestion of HRW (1.0 ppm) using the same method as that in Experiment 1. Based on maximal oxygen consumption and the Borg's scale, significant improvements were observed in endurance and fatigue in the HRW group (51). Therefore, Mikami et al. suggested that HRW may contribute to recovery and better endurance (51). Since the effects of HRW in the present study were clinically negligible, the anti-fatigue and endurance-enhancing effects of HRW have been questioned, although this has been refuted by the authors (79, 80).
HRW may be useful for recovery and enhancing performance. Botek et al. evaluated the physiological and perceptual efficacies of HRW in a protocol in which HRW (0.5 ppm) was administered to healthy volunteers within 30 min before exercise and exercise intensity was progressively increased (52). Cardiopulmonary function, lactate levels, and the rating of perceived exertion (RPE) were examined during the last minute of each exercise step. The findings obtained revealed that blood lactate levels, the ventilatory equivalent of oxygen, and RPE were significantly lower in the HRW group than in the PW group (52). These findings suggest that HRW reduced blood lactate levels at higher exercise intensities and enhanced the sense of effort and ventilatory efficiency of exercise (52).
Shibayama et al. examined the effects of acute H2 gas inhalation on subsequent oxidative stress, muscle damage, and exercise performance during recovery after intense exercise (53). Volunteers performed oxidative stress-inducing exercise consisting of treadmill running and squat jumps for 30 min, and then inhaled H2 gas (68%) or placebo gas for 60 min (recovery period) (53). In comparisons with the placebo, H2 gas significantly reduced the urinary 8-OHdG excretion rate and increased the height of countermovement jumps (53). These findings suggest that H2 gas enhanced exercise performance by reducing systemic oxidative damage.
Continuous sprinting exercise may disrupt the redox balance in muscles, causing systemic oxidative stress and muscle damage. Dobashi et al. investigated the effects of HRW on oxidative stress and muscle fatigue in healthy subjects subjected to 3 days of continuous exercise loading (54). PW and HRW (5 ppm) were consumed before and after each exercise session. Blood samples were collected 7 h before the first exercise session (day 1) and 16 h after each exercise session. The findings obtained showed that the relative change from baseline in BAP/d-ROM, an index of antioxidant capacity, decreased over time in the PW group (54). However, in the HRW group, the decrease in BAP/d-ROM observed in the PW group was significantly suppressed (54). These findings suggest that HRW contributed to maintaining the redox status during continuous intense exercise and prevented the accumulation of muscle fatigue.
Although animal studies reported that H2 enhanced mitochondrial metabolism, the effects of H2 on aerobic capacity during exercise in humans remain unclear. Hori et al. investigated whether the continuous intake of HRW (5.9 ppm) for 2 weeks by healthy subjects increased aerobic capacity during gradual cycling exercise (55). In comparisons with PW, HRW significantly increased peak oxygen uptake and slightly increased the peak load (55). Hori et al. suggested that HRW enhanced aerobic exercise performance and physical health. They also indicated that the enhancement in aerobic capacity during exercise with HRW was due to increased mitochondrial energy production (55).
Timón et al. examined the effects of the weekly intake of HRW on aerobic and anaerobic exercise performance in both trained and untrained humans (56). Two experimental groups, trained cyclists and untrained subjects, ingested PW and nanobubbled HRW (1.9 ppm). The findings obtained showed enhanced performance in trained cyclists only in the anaerobic test, with increased peak and average power as well as a reduced fatigue index (56). These findings indicate that the ergogenic effects of HRW are dependent on the state of training. Furthermore, HRW appeared to effectively enhance the anaerobic performance of trained cyclists (56).
Da Ponte et al. investigated the effects of 2 weeks of HRW ingestion on repetitive sprint performance and the acid-base status during prolonged intermittent cycling exercise (57). Trained male cyclists were given PW or HRW (0.45 ppm) daily and tested at baseline and after each 2-week period of treatment. The findings obtained showed that the absolute value of peak power output (PPO) was significantly reduced in the PW group on the 8th and 9th out of 10 sprints, and the relative value of ΔPPO was significantly reduced on the 6th, 8th, and 9th sprints; however, these decreases were significantly attenuated in the HRW group (57). These findings indicate that HRW contributed to the maintenance of PPO in repeated sprints to exhaustion (57).
Possible Mechanisms Underlying Fatigue-Ameliorating Effects
H2 has been reported to exert anti-fatigue effects in experimental animals and healthy subjects by increasing exercise capacity (46, 50–53, 55–57), reducing fatigue indices (51, 56), and inhibiting increases in blood lactate levels due to muscle fatigue (46, 50, 52). H2 was also found to inhibit increases in cAMP-responsive element-binding protein (CREB), a marker of the phosphorylation of intracellular signaling proteins in skeletal muscle associated with exercise (47). On the other hand, d-ROM, 8-OHdG, and TBARS have been used as oxidative stress markers, and BAP, GPx, BAP/d-ROM, and SOD as antioxidant markers, and the effects of H2 on these markers has also been evaluated. H2 reduced TBARS (47), d-ROM (49), and 8-OHdG (48, 53), and increased GPx (46), SOD (47), and BAP/d-ROM (49, 54). Furthermore, H2 decreased inflammatory markers, such NO, TNF-α, IL-1β, IL-6, and IL-17 (46, 47). These findings suggest that enhancements in athletic performance and anti-fatigue effects by H2 are due to its antioxidant and anti-inflammatory effects (Table 1).
