- 1Department of Gerontology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Nephrology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Gerontology, Nanjing General Hospital, Nanjing, China
- 4Department of Physiology, University of Cincinnati, Cincinnati, OH, United States
Background: Although endothelial cell protein C receptor (EPCR) gene Ser219Gly polymorphism has been associated with venous thromboembolism (VTE) susceptibility, no clear consensus has yet been reached.
Objective and methods: A meta-analysis of 9,494 subjects from 13 individual studies was conducted to better elucidate the potential relationship between the EPCR gene Ser219Gly polymorphism and VTE. Pooled odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were evaluated by using fixed or random effect models.
Results: The current meta-analysis suggested that there was a significant association between EPCR gene Ser219Gly polymorphism and VTE under allelic (OR: 1.42, 95% CI: 1.21–1.66, P = 1.30 × 10−5), recessive (OR: 2.02, 95% CI: 1.44–2.85, P = 5.35 × 10−5), homozygous (OR: 2.24, 95% CI: 1.59–3.16, P = 3.66 × 10−6), and additive genetic models (OR: 1.63, 95% CI: 1.30–2.04, P = 2.24 × 10−5).
Conclusions: EPCR gene Ser219Gly polymorphism was associated with an elevated risk of VTE and the Gly residue carriers of the EPCR gene might be predisposed to VTE.
Introduction
Venous thromboembolism (VTE) encompasses deep vein thrombosis (DVT) and pulmonary empolism (PE). DVT indicates the formation of a venous thrombus typically in the deep veins of the legs, which may undergo embolization when the clot is dislodged from the underlying endothelium and carried downstream by circulatory system potentially reaching the lungs where it can cause a sudden occlusion of pulmonary circulation, referred to as PE. This event presents as a clinical emergency that can endanger the patient's life. In Western countries, VTE morbidity is around 1–2% in the whole population and rising. The early mortality of the DVT and PE are as high as 3.8 and 38.9%, respectively (Naess et al., 2007).
Hypercoagulability plays a major role in clot formation and can be mediated by impaired functioning of anti-coagulant pathways, such as the Protein C system. Protein C interacts with the endothelial protein C receptor (EPCR) expressed on the endothelium of the great vessels to cleave plasminogen to plasmin, a potent anti-coagulant (Castellino and Ploplis, 2009). EPCR is a Vitamin K-dependent Type-1 transmembrane protein that exhibits the same homology as the MHC class I/CD1 family of proteins (Oganesyan et al., 2002). It can also exist in a soluble form in the plasma (sEPCR) when cleaved by matrix metalloproteinases (MMP) (Xu et al., 2000; Bae et al., 2007).
Gene mutations in the EPCR gene, which is located on 20q11.2, may push hemostasis toward hypercoagulable state. It spans 6 kb, encodes for 241 amino acids, and is organized into 4 exons and 3 introns (Li et al., 2015). The Ser219Gly polymorphism that we are interested in involves an A-G base transition at either the 6,936th or 4,600th position. This mutation results in a Gly-Gly structure in the transmembrane region of the protein that could potentially reduce the stability of the helical structure and make it more vulnerable to MMP-mediated cleavage. Decreasing the amount of EPCR expressed on the cytomembrane could limit Protein C activity, resulting in a pro-thrombotic state in the body (Chen et al., 2011).
The frequent of Ser219Gly polymorphism in the general population is about 12% (Wang and Hu, 2008). Many studies have been conducted to elucidate the relationship between the EPCR Ser219Gly gene polymorphism and VTE, but the research has yet to present a clear consensus. In 2015, Sun et al. found that individuals with DVT exhibited higher Gly residue frequencies and concluded that the EPCR gene Ser219Gly polymorphism was associated with the DVT risk in a Chinese population (Sun and Yin, 2015). Looking at an Egyptian population, Zoheir et al. also found that mutant genotypes of EPCR gene 6936AG polymorphism (AG, GG) were associated with an increased risk for DVT as well as its mutant allele G (Zoheir et al., 2016). On the other hand, Anastasiou et al. found prevalence of the rare EPCR 219Gly residues to be comparable between patients with thrombotic disorders and controls in a Greek population. The EPCR gene Ser219Gly polymorphism seemed to have no impact on VTE recurrence (Anastasiou et al., 2016).
