- 1Auckland Cancer Society Research Centre, University of Auckland, Auckland, New Zealand
- 2Molecular Medicine and Pathology Department, University of Auckland, Auckland, New Zealand
- 3Cancer Research Center of Lyon, INSERM 1052, CNRS5286, Lyon, France
- 4Faculty of Pharmacy, University of Lyon, Claude Bernard Lyon 1 University, Lyon, France
- 5Molecular Biology of Tumors, Hôpital Edouard Herriot, Hospices Civils de Lyon, Lyon, France
Introduction: Endocrine therapy of breast cancer, which either deprives cancer tissue of estrogen or prevents estrogen pathway signaling, is the most common treatment after surgery and radiotherapy. We have previously shown for the estrogen-responsive MCF-7 cell line that exposure to tamoxifen, or deprivation of estrogen, leads initially to inhibition of cell proliferation, followed after several months by the emergence of resistant sub-lines that are phenotypically different from the parental line. We examined the early responses of MCF-7 cells following either exposure to 4-hydroxytamoxifen or deprivation of estrogen for periods of 2 days–4 weeks.
Methods: Endocrine-sensitive or -resistant breast cancer cell lines were used to examine the expression of the stem cell gene SOX2, and the Wnt effector genes AXIN2 and DKK1 using quantitative PCR analysis. Breast cancer cell lines were used to assess the anti-proliferative effects (as determined by IC50 values) of Wnt pathway inhibitors LGK974 and IWP-2.
Results: Hormone therapy led to time-dependent increases of up to 10-fold in SOX2 expression, up to threefold in expression of the Wnt target genes AXIN2 and DKK1, and variable changes in NANOG and OCT4 expression. The cells also showed increased mammosphere formation and increased CD24 surface protein expression. Some but not all hormone-resistant MCF-7 sub-lines, emerging after long-term hormonal stress, showed up to 50-fold increases in SOX2 expression and smaller increases in AXIN2 and DKK1 expression. However, the increase in Wnt target gene expression was not accompanied by an increase in sensitivity to Wnt pathway inhibitors LGK974 and IWP-2. A general trend of lower IC50 values was observed in 3-dimensional spheroid culture conditions (which allowed enrichment of cells with cancer stem cell phenotype) relative to monolayer cultures. The endocrine-resistant cell lines showed no significant increase in sensitivity to Wnt inhibitors.
Conclusion: Hormone treatment of cultured MCF-7 cells leads within 2 days to increased expression of components of the SOX2 and Wnt pathways and to increased potential for mammosphere formation. We suggest that these responses are indicative of early adaptation to endocrine stress with features of stem cell character and that this facilitates the survival of emerging hormone-resistant cell populations.
Introduction
Endocrine therapy of breast cancer, involving either deprivation of estrogen or the administration of drugs such as tamoxifen to prevent pathway signaling, is the most common treatment of breast cancer after surgery and radiotherapy. Treatment is thought to induce progressive death of cells, commencing after 3 days, in cells expressing estrogen receptor (ER) (1), followed by the gradual emergence, over several months, of hormone-resistant tumor cell populations (2–4). These populations are associated in vivo with both disease relapse and increased metastasis (5–7). Two changes in cell populations might be expected following therapy: an initial adaptive response of the original population to pathway inhibition, and the emergence of drug-resistant populations with altered properties. We (3, 4, 8–11) and others (12, 13) have characterized a number of endocrine therapy-resistant populations of the MCF-7 human breast cancer cell line, but these emerge after several months of exposure to hormone therapy. Here, we have sought to investigate adaptive responses that occur within the first few weeks of exposure to hormone therapy, in order to gain insights into the mechanisms of the adaptive response, and their relationship to stable, long-term resistant phenotypes.
The proliferation of breast cancer is thought to be driven by stem cell populations (14). Stem cell character is often associated with increased expression of genes, such as SOX2, OCT4, and NANOG, which also have roles in embryonic development (15). It is also associated with increased activity of the Wnt signaling pathway, which in turn is associated with the epithelial to mesenchymal transition (16). Wnt signaling involves a complex network of effectors but it can be assessed by measuring expression of the Wnt target genes DKK1 (Dickkopf-1) and AXIN2 (17). Here, we have used the MCF-7 cell line model to investigate whether cells undergo early (adaptive) changes in expression of SOX2, DKK1, and AXIN2 when they have been either treated with 4-hydroxytamoxifen or deprived of estrogen. We have also measured upregulation of these markers in a series of hormone-resistant MCF-7 cell sub-lines developed by long-term selection in previous studies (3, 4, 8–11, 18). We recently showed that SOX2 was expressed at higher levels in estrogen receptor-positive (ER+) breast tumor tissue samples from The Cancer Genome Atlas (TCGA) data set and also in tamoxifen-resistant MCF-7 breast cancer sub-lines (19).
Early changes of stem cell markers in response to therapy may provide a basis for therapy involving inhibition of the corresponding signaling pathways. Suitable inhibitors to test this hypothesis are still under development but we have carried out preliminary studies on two candidate inhibitors. The Porcupine inhibitors IWP-2 (20) and LGK974 (21) block Wnt secretion and reduce DKK1 and AXIN2 expression (20, 21). LGK974 is currently under clinical investigation for antitumor (including anti-breast cancer) efficacy (Trial NCT01351103). We have investigated, first, whether these drugs selectively inhibit the proliferation of hormone-resistant MCF-7 sub-lines and, second, whether the drug sensitivity correlates with the expression of the Wnt target genes DKK1 and AXIN2.
Materials and Methods
Cell Lines and Culture Conditions
The MCF-7 human breast cancer line (22) was chosen because it is ER-positive and has been studied extensively by others as well as by us. The MCF-7 (parental) line was purchased from the ATCC, grown in alpha-MEM containing 5% fetal calf serum (FCS), penicillin/streptomycin (100 U/ml and 100 µg/ml), and insulin/transferrin/selenium supplement (Roche). We have generated tamoxifen-resistant (sub-lines TamR3, TamR6) and fulvestrant-resistant (FulvR1a, FulvR1c, FulvR2a, and FulvR3a) MCF-7 sub-lines by growing the parental cells in 1,000 nM tamoxifen or 100 nM fulvestrant in estrogen-deprived medium (EDM) (phenol red-free RPMI 1640 with 5% charcoal-stripped fetal bovine serum) and penicillin/streptomycin (100 U/ml and 100 µg/ml) for over 12 months. The sub-line TamR7 was grown in tamoxifen-supplemented complete medium with normal serum. Long-term estrogen-deprived MCF-7 sub-lines (TamC3, TamC6, FulvC1a, FulvC2, FulvC3, and FulvC4) were generated as vehicle controls by exposure to 0.1% ethanol in the same growth medium in the absence of fulvestrant or tamoxifen for the same duration. MCF-7 tamoxifen-resistant sub-lines were generated as previously described (3).
These MCF-7 sub-lines have been derived by selective culture for a prolonged period of time (over 12 months) in the presence (three sub-lines FulvR1c, FulvR2a, and FulvR3a) of fulvestrant, a “pure” anti-estrogen (4). The other five endocrine-resistant MCF-7-derived cell lines (TamR7, TamC3, TamR3, TamC6, and TamR6) have been extensively characterized (3, 4, 8–11, 18, 19, 23). Surprisingly, all of the emerging fulvestrant-treated sub-lines showed the triple negative (ER− progesterone receptor− HER2−) phenotype (4) (Figure S1 in Supplementary Material). Breast cancer cell lines (T47D, SKBR3, HCC70, MDA-MB-231, MDA-MB-468, BCC1143, and BT20) were cultured according to ATCC recommendations or as described previously (24).