Recent studies demonstrated that H2 exhibited antioxidant and other biological activities through the regulation of various types of gene expression, in addition to its scavenging effects on ROS, which are often produced by mitochondria (47, 51, 64–66). Nogueira et al. (47) suggested that the mechanism by which H2 inhibits oxidation, inflammation, and cellular stress in rats subjected to acute exercise stress involved the regulation of gene expression. Furthermore, Sobue et al. (81) proposed that H2 activates mitochondrial unfolded protein responses and exerts biological effects via epigenetic histone modifications and changes in gene expression. Hori et al. (56) indicated that the continuous intake of HRW increased mitochondrial energy production via the expression of these genes and proteins, resulting in an increased peak oxygen consumption during progressive exercise. On the other hand, Mizuno et al. reported the ameliorating effects of HRW on mood, anxiety, and autonomic nerve function in daily life (82), and Hu et al. demonstrated that electrolyzed H2 water attenuated chronic stress due to its antioxidant and anti-inflammatory effects (83). We also demonstrated in a recent review that the radio-protective and anti-tumor effects of H2 may involve not only its direct scavenging effects on ·OH, but also its antioxidant and anti-inflammatory effects through the regulation of gene expression as indirect effects (31, 71). Therefore, although further genetic studies are needed, enhancements in athletic performance by H2 and the anti-fatigue effects of H2 involve not only direct scavenging effects on mitochondria-generated ROS, but also antioxidant and anti-inflammatory effects through the regulation of gene expression as indirect effects.
Dose H2 Improve Mitochondrial Disorders in ME/CFS?
Abnormalities in the structure and functions of mitochondria have been detected in patients with ME/CFS (5–25). Studies on the structure of mitochondria in the muscles and leukocytes of patients with ME/CFS revealed the enrichment of mitochondrial cristae (5), polymorphisms in mitochondrial DNA (6), and relationships between specific haplotypes in mitochondrial DNA and specific symptoms (7). Regarding mitochondrial dysfunction, metabolomics studies suggested abnormalities in energy production pathways from monosaccharides, fatty acids, and amino acids (5–11). Previous studies on patients with ME/CFS reported increases (5) and decreases in ATP synthesis (17) in leukocytes, while elevated lactate levels in cerebrospinal fluid indicated impaired oxidative phosphorylation and, as a result, increased anaerobic metabolism (84). Furthermore, decreases in NADH and CoQ10 have been observed in patients with ME/CFS (85).
Mandarano et al. reported that the mitochondrial membrane potential was reduced in the CD8+ T cells of patients with ME/CFS both at rest and during activation (86). Hornig also showed that resting glycolysis was impaired in the CD4+ and CD8+ T cells of patients with ME/CFS, and CD8+ T cells showed impaired activation-related metabolic remodeling and a reduced mitochondrial membrane potential (87). Furthermore, Hornig suggested that mitochondrial ROS induced the activation of NLRP3 in ME/CFS patients, and that the release of IL-1β and IL-18 may induce inflammation (87). We showed in a recent review that ·OH may mainly promote the oxidation of mitochondrial DNA (44). We also reported that the amelioration of chronic inflammation by H2 may be explained by the scavenging of ·OH in mitochondria, which inhibits the cascade from the activation of NLRP3 inflammasomes to the release of IL-1β and IL-18 (44). These findings suggest that H2 protects against mitochondrial dysfunction by scavenging mitochondria-produced ·OH in patients with ME/CFS (Figure 2).
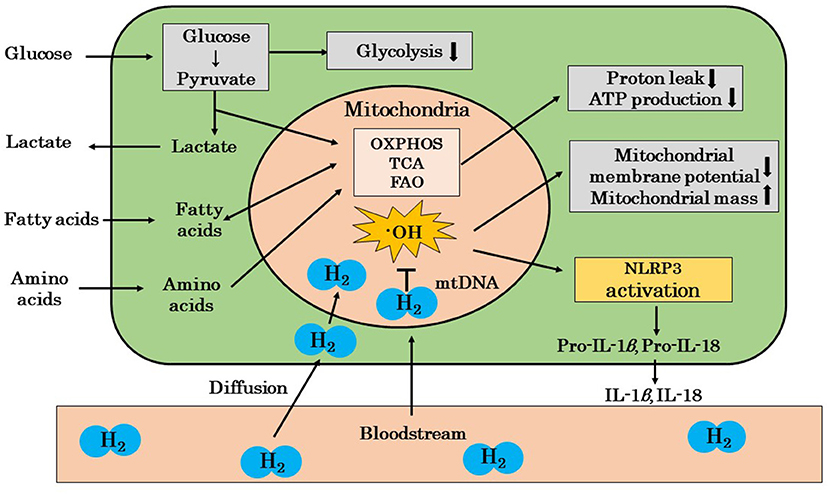
Figure 2. A possible mechanism by which H2 ameliorates mitochondrial dysfunction in ME/CFS patients. The mitochondria of ME/CFS patients show a reduced glycolytic capacity and abnormal metabolism. These mitochondria show decreased proton leakage, ATP production, and mitochondrial membrane potential and an increased mitochondrial mass. H2 ameliorates mitochondrial dysfunction by selectively scavenging ·OH, which is the cause of mitochondrial damage, and blocks the cascade from NLRP3 activation to the release of inflammatory cytokines, such as IL-1β and IL-18. H2, molecular hydrogen; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; ·OH, hydroxyl radicals; OXPHOS, oxidative phosphorylation; TCA, tricarboxylic acid cycle; FAO, fatty acid oxidation; mtDNA, mitochondrial DNA; IL, interleukin; NLPR3, nucleotide-binding and oligomerization domain-like receptor family pyrin domain-containing 3 (inflammasome); ↓, decrease; ↑, increase.