To determine whether the EPCR gene Ser219Gly polymorphism was associated with VTE susceptibility, the current meta-analysis of 9,494 subjects from 13 individual studies was conducted (Supplements S1).
Materials and Methods
Publication Search and Inclusion Criteria
We conducted a primary search using the terms “EPCR,” “Ser219Gly,” “6936A/G,” “4600A/G,” “rs867186,” “venous thromboembolism,” and “polymorphism,” through PubMed, WanFang database, the VIP database, the China National Knowledge Infrastructure, Embase, and the Web of Science. This search yielded papers published between 2004 and 2016 with the most recent update occurring on May 06, 2017.
The following inclusion criteria had to be met by the selected studies for our meta-analysis. Studies selected must: (a) assess the association of VTE and EPCR gene Ser219Gly polymorphism, (b) diagnose DVT by Doppler ultrasonography of the lower extremities, or diagnose PE by compression or ventilation lung ultrasonography, contrast venography, lung ventilation/perfusion scan, conventional pulmonary angiography, or computed tomographic angiography, (c) have control groups at Hardy-Weinberg equilibrium (HWE). The studies must be officially published cohort or case-control studies.
Data Extraction
The data was abstracted according to a standardized protocol by three authors. Two were responsible for removing duplicate studies while the third acted as an arbitrator to resolve any disagreements. Studies that did not meet inclusion criteria, were published repeatedly, or did not supply sufficient data were removed. Similar data sets presented in multiple publications by a single author group were represented once in the current meta-analysis. The listed data table also had to include items such as the first author's name, publication year, region, genotyping method, matching criteria, the genotype number in the VTE and control groups, and sample size of VTE and controls (Table 1).
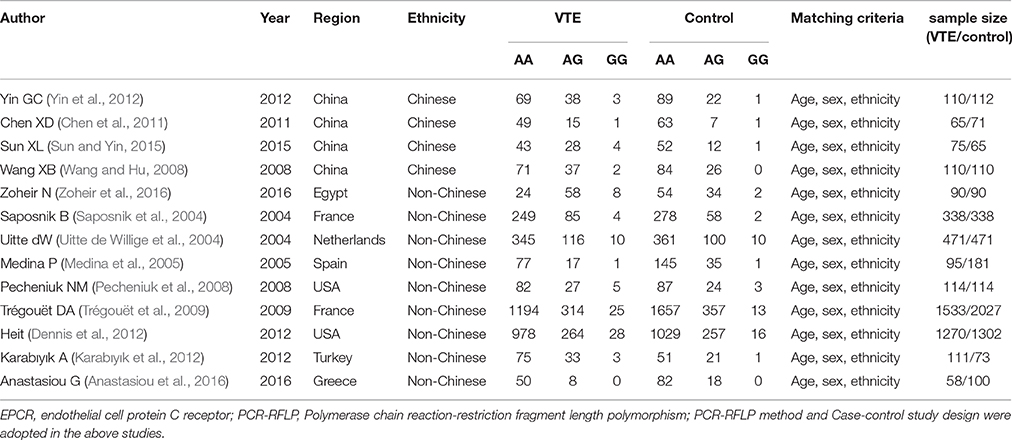
Table 1. Characteristics of the investigated studies of the association between the endothelial cell protein C receptor (EPCR) gene rs867186 polymorphism and venous thromboembolism (VTE).
Statistical Analyses
Four genetic models were used in the current meta-analysis: allelic (G allele distribution frequency), recessive (GG vs. AG and AA), homozygous (GG vs. AA), and additive (G vs. A). The relationship of EPCR gene Ser219Gly polymorphism and VTE was compared by using odds ratios (ORs) and their corresponding 95% confidence intervals (CIs). Chi-square-based Q-tests were used to calculate the heterogeneity among the studies with significance set at P < 0.05 level (Cochran, 1968). If no heterogeneity was detected among the included studies, the fixed-effect model (the Mantel–Haenszel method) would be used (Mantel and Haenszel, 1959). Otherwise, the random-effect model (the DerSimonian and Laird method) would be used (DerSimonian and Laird, 1986). Z-test was used to assess the pooled OR and the significance was set at P < 0.05 level. The effects of population stratification have also been conducted. The sensitivity analysis has been performed to determine the pooled results stability.