For short-term tamoxifen treatment, cells were grown in alpha-MEM containing 5% FCS, penicillin/streptomycin (100 U/ml and 100 µg/ml), and insulin/transferrin/selenium supplement (Roche), and incubated with 4-hydroxytamoxifen (100 nM) for 2 days–1 month.
Short Tandem Repeat Profiling
The relationship between the MCF-7-derived cell lines and the parental line was established using the PCR Amplification kit that amplifies 15 tetranucleotide repeat loci plus the amelogenin gender-determining marker, performed by DNA Diagnostics laboratory (Auckland, New Zealand). The combination of markers selected was consistent with the National Institute of Standards and Technology database recommendations for identity testing.
Reverse Transcription, cDNA Synthesis, and Quantitative PCR and PCR
As described in detail previously (19), oligo-dT and random primers were used to reverse transcribe RNA with qScript Flex cDNA kit (Quantabio) according to the manufacturer’s instructions. To investigate whether increased Wnt pathway activation led to increased sensitivity to drugs that affected the Wnt pathway, quantitative RT-PCR (qRT-PCR) was performed using gene-specific primers (Table S1 in Supplementary Material) and Sybr Green MasterMix (Life Technologies), and expression values were normalized relative to GAPDH and HPRT RNA expression.
Cell Proliferation Assay
As described in detail previously (10), cell proliferation was measured by the degree of incorporation of 3H-thymidine into DNA of S-phase cells. Briefly, 3,000 cells per well were seeded in 96-well plates that were tissue culture-treated for monolayer culture and incubated for 3 days. Alternatively, 6,000 cells per well were seeded in 96-well plates (Corning Costar Ultra-Low attachment) for 3 days spheroid culture. 3H-thymidine (0.04 μCi per well for monolayer culture or 0.08 μCi per well for spheroid culture) was added (5 h for monolayer culture or 7 h for suspension culture) prior to harvest.
Mammosphere Formation
For mammosphere formation efficiency, MCF-7 cells in monolayer culture were exposed to 4-hydroxytamoxifen (100 nM) or solvent for 2 days, trypsinized and seeded as cell suspensions in 96-well plates coated with poly(2-hydroxyethyl methacrylate) (polyHEMA; to prevent cell attachment) (25), with 1,000 cells per well in six replicates per experiment. Mammospheres were counted after 6 days.
For mammosphere morphology and size, MCF-7 control or 4-hydroxytamoxifen-incubated cells (2 days) were trypsinized from the monolayer culture, and cell suspensions were seeded in 96-well plates (Corning Costar Ultra-Low attachment) with 2,000, 1,000 or 500 cells per well. After 4-day incubation, images were captured using FLoid Cell Imaging Station (ThermoFisher Scientific) (460× magnification). Representative images are shown. All experiments were performed at least two times.
Flow Cytometric Analysis
Cells were grown in 25 cm2 culture flasks and incubated with 100 nM 4-hydroxytamoxifen for 2 days. Cells (106 cells) were harvested, washed with blocking buffer [1% FCS/phosphate-buffered saline (PBS)], and incubated with 0.4 ml of antibody to CD24 (APC-H7 CD24, BD Pharmingen) or CD44 (APC CD44, BD Pharmingen) in blocking buffer (1:400 dilution) on ice for 30 min. Cells were washed and resuspended in 0.5 ml of blocking buffer. Cells were analyzed in a BD Accuri™ Flow Cytometer and profiles were analyzed with FlowJo V10 software.
Viability Assay
Mitochondrial activity was measured by the Alamar Blue assay (Life Technologies). Superoxide dismutase was measured using a water-soluble tetrazolium salt (WST-1) (purchased from Roche) (26). The stable tetrazolium salt WST-1 is cleaved to a soluble formazan by a complex cellular mechanism that occurs primarily at the cell surface. This bioreduction is largely dependent on the glycolytic production of NAD(P)H in viable cells. Therefore, the amount of formazan dye formed directly correlates with the number of metabolically active cells in the culture. All experiments were done in triplicate wells and performed at least two times.
Immunoblotting
As described in detail previously (10), breast cancer cell lines were grown to log-phase, washed twice with ice-cold PBS, and lysed in sodium dodecyl sulfate (SDS) lysis buffer according to the manufacturer’s protocol (Cell Signaling Technology, Danvers, MA, USA). Protein concentration was quantified using the bicinchoninic acid reagent (Sigma). Cell lysates containing 25 µg of protein were separated by SDS-polyacrylamide gel electrophoresis, and transferred to PVDF membranes (Millipore). Membranes were immunoblotted with antibodies against SOX2 (Cell Signaling Technology) and tubulin (Sigma). Bound antibody was visualized using SuperSignal West Pico (Thermo Scientific, Waltham, MA) or ECL plus (GE Healthcare, Auckland, New Zealand) and the chemiluminescence detection system by Fujifilm Las-3000.
Data Analysis
T-test or Mann–Whitney rank sum test was used for comparison between two groups. Correlation analysis was performed with Spearman’s rank order correlation coefficient (R) and statistical significance (P) using SigmaPlot (Systat Software, Inc.). Values of p < 0.05 were considered to be statistically significant.
Results
Expression of SOX2, AXIN2, and DKK1: An Early Response to Endocrine Therapy in the MCF-7 Breast Cancer Cell Line
SOX2 expression was found to be significantly increased when MCF-7 cells were either exposed to 4-hydroxytamoxifen or grown in EDM for up to 1 month (Figure 1A). Expression of the Wnt target genes AXIN2 and DKK1 was also investigated, since they were reported to be significantly upregulated in stably MCF-7 tamoxifen-resistant MCF-7 cells (>4 months selection) that overexpressed SOX2 (12). AXIN2 was significantly increased when MCF-7 cells were grown in EDM for 1 month and DKK1 was also significantly increased in the treatment group after 1 week (Figures 1B,C).