Prospects of H2 as a Therapeutic Substance for ME/CFS
The development of therapies and drugs for ME/CFS has been vigorously pursued. Chinese herbal medicine has been applied as a treatment for decreased immunity, such as the decreased activation of natural killer cells, and vitamin C, NADH, and CoQ10 have been administered as antioxidant-enhancing drugs for increased oxidative stress and decreased antioxidant capacity (68–70). Furthermore, non-steroidal anti-inflammatory drugs have been administered to patients with severe myalgia, arthralgia, and headache, and vaccinia virus-inoculated rabbit inflammatory skin extracts have been applied to the treatment of patients with concomitant neuropathic pain (88). However, all of these therapies are symptomatic and not curative treatments that focus on the etiology of ME/CFS.
In this study, we reported that H2 was effective against fatigue induced by acute and chronic exercise stress in animals and healthy subjects by a literature review (46–57). In addition to the direct scavenging of mitochondria-generated ROS, H2 may also exert indirect antioxidant and anti-inflammatory effects through the regulation of various types of gene expression as the mechanisms of anti-fatigue effects (31, 47, 51). The findings of a literature review also suggested that the protection against mitochondrial dysfunction may be partly involved in the amelioration of H2 on the acute and chronic fatigue in animals and healthy subjects. Since mitochondrial dysfunction plays a major role in abnormal energy metabolism in ME/CFS, our literature review suggested that H2 gas may be an effective medical gas for the treatment of ME/CFS (58, 59, 62, 63). More than 1,300 studies on H2 have been reported to date, including ~100 clinical trials, and research on the medical use of H2 is being conducted worldwide (43). Since H2 is produced by intestinal bacteria (89) and is recognized as a food additive in Japan, the U.S., and European Union (EU), and H2 gas has been applied to the treatment of treat caisson disease (90), there are no safety issues associated with H2. Therefore, further clinical studies to evaluate the efficacy of H2 gas as a therapeutic substance for ME/CFS are needed.
Although the anti-fatigue effects of H2 have been reported in animal studies, these experiments were conducted using HRW or H2-saline. These effects have been reported in clinical trials; however, only Nogueira et al. (47) and Shibayama et al. (53) used H2 gas, while the others used HRW or H2-saline. Moreover, Morris et al. (58) and Lucas et al. (59) indicated the efficacy of HRW in ME/CFS patients in their reviews. These studies showed the potential anti-fatigue effects of HRW or the possible efficacy of HRW in ME/CFS patients, but not the efficacy of H2 gas inhalation. Liu et al. (91) examined the effects of the oral administration of HRW and H2 gas inhalation in rats and measured the blood and tissue concentrations of H2 over time. The findings obtained showed that the maximum values of blood and tissue concentrations were higher with the oral administration of HRW than with H2 gas inhalation, while the area under the curve of these values markedly increased with H2 gas inhalation in a time-dependent manner. Therefore, the efficacy of H2 in patients with ME/CFS appears to be greater following H2 gas inhalation than with HRW.
Ameliorative Effect of H2 on COVID-19 “Sequelae”
COVID-19 is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a viral infection that induces a number of respiratory, digestive, and vascular symptoms. Symptoms in the acute phase generally subside within 2–3 weeks. However, some patients with COVID-19 have a prolonged recovery period and may continue to have “sequelae” for months after the initial infection. A number of chronic symptoms have been reported as these “sequelae,” including fatigue, dyspnea, myalgia, exercise intolerance, sleep disturbances, poor concentration, anxiety, fever, headache, and malaise (64, 65). These symptoms have been described as “long COVID” or “post COVID” and are similar to those observed in ME/CFS (64, 65). However, despite these similarities, there is currently no evidence to show that COVID-19 is a trigger for ME/CFS (19).