The Fisher's exact test was used to evaluate the HWE and the significance was set at P < 0.05 level. The funnel plot was used to assess the potential publication bias. The Egger's linear regression test on a natural log scale of the OR was used to evaluate the funnel plot symmetry and the significance was set at P < 0.05 level (Egger et al., 1997). The softwares Stata 12.0 and Review Manager 5.0 were used to perform the statistical analyses (StataCorp, College Station, TX, USA).
Results
Studies and Populations
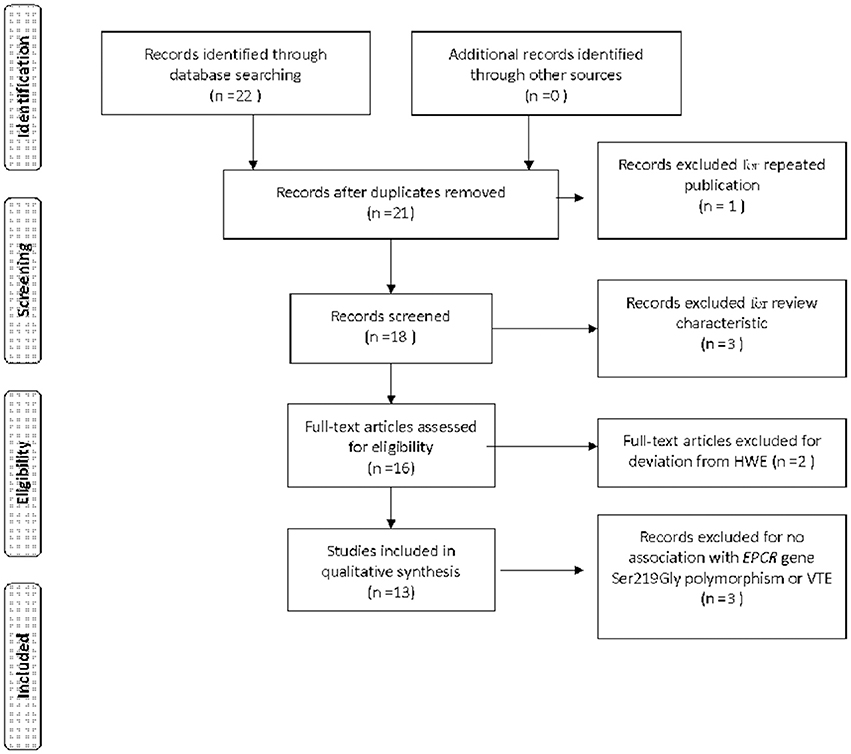
Data was extracted from 4,440 VTE cases and 5,054 controls (Table 1) (Saposnik et al., 2004; Uitte de Willige et al., 2004; Medina et al., 2005; Pecheniuk et al., 2008; Wang and Hu, 2008; Trégouët et al., 2009; Chen et al., 2011; Dennis et al., 2012; Karabıyık et al., 2012; Yin et al., 2012; Sun and Yin, 2015; Anastasiou et al., 2016; Zoheir et al., 2016). Of the 22 papers acquired through the initial retrieval process, 13 were recruited for the current meta-analysis. Among the nine rejected studies, three papers were reviews and two did not meet HWE (Medina et al., 2004; Navarro et al., 2008). One paper was published by the same author group to that by Medina et al. in 2005 (Yamagishi et al., 2009). Three papers were not related to the topic at hand (Figure 1).
Pooled Analyses
Our meta-analysis suggests a significant association between the EPCR gene Ser219Gly polymorphism and VTE under allelic (OR: 1.42, 95% CI: 1.21–1.66, P = 1.30 × 10−5), recessive (OR: 2.02, 95% CI: 1.44–2.85, P = 5.35 × 10−5), homozygous (OR: 2.24, 95% CI: 1.59–3.16, P = 3.66 × 10−6), and additive genetic models (OR: 1.63, 95% CI: 1.30–2.04, P = 2.24 × 10−5, Table 2, Figures 2–5).