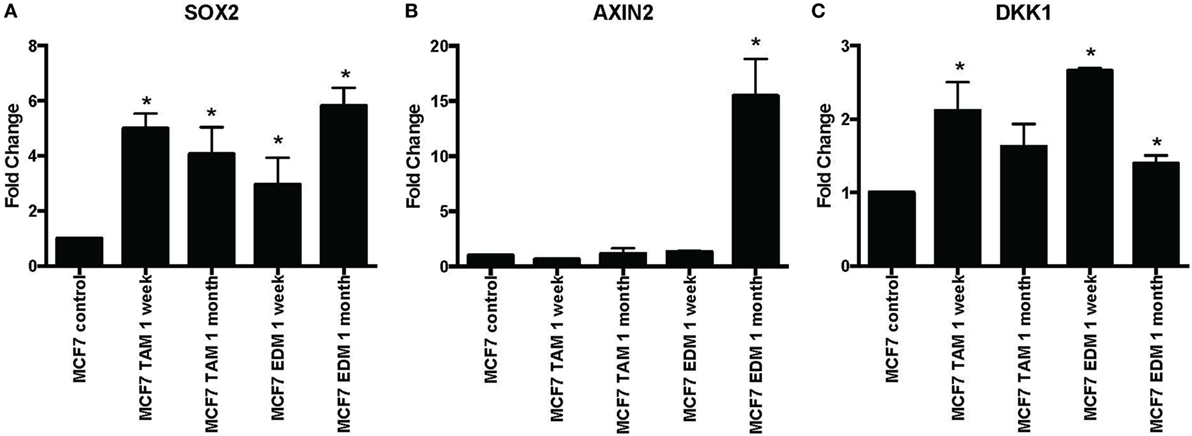
Figure 1. Increased expression of SOX2, AXIN2, and DKK1 in MCF-7 breast cancer cells in 4-hydroxytamoxifen-supplemented or estrogen-deprived medium (EDM). The expression of (A) SOX2, (B) AXIN2, and (C) DKK1 mRNA relative to that of MCF-7 control was measured by quantitative RT-PCR in MCF-7 breast cancer cells either exposed to 4-hydroxytamoxifen (TAM; 100 nM) or grown in EDM for 1 week and 1 month, relative to the MCF-7 parental line. Error bars represent the SEM of three biological replicates, *p < 0.05.
We also examined earlier responses to hormone therapy of MCF-7 cells. The expression of SOX2, AXIN2, and DKK1 was determined in MCF-7 cells exposed to 4-hydroxytamoxifen for 5 days or less. As expected, both SOX2 and DKK1 were significantly upregulated in tamoxifen-treated cells, while AXIN2 showed a general trend toward upregulation with tamoxifen treatment (Figures 2A–C). SOX2 protein was also induced in parallel with the upregulated SOX2 mRNA expression (Figure 2D). We also investigated whether NANOG and OCT4 were differentially modulated during the development of tamoxifen resistance (Figure S2 in Supplementary Material). OCT4 expression appeared to increase significantly at the later time (1 month) with tamoxifen exposure, while NANOG showed significant increase in 1 week of 4-hydroxytamoxifen exposure and also when grown in EDM for 1 month. However, expression levels of SOX2, NANOG, and OCT4 were not correlated (Table S2 in Supplementary Material).
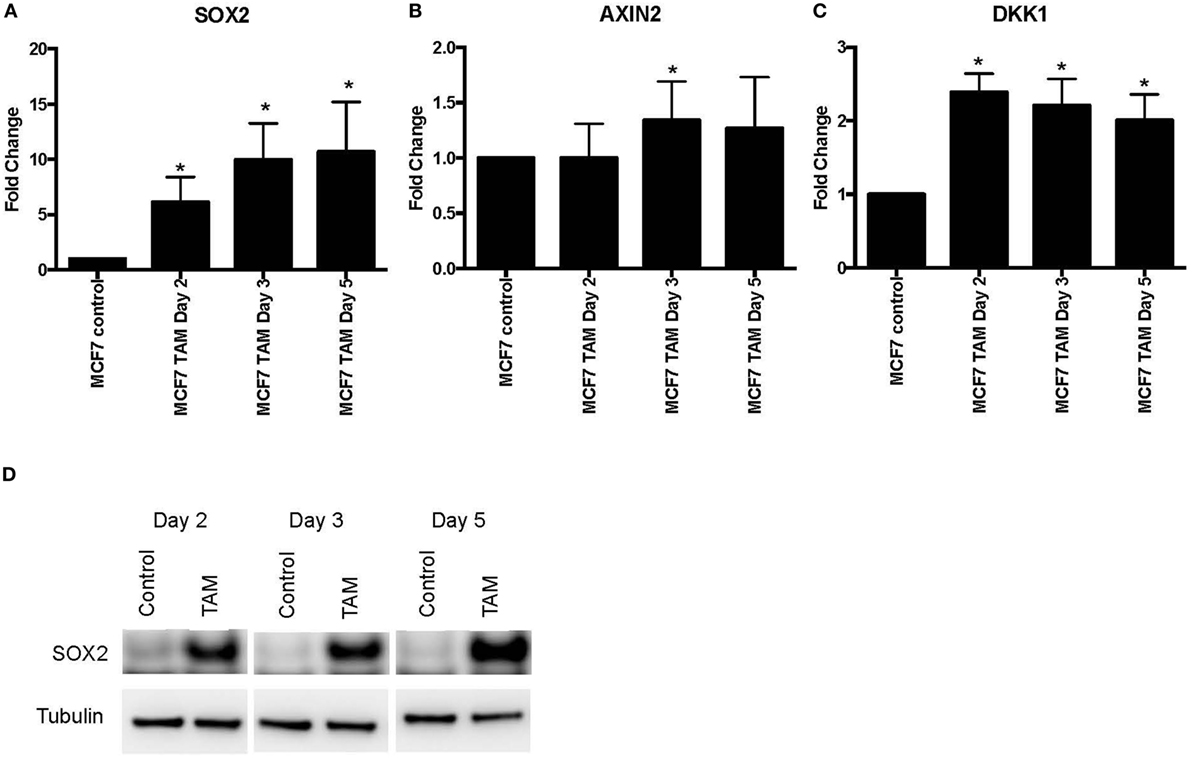
Figure 2. Increased expression of SOX2, AXIN2, and DKK1 in MCF-7 breast cancer cells with short-term 4-hydroxytamoxifen treatment. The expression of (A) SOX2, (B) AXIN2, and (C) DKK1 relative to MCF-7 control measured by quantitative RT-PCR in MCF-7 breast cancer cells exposed to 4-hydroxytamoxifen (100 nM) for 2–5 days, relative to the MCF-7 parental line. (D) Western blot analysis showing expression of SOX2 in 4-hydroxytamoxifen (TAM; 100 nM)-treated MCF-7 cells. Tubulin is the loading control.
Expression of Stem Cell Character in Response to Endocrine Therapy in the MCF-7 Breast Cancer Cell Line
We next examined the stem-like character (mammosphere formation efficiency) as an early response to tamoxifen exposure in MCF-7 cells. As expected, the increase of SOX2 expression was associated with an increased ability to form mammospheres (Figures 3A,B). An increase in CD24, but not in CD44, expression was also detected in MCF-7 cells exposed to 4-hydroxytamoxifen for 2 days (Figure 3C).