Guan et al. (37) examined the effects of a H2/O2 mixed gas (67% H2, 33% O2) on patients with COVID-19 by an open-label multicenter clinical trial and showed that the improvements in disease severity, dyspnea, cough, chest distress, chest pain, and oxygen saturation were significantly greater in H2/O2 treatment group (44 patients) than those in control group (46 patients). In addition, Botek et al. recently reported the results of a randomized, single-blind, placebo-controlled study of the effects of 14 days of H2 gas inhalation (2 × 60 min/day) on the physical and respiratory status of 50 acute “post COVID-19” patients. Compared to the placebo gas, H2 gas inhalation significantly improved physical and respiratory function on gait and pulmonary function tests, suggesting that H2 gas may improve the symptoms of acute “post COVID-19” patients (92). These results indicate that inhalation of H2 gas may have a therapeutic effect not only on patients with COVID-19 but also on those with “post COVID-19.”
Although further research is needed in the future, if we assume that “long COVID” or post COVID” develops through a similar mechanism to ME/CFS, H2 gas inhalation may be useful for the treatment of ME/CFS including “long COVID” or “post COVID.”
Conclusion
Since H2 ameliorates mitochondrial dysfunction (43), we herein reviewed the literature for the anti-fatigue effects of H2 in animal studies and human clinical trials. The findings of the literature review suggested that H2 exerts anti-fatigue effects, and that these effects may involve not only the direct scavenging of mitochondria-generated ROS by H2, but also its antioxidant and anti-inflammatory effects through the regulation of gene expression (31, 46, 50). Since mitochondrial dysfunction is also involved in the etiology of ME/CFS (5–25), the literature review also suggested that the anti-fatigue effects of H2 in animal and human clinical studies indicate a possible ameliorative effect of H2 on ME/CFS (57, 58, 62, 63). Since “long COVID” or “post COVID,” the “sequelae” of COVID-19, may be similar to ME/CFS (64, 65), there is an urgent need to develop precise therapies and substances for ME/CFS. H2 gas may be an effective medical gas for the treatment of ME/CFS.
Author Contributions
S-iH performing study and design and drafting the manuscript. YI, BS, YT, and FS revising the manuscript for important intellectual content. All authors have read and agree to the published version of the manuscript.
Conflict of Interest
S-iH, YI, BS, and FS are employed by MiZ Company Limited.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Dr. Takashi Yamamura of National Center of Neurology and Psychiatry (NCNP) for his advice throughout the submission of this review. The authors would also like to thank Mr. Rei Takusagawa for his help.
References
1. Carruthers BM, Van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: International consensus criteria. J Intern Med. (2011) 270:327–38. doi: 10.1111/j.1365-2796.2011.02428.x
2. Fukuda K, Straus SE, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International chronic fatigue syndrome study group. Ann Intern Med. (1994) 121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009
3. Komaroff A, Takahashi R, Yamamura T, Sawamura M. Neurologic abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: A review. Brain Nerve. (2018) 70:41–54. doi: 10.11477/mf.1416200948
4. Committee Committee on the diagnostic criteria for myalgic encephalomyelitis/chronic fatigue syndrome Board Board on the Health of Select Populations Institute Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press. (2015). Available online at: https://pubmed.ncbi.nlm.nih.gov/25695122/ (accessed February 18, 2022).
5. Lawson N, Hsieh CH, March D, Wang X. Elevated energy production in myalgic encephalomyelitis/chronic fatigue syndrome. J Nat Sci. (2016) 2:e221.
6. Boles RG, Zaki EA, Kerr JR, Das K, Biswas S, Gardner A. Increased prevalence of two mitochondrial DNA polymorphisms in function disease: are we describing different parts of an energy-deleted elephant? Mitochondrion. (2015) 23:1–6. doi: 10.1016/j.mito.2015.04.005
7. Billing-Ross P, Germain A, Ye K, Keinan A, Gu Z, Hanson MR. Mitochondrial DNA variants correlate with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. (2016) 14:19. doi: 10.1186/s12967-016-0771-6
8. Naviaux RK, Naviaux JC, Li K, Bright AT, Alaynick WA, Wang L, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA. (2016) 113:E5472–80. doi: 10.1073/pnas.1607571113
9. Yamano E, Sugimoto M, Hirayama A, Kume S, Yamato M, Jin G, et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci Rep. (2016) 6:34990. doi: 10.1038/srep34990
10. Fluge Ø, Mella O, Bruland O, Risa K, Dyrstad SE, Alme K, et al. Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalomyelitis/chronic fatigue syndrome. JCL Insight. (2016) 1:e89376. doi: 10.1172/jci.insight.89376
11. Garmain A, Ruppert D, Levine SM, Hanson MR. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol Biosyst. (2017) 13:371–9. doi: 10.1039/C6MB00600K
12. Missailidis D, Annesley SJ, Allan CY, Sanislav O, Lidbury BA, Lewis DP, et al. An isolated complex v inefficiency and dysregulated mitochondrial function in immortalized lymphocytes from ME/CFS patients. Int J Mol Sci. (2020) 21:3. doi: 10.3390/ijms21031074
13. Plioplys AV, Plioplys S. Electron-microscopic investigation of muscle mitochondria in chronic fatigue syndrome. Neuropsychobiology. (1995) 32:175–81. doi: 10.1159/000119233
14. Holden S, Maksoud R, Eaton-Fitch N, Cabanas H, Staines D, Marshall-Gradisnik S. A systematic review of mitochondrial abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med. (2020) 18:290. doi: 10.1186/s12967-020-02452-3
15. Venter M, Tomas C, Pienaar IS, Strassheim V, Erasmus E, Ng W-F, et al. MtDNA population variation in myalgic encephalomyelitis/chronic fatigue syndrome in two populations: a study of mildly deleterious variants. Sci Rep. (2019) 9:1. doi: 10.1038/s41598-019-39060-1
16. Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuroendocrinol Lett. (2009) 30:470–6.