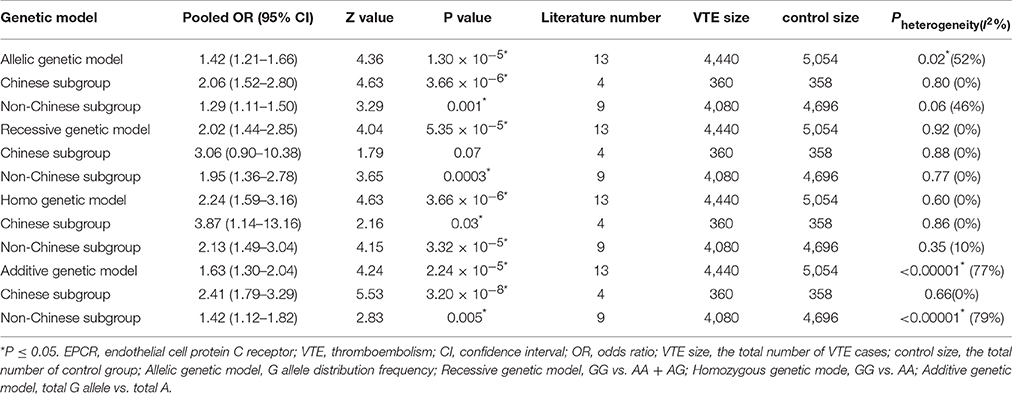
Table 2. Summary of meta-analysis of association between endothelial cell protein C receptor (EPCR) gene rs867186 polymorphism and VTE.
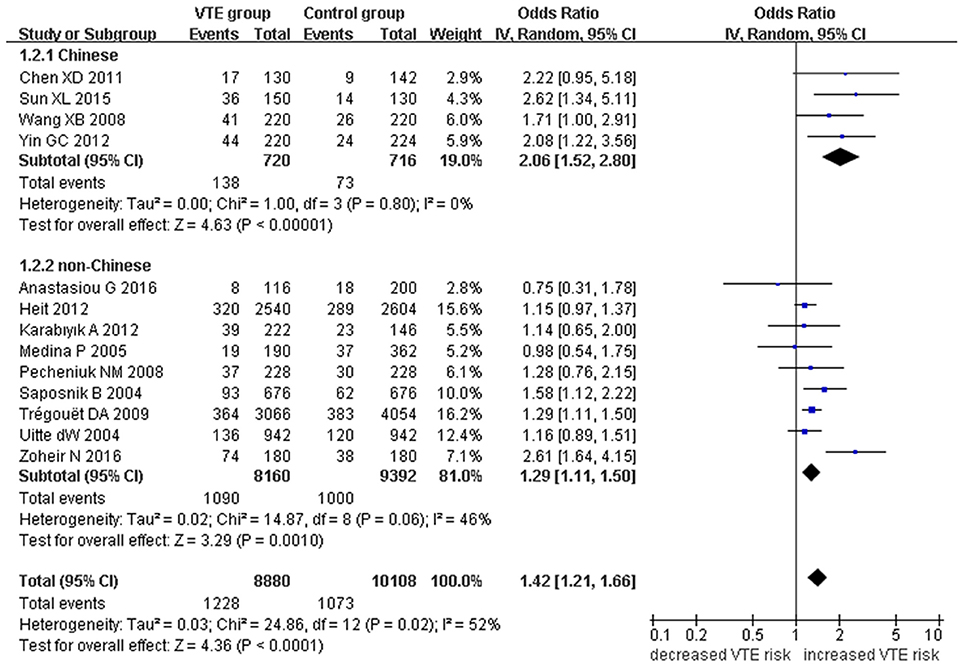
Figure 2. Forest plot of VTE associated with EPCR gene Ser219Gly polymorphism under an allelic genetic model (distribution of Gly residue frequency of EPCR gene Ser219Gly polymorphism).