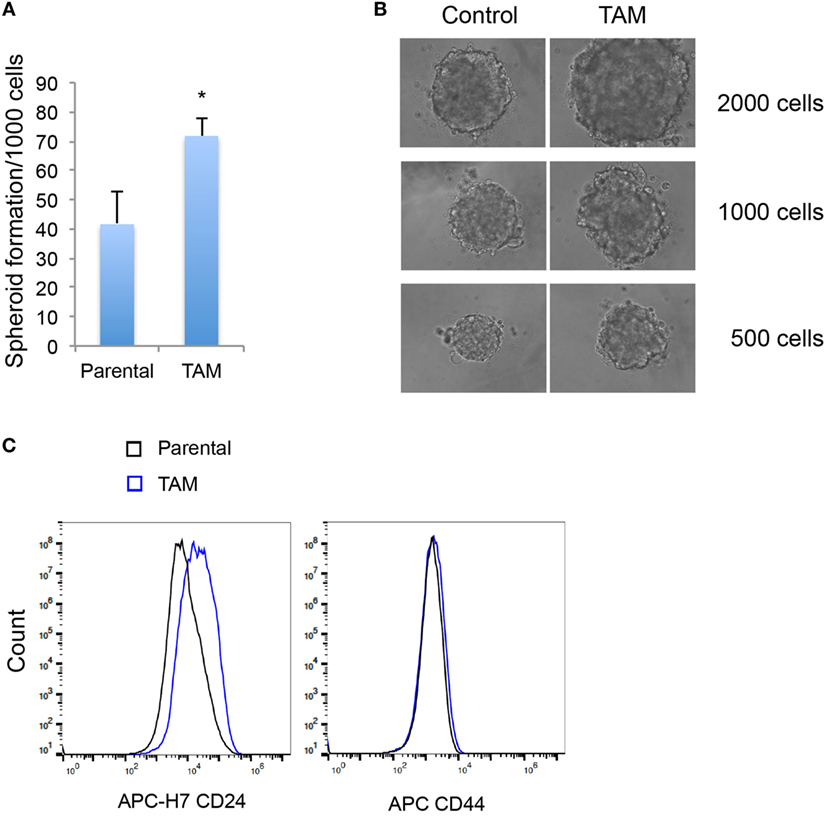
Figure 3. (A) Mammosphere formation efficiency of MCF-7 cells exposed to 4-hydroxytamoxifen relative to that of vehicle-exposed cells. (B) Mammosphere morphology of MCF-7 cells exposed to 4-hydroxytamoxifen (TAM; 100 nM) compared with the MCF-7 parental line. (C) Increased expression of CD24, but not CD44, in MCF-7 cells exposed to 4-hydroxytamoxifen (100 nM) for 2 days. *Significantly different from MCF-7 parental line (p < 0.05). Error bars represent the SEM of three biological replicates, *p < 0.05.
Expression of SOX2, AXIN2, and DKK1 in Endocrine-Resistant MCF-7 Breast Cancer Cells
Using our panel of endocrine therapy-resistant breast cancer cell lines generated by long-term estrogen-deprived culture conditions, or anti-estrogen treatment (3, 4, 8–11, 18, 27), we examined the gene expression pattern of SOX2, together with the WNT effector genes DKK1 and AXIN2, to assess long-term expression changes of these stem cell markers. All except two endocrine therapy-resistant breast cancer lines showed significant upregulation of SOX2 expression (Figure 4A). The ER− endocrine-resistant breast cancer lines (generated by fulvestrant treatment) showed significant upregulation of SOX2 expression as compared to the ER+ cell lines (Figure 4B). Four resistant sub-lines showed a significantly increased AXIN2 expression (Figure 5A). Although there was a general trend of reduction of DKK1 expression, only five sub-lines showed significant reduction in DKK1 expression and one showed a significant increase in DKK1 expression (Figure 5B). Contrary to the published data (12), we found that in the MCF-7 endocrine therapy-resistant sub-lines, the expression levels of SOX2, DKK1, and AXIN2 were not correlated (Table 1). In addition to the MCF-7 cell lines, we used seven additional breast cancer cell lines to compare the expression of SOX2 and AXIN2 or DKK1 but no significant correlations were found (Table S3 in Supplementary Material).
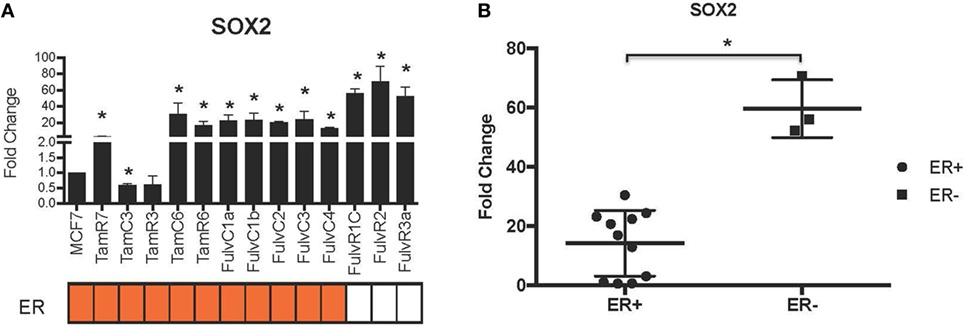
Figure 4. Increased expression of SOX2 in 13 MCF-7 endocrine therapy-resistant sub-lines. (A) The expression of SOX2 relative to MCF-7 control measured by quantitative RT-PCR in MCF-7 breast cancer cells with either long-term exposure to tamoxifen or fulvestrant, or growth in estrogen-deprived medium. (B) Box plot indicating expression of SOX2 in estrogen receptor (ER+) and ER− MCF-7 breast cancer sub-lines. Error bars represent the SEM of three biological replicates, *p < 0.05.
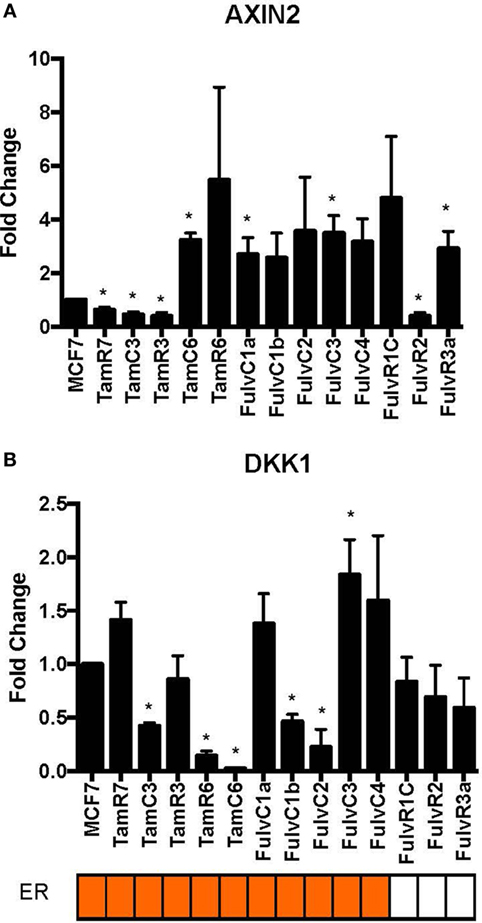
Figure 5. Variations in expression of AXIN2 and DKK1 in 13 MCF-7 endocrine therapy-resistant sub-lines. The expression of (A) AXIN2 and (B) DKK1 relative to MCF-7 control measured by quantitative RT-PCR in MCF-7 breast cancer cell lines with either long-term exposure to tamoxifen or fulvestrant, or growth in estrogen-deprived medium. Error bars represent the SEM of three biological replicates, *p < 0.05.
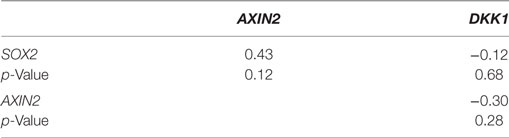
Table 1. Spearman’s rank order correlation of relative gene expression values for SOX2, AXIN2, and DKK1 in MCF-7 parent and endocrine therapy-resistant sub-lines (n = 14).