17. Armstrong CW, McGregor NR, Lewis DP, Butt HL, Gooley PR. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics. (2015) 11:1626–39. doi: 10.1007/s11306-015-0816-5
18. Booth NE, Myhill S, McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Int J Clin Exp Med. (2012) 5:208−20.
19. Tomas C, Brown A, Strassheim V, Elson J, Newton J, Manning P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS ONE. (2017) 12:e018802. doi: 10.1371/journal.pone.0186802
20. Nguyen T, Staines D, Nilius B, Smith P, Marshall-Gradisnik S. Novel identification and characterisation of Transient receptor potential melastatin 3 ion channels on Natural Killer cells and B lymphocytes: Effects on cell signalling in chronic fatigue syndrome/myalgic encephalomyelitis patients. Biol Res. (2016) 49:1. doi: 10.1186/s40659-016-0087-2
21. Castro-Marrero J, Cordero MD, Sáez-Francas N, Jimenez-Gutierrez C, Aguilar-Montilla FJ, Aliste L, et al. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal. (2013) 19:1855–60. doi: 10.1089/ars.2013.5346
22. Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. (2012) 25:1073–87. doi: 10.1002/nbm.2772
23. Light KC, Agarwal N, Iacob E, White AT, Kinney AY, VanHaitsma TA, et al. Differing leukocyte gene expression profiles associated with fatigue in patients with prostate cancer versus chronic fatigue syndrome. Psychoneuroendocrinology. (2013) 38:2983–95. doi: 10.1016/j.psyneuen.2013.08.008
24. Nguyen T, Staines D, Johnston S, Marshall-Gradisnik S. Reduced glycolytic reserve in isolated natural killer cells from myalgic encephalomyelitis/chronic fatigue syndrome patients: a preliminary investigation. Asian Pac J Allergy Immunol. (2019) 37:102–8. doi: 10.12932/AP-011117-0188
25. Sweetman E, Ryan M, Edgar C, Mackay A, Vallings R, Tate W. Changes in the transcriptome of circulating immune cells of a New Zealand cohort with myalgic encephalomyelitis/chronic fatigue syndrome. Int J Immunopathol Pharmacol. (2019) 33:1. doi: 10.1177/2058738418820402
26. Rowe PC, Underhill RA, Friedman KJ, Gurwitt A, Medow MS, Schwartz MS. Myalgic encephalomyelitis/chronic fatigue syndrome diagnosis and management in young people: A primer. Front Pediatr. (2017) 5:121. doi: 10.3389/fped.2017.00121
27. Reeves WC, Jones JF, Maloney E, Heim C, Hoaglin DC, Boneva S, et al. Prevalence of chronic fatigue syndrome in metropolitan, urban, and rural Georgia. Popul Health Metr. (2007) 5:1–10. doi: 10.1186/1478-7954-5-5
28. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. (2007) 13:688–94. doi: 10.1038/nm1577
29. Chen JB, Kong XF, Lv YY, Qin SC, Sun XJ, Mu F, et al. “Real world survey” of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients. Med Gas Res. (2019) 9:115–21. doi: 10.4103/2045-9912.266985
30. Chen JB, Kong XF, Mu F, Lu TY, Lu YY, Xu KC. Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer. Med Gas Res. (2020) 10:75–80. doi: 10.4103/2045-9912.285560
31. Hirano S-I, Yamamoto H, Ichikawa Y, Sato B, Takefuji Y, Satoh F. Molecular hydrogen as a novel antitumor agent: possible mechanism underlying gene expression. Int J Mol Sci. (2021) 22:8724. doi: 10.3390/ijms22168724
32. Akagi J, Baba H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol Rep. (2018) 41:301–11. doi: 10.3892/or.2018.6841
33. Katsumata Y, Sano F, Abe T, Tamura T, Fujisawa T. The effects of hydrogen gas inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-elevated myocardial infraction. First pilot study in humans. Circ J. (2017) 81:940–7. doi: 10.1253/circj.CJ-17-0105
34. Tamura T, Hayashida K, Sano M, Suzuki M, Shibusawa T, Yoshizawa J, et al. Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome. Circ J. (2016) 80:1870–3. doi: 10.1253/circj.CJ-16-0127
35. Ono H, Nishijima Y, Adachi N, Tachibana S, Chitoku S, Mukaihara S, et al. Improved brain MRI indices in the acute brain stem infarct sites treated with hydroxyl radical scavengers, Edaravone and hydrogen, as compared to Edaravone alone. A non-controlled study. Med. Gas Res. (2011) 1:12. doi: 10.1186/2045-9912-1-12
36. Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N. Pilot study of H2 therapy in Parkinson's disease. A randomized double-blind placebo-controlled trial. Mov Disord. (2013) 28:836–9. doi: 10.1002/mds.25375