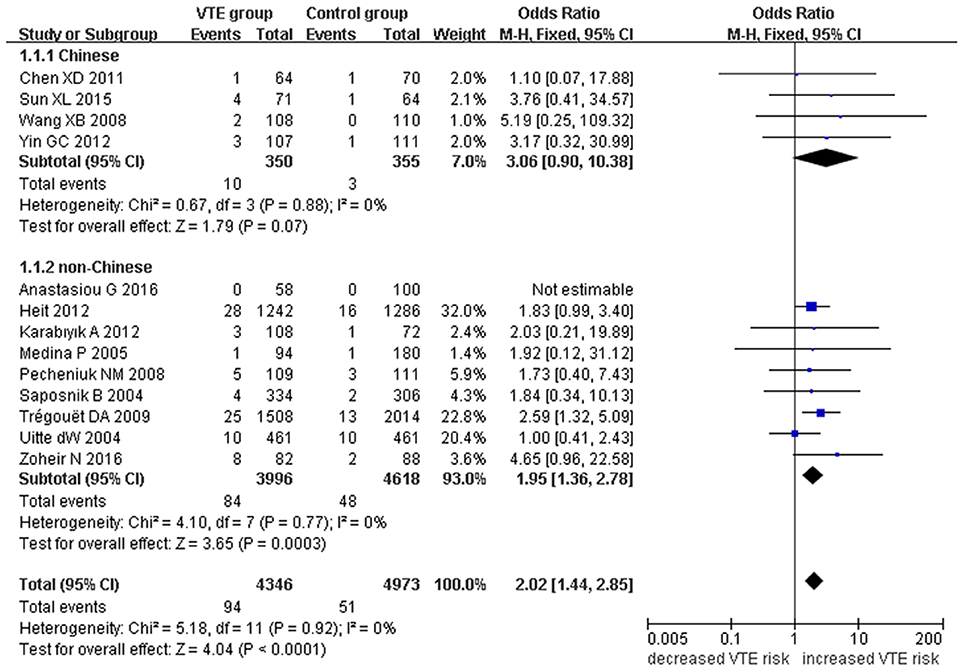
Figure 3. Forest plot of VTE associated with EPCR gene Ser219Gly polymorphism under a recessive genetic model (GG vs. AA + AG).
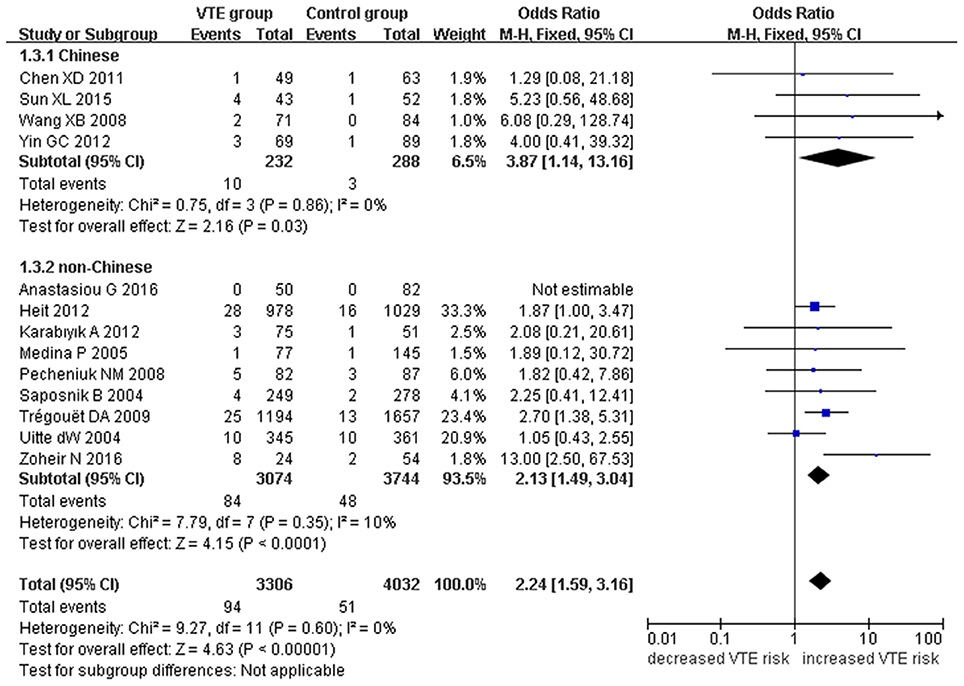
Figure 4. Forest plot of VTE associated with EPCR gene Ser219Gly polymorphism under a homozygous genetic model (GG vs. AA).