Correlation of Stem Cell Genes SOX2 and Wnt Pathway Genes AXIN2 and DKK1 in Breast Cancer Samples from TCGA Data Set
We examined the correlation of the expression of SOX2 and Wnt pathway effector genes AXIN2 and DKK1 in the genome-wide RNA transcript profile from TCGA (breast invasive carcinoma gene expression) using the RNAseq data set (TCGA_BRCA_exp_HiSeqV2-2015-02-24) in 1,009 tumor tissue samples from breast cancer patients. A weak but significant negative correlation was observed in the expression of AXIN2 and DKK1 (Spearman’s rank order correlation coefficient, r = −0.15, p = 2 × 10−6). Expression of SOX2 and AXIN2 or DKK1 was not significantly correlated (Table 2). We next stratified the dataset into the ER+ and ER− breast cancer samples, but no correlation was found between SOX2 and AXIN2 or DKK1, or between AXIN2 and DKK1 expression in ER+ (Table S4 in Supplementary Material) or ER− samples (Table S5 in Supplementary Material).
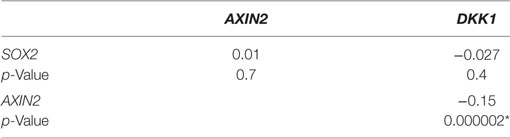
Table 2. Spearman’s rank order correlation coefficients of relative gene expression values for SOX2, AXIN2, and DKK1 in breast cancer samples from The Cancer Genome Atlas (TCGA_BRCA_exp_HiSeqV2-2015-02-24) data set (n = 1,009), *p < 0.05.
Responses in a Panel of MCF-7 Breast Cancer Sub-Lines to Wnt Pathway Inhibitors LGK974 and IWP-2 under Monolayer and Spheroid Culture Conditions
An endocrine therapy-resistant MCF-7 cell line has been reported to be sensitive to Wnt inhibitors (12). The Wnt pathway inhibitors LGK974 and IWP-2 inhibit palmitoylation of Wnt ligands, thus blocking Wnt secretion (20, 21). MCF-7 and its endocrine therapy-resistant sub-lines were assayed for sensitivity to LGK974 and IWP-2 (as measured by IC50 values) (Figure 6). Endocrine-resistant cell lines did not show increased sensitivity to Wnt inhibitors as compared to parental cell line. In MCF-7 parental and endocrine therapy-resistant sub-lines, the IC50 values of the Wnt inhibitors LGK974 (range from 1.4 to 6.8 µM) and IWP-2 (0.7–4 µM) were not significantly correlated (Spearman’s rank order correlation coefficient, r = 0.28, p = 0.33). Neither LGK974 nor IWP-2 (10 µM) reached the IC50 with any cell line, using the WST-1 and Alamar Blue viability assays (Figure S3 in Supplementary Material).
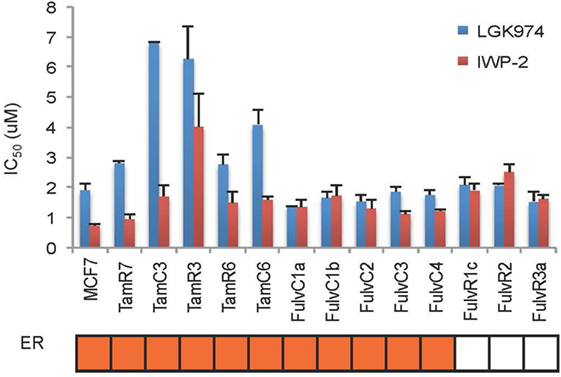
Figure 6. Comparison of LGK974 and IWP-2 drug sensitivity of breast cancer endocrine-resistant cell lines. IC50 values (50% inhibition of thymidine incorporation relative to non-drug-treated cells) for LGK974 and IWP-2 are represented on the y-axis and individual cell lines on the x-axis. Cell lines are colored orange for estrogen receptor-positive status (ER). Results are shown as the mean ± SE from triplicate experiments. IC50 values for LGK974 and IWP-2 were not significantly correlated (Spearman’s rank correlation coefficient, r = 0.28; p = 0.33).
As three dimension (3D) spheroid culture conditions allowed enrichment of cancer cells with a cancer stem cell phenotype, we next examined the sensitivities of our MCF-7 sub-lines to the LGK974 inhibitor in 3D spheroid culture and compared them to the sensitivities of the same cells grown in adherent monolayer culture conditions. Although there was no significant difference (T test, p > 0.05) in the IC50 of the 2D monolayer (range from 1.5 to 6.3 µM) and 3D spheroid culture conditions (range from 1.1 to 4.4 µM), a general trend of lower IC50 was observed in 3D spheroid culture conditions (Figure 7). The IC50 values arising from the two culture conditions were positively correlated (Spearman’s rank order correlation coefficient, r = 0.69, p = 0.01).
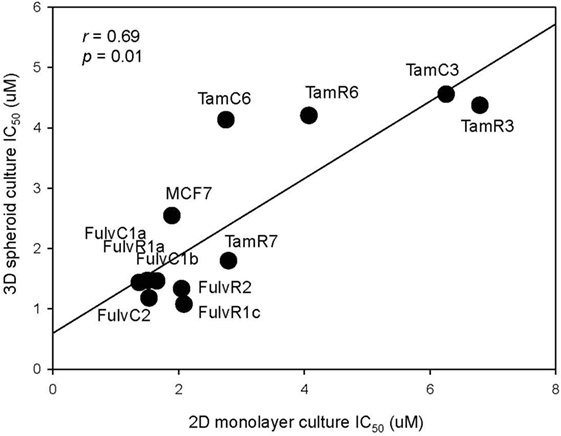
Figure 7. Correlation of drug sensitivity in monolayer cultures and in three dimension (3D) spheroid cultures. Plot of IC50 values for LGK974 in 2D monolayer culture (x-axis) and 3D spheroid culture (y-axis). Experiments were performed in triplicate wells and performed twice (Spearman’s rank correlation coefficient, r = 0.69, p = 0.01).
We next examined whether the expression levels of SOX2, AXIN2, or DKK1 were associated with altered drug sensitivity of the MCF-7 endocrine therapy-resistant sub-lines to LGK974. Expression of SOX2, AXIN2, and DKK1 and the growth inhibitory effects (IC50 value) of the Wnt inhibitor LGK974 were not significantly correlated (Table 3).
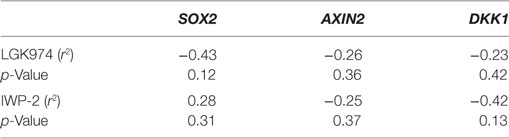
Table 3. Spearman’s rank order correlation coefficients of relative gene expression values (fold change) for AXIN2, DKK1, and SOX2 versus IC50 value of the WNT inhibitors LGK974 and IWP-2 in MCF-7 parental and endocrine therapy-resistant breast cancer cell lines (n = 14).