37. Guan WJ, Wei CH, Chen AL, Sun XC, Guo GY, Zou X, et al. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis. (2020) 12:3448–52. doi: 10.21037/jtd-2020-057
38. Gong Z, Guan J, Ren X, Meng D, Zhang H, Wang B, et al. Protective effect of hydrogen on the lung of sanitation workers exposed to haze. Chin J Tuberc Respir Dis. (2016) 39:916–23. doi: 10.3760/cma.j.issn.1001-0939.2016.12.003
39. Kajiyama S, Hasegawa G, Asano M, Hosoda H, Fukui M, Nakamura N, et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutri Res. (2008) 28:137–43. doi: 10.1016/j.nutres.2008.01.008
40. Song G, Li M, Sang H, Zhang L, Li X. Hydrogen-rich water decreases serum low-density lipoprotein cholesterol levels and improves high-density lipoprotein function in patients with potential metabolic syndrome. J Lipid Res. (2013) 54:1884–93. doi: 10.1194/jlr.M036640
41. Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. (2014) 144:1–11. doi: 10.1016/j.pharmthera.2014.04.006
42. Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. (2015) 555:289–317. doi: 10.1016/bs.mie.2014.11.038
43. Hirano S-i, Ichikawa Y, Kurokawa R, Takefuji Y, Satoh F. A “philosophical molecule,” hydrogen may overcome senescence and intractable diseases. Med Gas Res. (2020) 10:47–9. doi: 10.4103/2045-9912.279983
44. Hirano S-i, Ichikawa Y, Sato B, Yamamoto H, Takefuji Y, Satoh F. Potential therapeutic applications of hydrogen in chronic inflammatory disease: possible inhibiting role on mitochondrial stress. Int J Mol Sci. (2021) 22:2549. doi: 10.3390/ijms22052549
45. Yamamoto H, Ichikawa Y, Hirano S-i, Sato B, Takefuji Y, Satoh F. Molecular hydrogen as a novel protective agent against pre-symptomatic diseases. Int J Mol Sci. (2021) 22:7211. doi: 10.3390/ijms22137211
46. Ara J, Fadriquela A, Ahmed MF, Bajgai J, Sajo MEJ, Lee SP, et al. Hydrogen water drinking exerts antifatigue effects and in chronic forced swimming via antioxidative and anti-inflammatory activities. Biomed Res Int. (2018) 2018:2571269. doi: 10.1155/2018/2571269
47. Nogueira JE, Passaglia P, Mota CMD, Santos BM, Batalhão ME, et al. Molecular hydrogen reduces acute exercise-induced inflammatory and oxidative stress status. Free Radic Biol Med. (2018) 129:186–93. doi: 10.1016/j.freeradbiomed.2018.09.028
48. Yamazaki M, Kusano K, Ishibashi T, Kikuchi M, Koyama K. Intravenous infusion of H2-saline suppresses oxidative stress and elevates antioxidant potential in Thoroughbred horses after racing exercise. Sci Rep. (2015) 5:15514. doi: 10.1038/srep15514
49. Tsubone H, Hanafusa M, Endo M, Manabe N, Hiraga A, et al. Effect of treadmill exercise and hydrogen-rich water intake on serum oxidative metabolites in serum of Thoroughbred horses. J Equine Sci. (2013) 24:1–8. doi: 10.1294/jes.24.1
50. Aoki K, Nakao A, Adachi T, Matsui Y, Miyakawa S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. (2012) 2:12. doi: 10.1186/2045-9912-2-12
51. Mikami T, Tano K, Lee H, Park J, Ohta F, LeBaron TW, et al. Drinking hydrogen water enhances endurance and relieves psychometric fatigue: a randomized, double-blind, placebo-controlled study. Can J Physiol Pharmacol. (2019) 97:857–62. doi: 10.1139/cjpp-2019-0059
52. Botek M, Krejčí J, McKune AJ, Sládečková B, Naumovski N. Hydrogen rich water improved ventilatory, perceptual and lactate responses to exercise. Int J Sports Med. (2019) 40:879–85. doi: 10.1055/a-0991-0268
53. Shibayama Y, Dobashi S, Arisawa T, Fukuoka T, Koyama K. Impact of hydrogen-rich gas mixture inhalation through nasal cannula during post-exercise recovery period on subsequent oxidative stress, muscle damage, and exercise performances in men. Med Gas Res. (2020) 10:155–62. doi: 10.4103/2045-9912.304222
54. Dobashi S, Takeuchi K, Koyama K. Hydrogen-rich water suppresses the reduction in blood total antioxidant capacity induced by 3 consecutive days of severe exercise in physically active males. Med Gas Res. (2020) 10:21–6. doi: 10.4103/2045-9912.279979
55. Hori A, Sobue S, Kurokawa R, Hirano S-i, Ichihara M, Hotta N. Two-week continuous supplementation of hydrogen-rich water increases peak oxygen uptake during an incremental cycling exercise test in healthy humans: a randomized, single-blinded, placebo-controlled study. Med Gas Res. (2020) 10:163–9. doi: 10.4103/2045-9912.304223