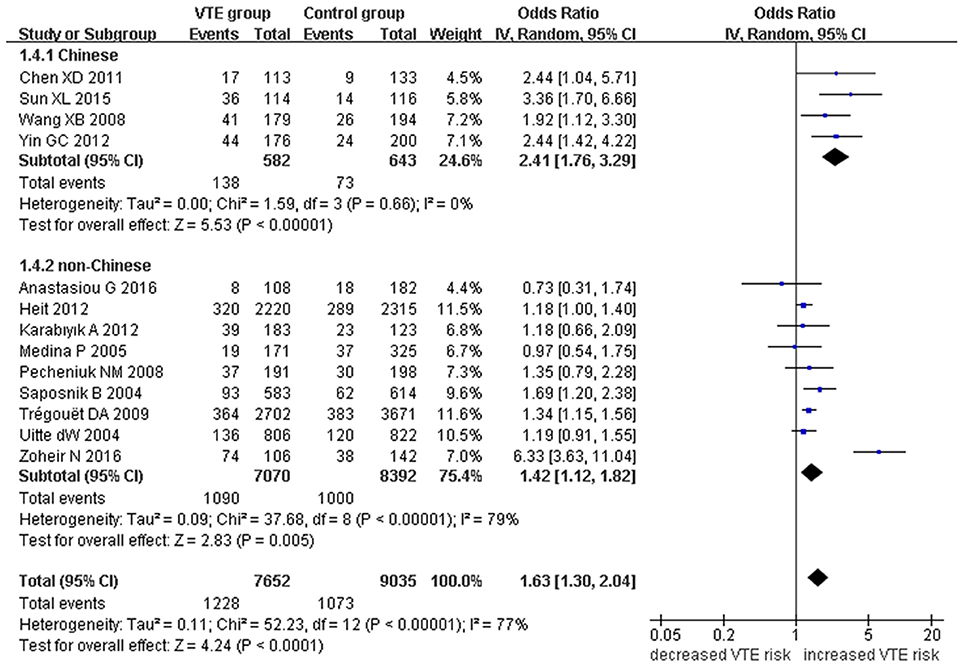
Figure 5. Forest plot of VTE associated with EPCR gene Ser219Gly polymorphism under an additive genetic model (total G allele vs. total A).
We also analyzed subgroups, stratified by ethnicity. In the Chinese subgroup, we found significant association between EPCR gene Ser219Gly polymorphism and VTE under allelic (OR: 2.06, 95% CI: 1.52–2.80, P = 3.66 × 10−6), homozygous (OR: 3.87, 95% CI: 1.14–13.16, P = 0.03), and additive genetic models (OR: 2.41, 95% CI: 1.76–3.29, P = 3.20 × 10−8). No significant association was detected under the recessive genetic model (OR: 3.06, 95% CI: 0.90–10.38, P = 0.07).
In the non-Chinese subgroup, a significant association between them was also observed under allelic (OR: 1.29, 95% CI: 1.11–1.50, P = 0.001), recessive (OR: 1.95, 95% CI: 1.36–2.78, P = 0.0003), homozygous (OR: 2.13, 95% CI: 1.49–3.04, P = 0.03), and additive genetic models (OR: 1.42, 95% CI: 1.12–1.82, P = 0.005).
In the whole population, no significant heterogeneity was detected under recessive or homozygous genetic models (P > 0.05). However, allelic and additive genetic models exhibited significant heterogeneity (P > 0.05). In the subgroup analysis, no significant heterogeneity was detected under all of the genetic models in the Chinese population (P > 0.05). Regard to the non-Chinese population, heterogeneity was detected under the additive genetic models (P < 0.05). This suggests that the source of heterogeneity was associated with ethnicity.
Sensitivity Analysis
Removing the study by Zoheir et al. (2016) eliminated the heterogeneity detected in the non-Chinese population while still providing results consistent with our initial findings. The sensitivity analysis has shown that a more significant association between them was found after the study by Uitte de Willige et al. (2004) was omitted under the recessive genetic model (OR: 2.26, 95% CI: 1.55–3.29). However, removing any study in the current meta-analysis does not generate the inconsistent results with the initial findings (Figure 6).