Discussion
The principal finding of this study is that it demonstrated the difference between early responses (Figures 1–3) and late stable phenotypes (Figures 4–7) following endocrine treatment of MCF-7 cells. Either exposure of cultured MCF-7 cells to 4-hydroxytamoxifen or depletion of estrogen from the growth medium leads after 7 days to threefold to sixfold increases in SOX2 expression. Increases are highly reproducible, evident within 2 days and persist for at least 1 month (Figures 1 and 2). These changes are accompanied by a decrease in proliferation rate, as shown by others (28). Longer term exposure to tamoxifen, or to estrogen deprivation, leads after several months to the emergence of discrete hormone-resistant subpopulations, distinguishable from the hormone-sensitive line by cellular DNA content and other properties (3, 4). Most but not all of these emerging populations continue to over-express SOX2 (Figure 4) and it was notable that the two lines (TamC3 and TamR3) that did not exhibit increased SOX2 expression instead showed increased PAX2 signaling (3). We hypothesize that upregulation of SOX2 expression is part of a stress response generated by endocrine therapy.
Several observations suggest that the stress response is associated with an increase in stem cell-like properties of the treated population. SOX2 is a recognized marker of stem cell properties (29) and we have found that components of the WNT pathway, which are also associated with stem cell-like properties (30), are increased in MCF-7 cells exposed to endocrine therapy. Significant increases in the Wnt target genes DKK1 and AXIN2 expression followed estrogen deprivation. Expression of DKK1 was increased transiently between 2 and 7 days (Figures 1 and 2) but not after 1 month of endocrine treatment. Some but not all sub-lines of MCF-7 emerging after endocrine therapy maintained upregulation of AXIN2 and DKK1 (Figure 5). SOX2 expression has previously been reported to be associated with increased expression of the Wnt effector genes AXIN2 and DKK1 (12), although in our analysis of TCGA tumor tissue samples, the expression levels of AXIN2 or DKK1 were not significantly correlated with those of SOX2. This lack of correlation suggests that the SOX2 and Wnt signaling pathways may be partially independent of each other; Wnt induction of AXIN2 and DKK1 may form negative feedback loops that dampen Wnt signaling, reducing the functional linkage to Wnt pathway inhibitor sensitivity (17, 31). The upregulation of AXIN2 expression in our endocrine therapy-resistant cell lines is in agreement with the findings of others (32). Expression of stem cell genes OCT4 and NANOG (transcription factors essential for self-renewal and pluripotency maintenance of stem cells) (33) was also increased in MCF-7 cells exposed to endocrine therapy as an early response. Increased efficiency of mammosphere formation and increased expression of the surface protein CD24 add further evidence in favor of the hypothesis that the MCF-7 breast cancer cells acquire stem cell-like properties as an early response to endocrine therapy. The increase in CD24 was consistent with the observation that estrogen mediated downregulation of CD24 in breast cancer cells (34). Knockdown of CD24 by siRNA is known to inhibit cell viability and invasion, to induce apoptosis, and to increase the sensitivity of MCF-7 cells to tamoxifen (35, 36).
The results with the MCF-7 cell line may provide insights into possible in vivo events in breast cancer patients undergoing hormone therapy. SOX2 levels are elevated in primary tumors of patients who do not respond to endocrine therapy (12), and SOX2 has been reported to maintain stem cell subpopulations in various cancers (37). Another report concludes that estrogen reduces the proportion of breast stem cells while tamoxifen increases it (38), consistent with our data. SOX2 overexpression has been correlated with breast cancer tumor grade (39), suggesting that it contributes to tumor aggression. SOX2 has also been implicated in endocrine resistance in breast cancer (12) and in the development of chemo-resistance in gastric cancer (40), glioblastoma (41), head and neck squamous cell carcinoma (42), lung cancer (43), and prostate cancer (44). Dysregulated Wnt signaling is observed in breast cancer (45, 46) and Wnt signaling has been reported to be an early event in a breast neoplasia model (47). The Wnt effector AXIN2 is upregulated in breast carcinoma (48, 49) and is involved in the Wnt-Axin2-GSK3beta cascade to regulate the epithelial–mesenchymal transition in breast cancer cells (50). Dickkopf-1 (DKK-1) is preferentially expressed in tumors with poor prognosis, ER− breast cancer and endocrine therapy-resistant tumors (51). Overexpression of DKK1 has also been reported in many cancers, including breast cancer, prostate cancer, lung cancer, and multiple myeloma (52–54). Activation of the Wnt pathway improves survival of human breast cancer cells and rescues ER+ tumor cells from tamoxifen (55).
In fact, CD44−/CD24+ is a marker of poor prognosis in early invasive breast cancer (56). CD24 overexpression has also been related to shorter distant metastasis-free survival in breast cancer patients (57). A proposed cancer stem cell model anticipates hierarchical organization but also constant clonal evolution of tumor cells (33). It is, therefore, plausible that early adaptive responses to tamoxifen favor the generation of CD44−/CD24+ cells and that continued therapy leads to stable phenotypes of CD44+/CD24− stem cell as reported (12). Alternatively, there may be technical differences as charcoal-stripped serum was used for our selection for stable phenotypes.
The above results suggest that targeting of the SOX2 and Wnt pathways might inhibit the emergence of endocrine-resistant breast cancer cells. We did not have inhibitors of SOX2 but did carry out preliminary studies on the inhibitory effects of the Porcupine inhibitors IWP-2 and LGK974, which target the Wnt pathway. LGK974 has been reported to inhibit Wnt secretion and reduce the expression of the Wnt target gene AXIN2 (21). The abilities of IWP-2 and LGK974 to inhibit cell proliferation were tested but the resulting IC50 values were not significantly correlated (Figure 6). We also measured the effect of LGK974 on MCF-7 cell lines in 3D spheroid culture, as compared to 2D monolayer culture and found significant correlation of the IC50 values (r = 0.69, p = 0.01), suggesting that further study of the Wnt pathway may be warranted as a therapeutic strategy. Alternatively, if there is a hierarchy of stem cells, Wnt inhibition may affect different signaling pathways at different times.
In conclusion, the large changes in expression of SOX2 in response to in vitro endocrine therapy, together with smaller changes in components of the Wnt pathway, especially those that are early in the response to 4-hydroxytamoxifen, suggest that they could be involved as an intrinsic part of the transition to hormone resistance. We suggest that a new state with increased stem cell-like character is induced and promotes phenotypic diversification such that the discrete emerging sub-lines show varying degrees of ER expression, EGFR expression, and mTOR pathway utilization (3). Further research on the mechanisms underlying such changes is warranted.
Author Contributions
EL and BB designed this study. EL, MA-A, DS, CF-P, and WJ carried out the study. EL, CF-P, and MA-A assisted with the data analysis. EL drafted the manuscript. GF and BB assisted with the manuscript preparation. All the authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AH and handling Editor declared their shared affiliation.
Funding
This work was supported by the Cancer Society New Zealand, the Cancer Society New Zealand − Auckland, the Maurice and Paykel Trust, Genesis Oncology Trust and the Auckland Cancer Society Research Centre.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fonc.2017.00184/full#supplementary-material.
Figure S1. Relative expression of estrogen receptor (ER), progesterone receptor (PR), and HER2 in SKRB3, MCF-7 and a selection of its sub-lines. Actin is shown as loading control.
Figure S2. Increased expression of NANOG and OCT4 in MCF-7 breast cancer cells in 4-hydroxytamoxifen or estrogen-deprived treatment. The expression of (A) NANOG and (B) OCT4 relative to MCF-7 control measured by qRT-PCR in MCF-7 breast cancer cells either exposed to 4-hydroxytamoxifen (100 nM) or grown in estrogen-deprived medium (EDM) for 1 week and 1 month, relative to the MCF-7 parental line, *p < 0.05.