56. Timón R, Olcina G, Gonzalez-Custodio A, Camecho-Cardenosa M, Camacho-Cardenosa A, Guardado IM. Effects of 7-day intake of hydrogen-rich water on physical performance of trained and untrained subjects. Biol Sport. (2021) 38:269–75. doi: 10.5114/biolsport.2020.98625
57. Da Ponte A, Giovanelli N, Nigris D, Lazzer S. Effects of hydrogen rich water on prolonged intermittent exercise. J Sports Med Phys Fitness. (2018) 58:612–21. doi: 10.23736/S0022-4707.17.06883-9
58. Morris G, Puri BK, Walker A, Maes M, Carvalho AF, Walder K, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: pathophysiological insights to novel therapeutic opportunities. Pharmacol Res. (2019) 48:104450. doi: 10.1016/j.phrs.2019.104450
59. Lucas K, Rosch M, Langguth P. Molecular hydrogen (H2) as a potential treatment for acute and chronic fatigue. Arch Phram. (2021) 354:e2000378. doi: 10.1002/ardp.202000378
60. Carruthers B, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome. J Chron Fatigue Syndr. (2003) 11:7–115. doi: 10.1300/J092v11n01_02
61. Jason LA, McManimen S, Sunnquis M, Brown A, Newton JL, Strand EB. Examining the Institute of Medicine's Recommendations regarding chronic fatigue syndrome: Clinical versus research criteria. J Neurol Phychol. (2015) 2015 (Suppl 2) Available online at: http://www.avensonline.org/wp-content/uploads/JNP-2332-3469-S2-0002.pdf
62. Hornig M, Montoya JG, Klimas NG, Levine S, Felsenstein D, Bateman L, et al. Distinct plasma immune signature in ME/CFS are present early in the course of illness. Sci Adv. (2015) 1:e1400121. doi: 10.1126/sciadv.1400121
63. Hornig M, Gottschalk G, Peterson DL, Knox KK, Schultz AF, Eddy ML, et al. Cytokine network analysis of cerebrospinal fluid in myalgic encephalomyelitis/chronic fatigue syndrome. Mol Phychiatry. (2016) 21:261–9. doi: 10.1038/mp.2015.29
64. Poenaru S, Abdallah SJ, Corrales-Medina V, Cowan J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review. Ther Adv Infectious Dis. (2021) 8:1–16. doi: 10.1177/20499361211009385
66. Fluge Ø, Rekeland IG, Lien K, Thürmer H, Borchgrevink PC, Schäfer C, et al. B-lymphocyte depletion in patients with myalgic encephalomyelitis/chronic fatigue syndrome: A randomized, double-blind, placebo-controlled trial. Ann Intern Med. (2019) 170:585–93. doi: 10.7326/M18-1451
67. Missailidis D, Annesley SJ, Fisher PR. Pathological mechanisms underlying myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics. (2019) 9:3. doi: 10.3390/diagnostics9030080
68. Castro-Marrero J, Sáez-Francàs N, Segundo MJ, Calvo N, Faro M, Aliste L., et al. Effect of coenzyme Q10 plus nicotinamide adenine dinucleotide supplementation on maximum heart rate after exercise testing in chronic fatigue syndrome - A randomized, controlled, double-blind trial. Clin Nutr. (2016) 35:826–34. doi: 10.1016/j.clnu.2015.07.010
69. Castro-Marrero J, Cordero MD, Segundo MJ, Sáez-Francàs N, Calvo N, Román-Malo L, et al. Does oral coenzyme Q10 plus NADH supplementation improve fatigue and biochemical parameters in chronic fatigue syndrome? Antioxid Redox Signal. (2015) 22:679–85. doi: 10.1089/ars.2014.6181
70. Forsyth LM, Preuss HG, MacDowell AL, Chiazze L, Birkmayer GD, Bellanti JA. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann Allergy Asthma Immunol. (1999) 82:185–91. doi: 10.1016/S1081-1206(10)62595-1
71. Hirano S-i, Ichikawa Y, Sato B, Yamamoto H, Takefuji Y, Satoh F. Molecular hydrogen as a potential clinically applicable radioprotective agent. Int J Mol Sci. (2021) 22:4566. doi: 10.3390/ijms22094566
72. Tschopp J. Mitochondria: Sovereign of inflammation? Eur J Immunol. (2011) 41:1196–202. doi: 10.1002/eji.201141436
73. Ismael S, Ahmed HA, Adris T, Parveen K, Thakor P, Ishrat T. The NLRP3 inflammasome: a potent therapeutic target for traumatic brain injury. Neural Regen Res. (2020) 16:49–57. doi: 10.4103/1673-5374.286951
74. Hosseinian N, Cho Y, Lockey RF, Kolliputi N. The role of the NLRP3 inflammasome in pulmonary diseases. Ther Adv Respir Dis. (2015) 9:188–97. doi: 10.1177/1753465815586335
75. Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. (2015) 8:15–27. doi: 10.2147/JIR.S51250
76. Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. (2012) 36:401–14. doi: 10.1016/j.immuni.2012.01.009
77. Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. (2018) 560:198–203. doi: 10.1038/s41586-018-0372-z
78. Wallet SM, Puri V, Gibson FC. Linkage of infection to adverse systemic complication: periodontal disease, toll-like receptors, and other pattern recognition systems. Vaccines (Basel). (2018) 6:21. doi: 10.3390/vaccines6020021
79. Falster C, Korfitzen S, Herold M, Lindebjerg J, Elsøe M. Discussion: Drinking hydrogen water enhances and relieves phychometric fatigue: a randomized, double-blind, placebo-controlled study. Can J Physiol Pharmacol. (2021) 99:1114–5. doi: 10.1139/cjpp-2021-0031
80. Ohta S, LeBaron TW. Reply to “Drinking hydrogen water enhances and relieves phychometric fatigue: a randomized, double-blind, placebo-controlled study”. Can J physiol pharmacol. (2021) 99:1116–17. doi: 10.1139/cjpp-2021-0151
81. Sobue S, Inoue C, Hori F, Qiao S, Murate T. Molecular hydrogen modulates gene expression via histone modification and induces the mitochondrial unfolded protein response. Biochem Biophys Res Commun. (2017) 493:318–24. doi: 10.1016/j.bbrc.2017.09.024
82. Mizuno K, Sasaki AK, Ebisu K, Tajima K, Kajimoto O, Nojima J, et al. Hydrogen rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med Gas Res. (2017) 7:247–55. doi: 10.4103/2045-9912.222448
83. Hu D, Li D, Shigeta M, Ochi Y, Okauchi T, Neyama H, et al. Alleviation of the chronic stress response attributed to the antioxidant and anti-inflammatory effects of electolyzed hydrogen water. Biochem Biophys Res Commun. (2021) 535:1–5. doi: 10.1016/j.bbrc.2020.12.035
84. Mathew SJ, Mao X, Keegan KA, Levine SM, Smith ELP, Heier LA, et al. Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: an in vivo 3.0 T 1H MRS imaging study. NMR Biomed. (2009) 22:251–8. doi: 10.1002/nbm.1315
85. Mikirova N, Casciari J, Hunninghake R. The assessment of energy metabolism in patients with chronic fatigue syndrome by serum fluorescence emission. Altern Ther Health Med. (2012) 18:36–40.
86. Mandarano AH, Maya J, Giloteaux L, Peterson D, Maynard M, Gottschalk CG, et al. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J Clin Invest. (2020) 130:1491–505. doi: 10.1172/JCI132185
87. Hornig M. Can the light of immunometabolism cut through “brain fog”? J Clin Invest. (2020) 130:1102–5. doi: 10.1172/JCI134985
88. Kuratsune H. Diagnosis and treatment of myalgic encephalomyelitis/chronic fatigue syndrome. Bain Nerve. (2018) 70:11–8. doi: 10.11477/mf.1416200944
89. Ichikawa Y, Yamamoto H, Hirano S-i, Sato B, Takefuji Y, Satoh F. The overlooked benefits of hydrogen-producing bacteria. Med Gas Res. (2022) 11.
90. Fontanari P, Baldier M, Guillot C, Tomei C, Barnet H, Gardette B, et al. Changes in maximal performance of inspiratory and skeletal muscles during and after the 7.1-MPa Hydra 10 record human dive. Eur J Appl Physiol. (2000) 81:325–8. doi: 10.1007/s004210050050
91. Liu C, Kurokawa R, Fujino M, Hirano Si, Sato B, Li XK. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci Rep. (2014) 4:5485. doi: 10.1038/srep05485
Keywords: molecular hydrogen, myalgic encephalomyelitis (ME), chronic fatigue syndrome (CFS), hydroxyl radicals, mitochondrial dysfunction, oxidative stress, post COVID, long COVID
Citation: Hirano S-i, Ichikawa Y, Sato B, Takefuji Y and Satoh F (2022) Molecular Hydrogen as a Medical Gas for the Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Possible Efficacy Based on a Literature Review. Front. Neurol. 13:841310. doi: 10.3389/fneur.2022.841310
Received: 22 December 2021; Accepted: 15 March 2022;
Published: 11 April 2022.
Edited by:
Ulises Gomez-Pinedo, Health Research Institute of Hospital Clínico San Carlos, SpainReviewed by:
Yasuyoshi Watanabe, RIKEN, JapanRodrigo Ramos-Zúñiga, University of Guadalajara, Mexico
Copyright © 2022 Hirano, Ichikawa, Sato, Takefuji and Satoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin-ichi Hirano, s_hirano@e-miz.co.jp; hirano_0719@yahoo.co.jp
 Shin-ichi Hirano
Shin-ichi Hirano Yusuke Ichikawa
Yusuke Ichikawa Bunpei Sato
Bunpei Sato Yoshiyasu Takefuji
Yoshiyasu Takefuji Fumitake Satoh
Fumitake Satoh