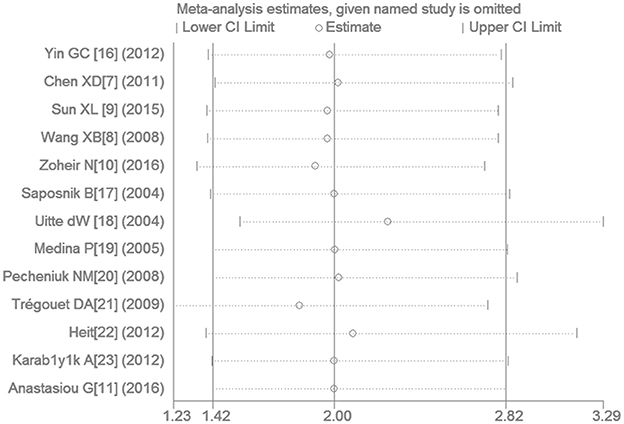
Figure 6. The sensitivity analysis for studies of the association of VTE and EPCR gene Ser219Gly polymorphism under recessive genetic model (GG vs. AA + AG).
Bias Diagnostics
No visible publication bias has been detected in the funnel plot under the recessive genetic model (Figure 7). Furthermore, no significant difference was found in the Egger's test, which implied that there was no publication bias in this meta-analysis under recessive genetic model (T = 1.13, P = 0.283, Figure 8).
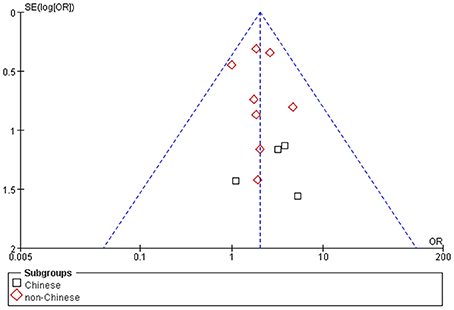
Figure 7. The funnel plot for studies of the association of VTE and EPCR gene Ser219Gly polymorphism under recessive genetic model (GG vs. AA + AG). The horizontal and vertical axis correspond to the OR and confidence limits. OR, odds ratio; SE, standard error.
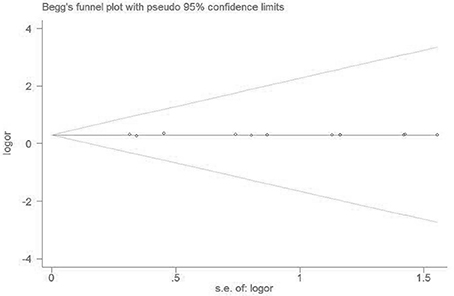
Figure 8. The Begg's funnel plot for studies of the association of VTE and EPCR gene Ser219Gly polymorphism under recessive genetic model (GG vs. AA + AG). The horizontal and vertical axis correspond to the LogOR and confidence limits. OR, odds ratio; SE, standard error.
Discussion
In the current meta-analysis, we found a significant association between the EPCR gene Ser219Gly polymorphism and VTE under allelic (OR: 1.42), recessive (OR: 2.02), homozygous (OR: 2.24), and additive genetic models (OR: 1.63). This suggests that the EPCR gene Ser219Gly polymorphism is associated with an elevated risk of VTE and that carriers of the G allele of the EPCR gene at the 6,936th or 4,600th position may be predisposed to VTE. Our analysis of the data by ethnicity echoed our initial findings with significant association detected in both the non-Chinese and Chinese ethnicities. Although heterogeneity was detected under allelic, and additive genetic models in the whole population (Pheterogeneity < 0.05), our subgroup analysis found it to be limited to the non-Chinese group, suggesting ethnicity to be the main source of heterogeneity (Pheterogeneity > 0.05).
EPCR is primarily expressed on the endothelial apical cell surface of the big vessels, but is also found in relatively high amounts in the placenta, lung, liver, and heart (Chen and Yuan, 2007; Iverson and Gomez, 2013). It also plays a role in many biological processes. Its canonical role is in the amplification of thrombin-activated Protein C activity, but EPCR has also demonstrated anti-apoptotic and inflammatory properties through the inhibition of the protease-activated receptor 1 and neutrophil granulocyte adhesion molecule CD11b/CD18, respectively (Riewald et al., 2002; Joyce et al., 2004).