Figure S3. Relative viability of LGK974 and IWP-2 among breast cancer endocrine-resistant cell lines. Viability was measured by (A) WST-1 and (B) Alamar blue. Results are shown as the mean ± SE from duplicate experiments.
Abbreviations
DKK1, Dickkopf-1; AXIN2, axis inhibition protein 2; SOX2, SRY (sex determining region Y)-box 2; OCT4, octamer-binding transcription factor 4, also known as POU5F1; NANOG, NANOG homeobox; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; qRT-PCR, quantitative reverse transcription polymerase chain reaction; PBS, phosphate-buffered saline; SDS, sodium dodecyl sulfate; TCGA, The Cancer Genome Atlas.
References
1. Bursch W, Ellinger A, Kienzl H, Torok L, Pandey S, Sikorska M, et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis (1996) 17(8):1595–607. doi:10.1093/carcin/17.8.1595
2. Martin LA, Pancholi S, Chan CM, Farmer I, Kimberley C, Dowsett M, et al. The anti-oestrogen ICI 182,780, but not tamoxifen, inhibits the growth of MCF-7 breast cancer cells refractory to long-term oestrogen deprivation through down-regulation of oestrogen receptor and IGF signalling. Endocr Relat Cancer (2005) 12(4):1017–36. doi:10.1677/erc.1.00905
3. Leung E, Kannan N, Krissansen GW, Findlay MP, Baguley BC. MCF-7 breast cancer cells selected for tamoxifen resistance acquire new phenotypes differing in DNA content, phospho-HER2 and PAX2 expression, and rapamycin sensitivity. Cancer Biol Ther (2010) 9(9):717–24. doi:10.4161/cbt.9.9.11432
4. Leung E, Kim JE, Askarian-Amiri M, Finlay GJ, Baguley BC. Evidence for the existence of triple-negative variants in the MCF-7 breast cancer cell population. Biomed Res Int (2014) 2014:7. doi:10.1155/2014/836769
6. Milani A, Geuna E, Mittica G, Valabrega G. Overcoming endocrine resistance in metastatic breast cancer: current evidence and future directions. World J Clin Oncol (2014) 5(5):990–1001. doi:10.5306/wjco.v5.i5.990
7. Stone A, Valdes-Mora F, Clark SJ. Exploring and exploiting the aberrant DNA methylation profile of endocrine-resistant breast cancer. Epigenomics (2013) 5(6):595–8. doi:10.2217/epi.13.70
8. Leung EY, Askarian-Amiri M, Finlay GJ, Rewcastle GW, Baguley BC. Potentiation of growth inhibitory responses of the mTOR inhibitor everolimus by dual mTORC1/2 inhibitors in cultured breast cancer cell lines. PLoS One (2015) 10(7):e0131400. doi:10.1371/journal.pone.0131400
9. Leung EY, Kim JE, Askarian-Amiri M, Joseph WR, McKeage MJ, Baguley BC. Hormone resistance in two MCF-7 breast cancer cell lines is associated with reduced mTOR signaling, decreased glycolysis, and increased sensitivity to cytotoxic drugs. Front Oncol (2014) 4:221. doi:10.3389/fonc.2014.00221
10. Leung EY, Kim JE, Askarian-Amiri M, Rewcastle GW, Finlay GJ, Baguley BC. Relationships between signaling pathway usage and sensitivity to a pathway inhibitor: examination of trametinib responses in cultured breast cancer lines. PLoS One (2014) 9(8):e105792. doi:10.1371/journal.pone.0105792
11. Leung E, Kim JE, Rewcastle GW, Finlay GJ, Baguley BC. Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol Ther (2011) 11(11):938–46. doi:10.4161/cbt.11.11.15527
12. Piva M, Domenici G, Iriondo O, Rabano M, Simoes BM, Comaills V, et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med (2014) 6(1):66–79. doi:10.1002/emmm.201303411
13. Kim SK, Yang JW, Kim MR, Roh SH, Kim HG, Lee KY, et al. Increased expression of Nrf2/ARE-dependent anti-oxidant proteins in tamoxifen-resistant breast cancer cells. Free Radic Biol Med (2008) 45(4):537–46. doi:10.1016/j.freeradbiomed.2008.05.011
14. Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol (2010) 28(25):4006–12. doi:10.1200/JCO.2009.27.5388
15. Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell (2012) 10(4):440–54. doi:10.1016/j.stem.2012.02.016
16. Jang GB, Kim JY, Cho SD, Park KS, Jung JY, Lee HY, et al. Blockade of Wnt/beta-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep (2015) 5:12465. doi:10.1038/srep12465
17. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell (2009) 17(1):9–26. doi:10.1016/j.devcel.2009.06.016
18. Leung E, Rewcastle GW, Joseph WR, Rosengren RJ, Larsen L, Baguley BC. Identification of cyclohexanone derivatives that act as catalytic inhibitors of topoisomerase I: effects on tamoxifen-resistant MCF-7 cancer cells. Invest New Drugs (2012) 30(6):2103–12. doi:10.1007/s10637-011-9768-4
19. Askarian-Amiri ME, Seyfoddin V, Smart CE, Wang J, Kim JE, Hansji H, et al. Emerging role of long non-coding RNA SOX2OT in SOX2 regulation in breast cancer. PLoS One (2014) 9(7):e102140. doi:10.1371/journal.pone.0102140
20. Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C-W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol (2009) 5(2):100–7. doi:10.1038/nchembio.137
21. Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A (2013) 110(50):20224–9. doi:10.1073/pnas.1314239110
22. Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst (1973) 51(5):1409–16.
23. Leung E, Hung JM, Barker D, Reynisson J. The effect of a thieno[2,3-b] pyridine PLC-gamma inhibitor on the proliferation, morphology, migration and cell cycle of breast cancer cells. MedChemComm (2014) 5(1):99–106. doi:10.1039/C3md00290j
24. Smart CE, Morrison BJ, Saunus JM, Vargas AC, Keith P, Reid L, et al. In vitro analysis of breast cancer cell line tumourspheres and primary human breast epithelia mammospheres demonstrates inter- and intrasphere heterogeneity. PLoS One (2013) 8(6):e64388. doi:10.1371/journal.pone.0064388
25. Fukazawa H, Mizuno S, Uehara Y. A microplate assay for quantitation of anchorage-independent growth of transformed cells. Anal Biochem (1995) 228(1):83–90. doi:10.1006/abio.1995.1318
26. Peskin AV, Winterbourn CC. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin Chim Acta; Int J Clin Chem (2000) 293(1–2):157–66.
27. Leung E, Baguley BC. mTOR signaling in endocrine resistance growth control. Res Biol Cancer (2013); mTOR Signaling in Endocrine Resistance Growth Control. Cervical, Breast and Prostate Cancer.
28. Osborne CK, Boldt DH, Clark GM, Trent JM. Effects of tamoxifen on human breast cancer cell cycle kinetics: accumulation of cells in early G1 phase. Cancer Res (1983) 43(8):3583–5.
29. Liu A, Yu X, Liu S. Pluripotency transcription factors and cancer stem cells: small genes make a big difference. Chin J Cancer (2013) 32(9):483–7. doi:10.5732/cjc.012.10282
30. Mohammed MK, Shao C, Wang J, Wei Q, Wang X, Collier Z, et al. Wnt/beta-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis (2016) 3(1):11–40. doi:10.1016/j.gendis.2015.12.004
31. Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell (2016) 165(1):45–60. doi:10.1016/j.cell.2016.02.025
32. Loh YN, Hedditch EL, Baker LA, Jary E, Ward RL, Ford CE. The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer (2013) 13:174. doi:10.1186/1471-2407-13-174
33. Fonseca NA, Cruz AF, Moura V, Simoes S, Moreira JN. The cancer stem cell phenotype as a determinant factor of the heterotypic nature of breast tumors. Crit Rev Oncol Hematol (2017) 113:111–21. doi:10.1016/j.critrevonc.2017.03.016
34. Kaipparettu BA, Malik S, Konduri SD, Liu W, Rokavec M, van der Kuip H, et al. Estrogen-mediated downregulation of CD24 in breast cancer cells. Int J Cancer (2008) 123(1):66–72. doi:10.1002/ijc.23480
35. Ma ZL, Chen YP, Song JL, Wang YQ. Knockdown of CD24 inhibits proliferation, invasion and sensitizes breast cancer MCF-7 cells to tamoxifen in vitro. Eur Rev Med Pharmacol Sci (2015) 19(13):2394–9.
36. Kim HJ, Kim JB, Lee KM, Shin I, Han W, Ko E, et al. Isolation of CD24(high) and CD24(low/-) cells from MCF-7: CD24 expression is positively related with proliferation, adhesion and invasion in MCF-7. Cancer Lett (2007) 258(1):98–108. doi:10.1016/j.canlet.2007.08.025
37. Weina K, Utikal J. SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med (2014) 3:19. doi:10.1186/2001-1326-3-19
38. Simoes BM, Piva M, Iriondo O, Comaills V, Lopez-Ruiz JA, Zabalza I, et al. Effects of estrogen on the proportion of stem cells in the breast. Breast Cancer Res Treat (2011) 129(1):23–35. doi:10.1007/s10549-010-1169-4
39. Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem (2008) 283(26):17969–78. doi:10.1074/jbc.M802917200
40. Tian T, Zhang Y, Wang S, Zhou J, Xu S. Sox2 enhances the tumorigenicity and chemoresistance of cancer stem-like cells derived from gastric cancer. J Biomed Res (2012) 26(5):336–45. doi:10.7555/JBR.26.20120045
41. Jeon HM, Sohn YW, Oh SY, Kim SH, Beck S, Kim S, et al. ID4 imparts chemoresistance and cancer stemness to glioma cells by derepressing miR-9*-mediated suppression of SOX2. Cancer Res (2011) 71(9):3410–21. doi:10.1158/0008-5472.CAN-10-3340
42. Lee SH, Oh SY, Do SI, Lee HJ, Kang HJ, Rho YS, et al. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br J Cancer (2014) 111(11):2122–30. doi:10.1038/bjc.2014.528
43. Chou YT, Lee CC, Hsiao SH, Lin SE, Lin SC, Chung CH, et al. The emerging role of SOX2 in cell proliferation and survival and its crosstalk with oncogenic signaling in lung cancer. Stem Cells (2013) 31(12):2607–19. doi:10.1002/stem.1518
44. Li D, Zhao LN, Zheng XL, Lin P, Lin F, Li Y, et al. Sox2 is involved in paclitaxel resistance of the prostate cancer cell line PC-3 via the PI3K/Akt pathway. Mol Med Rep (2014) 10(6):3169–76. doi:10.3892/mmr.2014.2630
45. Dey N, Young B, Abramovitz M, Bouzyk M, Barwick B, De P, et al. Differential activation of Wnt-beta-catenin pathway in triple negative breast cancer increases MMP7 in a PTEN dependent manner. PLoS One (2013) 8(10):e77425. doi:10.1371/journal.pone.0077425
46. Lamb R, Ablett MP, Spence K, Landberg G, Sims AH, Clarke RB. Wnt pathway activity in breast cancer sub-types and stem-like cells. PLoS One (2013) 8(7):e67811. doi:10.1371/journal.pone.0067811
47. Khalil S, Tan GA, Giri DD, Zhou XK, Howe LR. Activation status of Wnt/ss-catenin signaling in normal and neoplastic breast tissues: relationship to HER2/neu expression in human and mouse. PLoS One (2012) 7(3):e33421. doi:10.1371/journal.pone.0033421
48. Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci U S A (2006) 103(10):3799–804. doi:10.1073/pnas.0600065103
49. Gabrovska PN, Smith RA, Tiang T, Weinstein SR, Haupt LM, Griffiths LR. Development of an eight gene expression profile implicating human breast tumours of all grade. Mol Biol Rep (2012) 39(4):3879–92. doi:10.1007/s11033-011-1167-6
50. Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol (2006) 8(12):1398–406. doi:10.1038/ncb1508
51. Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, et al. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer (2007) 96(4):646–53. doi:10.1038/sj.bjc.6603579
52. Bu G, Lu W, Liu CC, Selander K, Yoneda T, Hall C, et al. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer (2008) 123(5):1034–42. doi:10.1002/ijc.23625
53. Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate (2008) 68(13):1396–404. doi:10.1002/pros.20805
54. Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res (2010) 70(13):5326–36. doi:10.1158/0008-5472.CAN-09-3879
55. Schlange T, Matsuda Y, Lienhard S, Huber A, Hynes NE. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res (2007) 9(5):R63. doi:10.1186/bcr1769
56. Ahmed MA, Aleskandarany MA, Rakha EA, Moustafa RZ, Benhasouna A, Nolan C, et al. A CD44(-)/CD24(+) phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat (2012) 133(3):979–95. doi:10.1007/s10549-011-1865-8
Keywords: breast cancer, endocrine resistance, SOX2, Wnt signaling pathway, stem cell CD24 CD44
Citation: Leung EY, Askarian-Amiri ME, Sarkar D, Ferraro-Peyret C, Joseph WR, Finlay GJ and Baguley BC (2017) Endocrine Therapy of Estrogen Receptor-Positive Breast Cancer Cells: Early Differential Effects on Stem Cell Markers. Front. Oncol. 7:184. doi: 10.3389/fonc.2017.00184
Received: 24 April 2017; Accepted: 08 August 2017;
Published: 04 September 2017
Edited by:
Valerie Malyvanh Jansen, Vanderbilt University, United StatesReviewed by:
Ariella Hanker, Vanderbilt University, United StatesNeil Bhola, University of California, San Francisco, United States
Copyright: © 2017 Leung, Askarian-Amiri, Sarkar, Ferraro-Peyret, Joseph, Finlay and Baguley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Euphemia Y. Leung, e.leung@auckland.ac.nz;
Bruce C. Baguley, b.baguley@auckland.ac.nz
 Euphemia Y. Leung
Euphemia Y. Leung Marjan E. Askarian-Amiri
Marjan E. Askarian-Amiri Debina Sarkar
Debina Sarkar Carole Ferraro-Peyret1,3,4,5
Carole Ferraro-Peyret1,3,4,5 Wayne R. Joseph
Wayne R. Joseph Graeme J. Finlay
Graeme J. Finlay Bruce C. Baguley
Bruce C. Baguley