In vitro and in vivo experiments show that EPCR expression is inhibited by various molecules, such as lipopolysaccharide (LPS), interleukin-1â (IL-1â), TNF-α, and thrombin. EPCR is also subject to MMP-mediated cleavage with the resultant sEPCR contributing to a pro-coagulation state through two mechanisms. Firstly, it acts as a competitive inhibitor by sEPCR by maintaining its interactions with activated Protein C, but no longer contributing to the thrombin-thrombomodulin (TM) system (Yin and Jin, 2013). sEPCR can also induce conformational changes in the APC active sites that reduce its ability to inhibit coagulation factor Va (Liaw et al., 2000).
The role of EPCR gene Ser219Gly mutation in facilitating the removal of EPCR from the cytomembrane and increasing levels of sEPCR in the plasma was fully verified through in vitro experiments. In a cell line with the Ser219Gly polymorphism, the EPCR drop rate was 5–7 times that of a WT cell line when stimulated with myristic acid-phorbol-acetic acid ester (Qu et al., 2006). This increase was also correlated to a hypercoagulation state.
We believe that the current meta-analysis offers an improved perspective on the subject. In 2012, Dennis et al. have performed a meta-analysis on the relationship between EPCR gene Ser219Gly variant and common thrombotic disorders risk (Dennis et al., 2012) that found a significant association between the EPCR rs867186 variant and VTE, but their analysis included studies by Medina et al. in 2004 and Yamagishi et al. in 2009 that had non-HWE controls (Medina et al., 2004; Yamagishi et al., 2009). The individual study by Navarro et al. (2008) was performed by the same author group to the study by Medina et al. (2005). However, both were adopted by their meta-analysis. By excluding studies that had non-HWE populations and adopting studies conducted by the same author groups to a single application, we believe our meta-analysis presents a more dependable conclusion. Our study also had broad representation of the Chinese and non-Chinese population. All four studies performed in Chinese population were used in our meta-analysis (Egger et al., 1997; Wang and Hu, 2008; Chen et al., 2011; Sun and Yin, 2015) and all studies in the non-Chinese population published after 2012 were also included in the current meta-analysis (Karabıyık et al., 2012; Anastasiou et al., 2016; Zoheir et al., 2016).
This study is not without limitations, however. We still lack the multiple large-scale studies necessary to completely understand the relationship between the EPCR gene Ser219Gly polymorphism and VTE. Furthermore, environmental factors that play significant roles in VTE development, such as trauma and surgery, were not controlled for. The micro-effects of many other related genes have yet to be fully understood (i.e., Methylenetetrahydropholate reductase gene C677T polymorphism, the coagulation factor XI gene rs2289252 and rs2036914 loci polymorphism) (Yin and Jin, 2013; Zhang et al., 2016).
In conclusion, EPCR gene Ser219Gly polymorphism was significantly associated with VTE susceptibility. The persons with the Gly residue of EPCR gene Ser219Gly polymorphism might be susceptible to VTE. More studies on the relationship between them should be carried out to further clarify this conclusion.
Author Contributions
YL and JW researched data. YL and HK wrote manuscript, researched data. YL, HG, and XY reviewed/edited manuscript. YL contributed to discussion, reviewed/edited manuscript. YL and GG researched data, contributed discussion.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (NSFC 81100073 to YL), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University for YL (2013-2015, JX2161015034), Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Thank all our colleagues working in the Department of geriatrics, the First Affiliated Hospital of Nanjing Medical University.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2017.00339/full#supplementary-material
References
Anastasiou, G., Politou, M., Rallidis, L., Grouzi, E., Karakitsos, P., Merkouri, E., et al. (2016). Endothelial protein C receptor gene variants and risk of thrombosis. Clin. Appl. Thromb. Hemost. 22, 199–204. doi: 10.1177/1076029614547261
Bae, J. S., Yang, L., and Rezaie, A. R. (2007). Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 104, 2867–2872. doi: 10.1073/pnas.0611493104
Castellino, F. J., and Ploplis, V. A. (2009). The protein C pathway and pathologic processes. J. Thromb. Haemost. 7(Suppl. 1), 140–145. doi: 10.1111/j.1538-7836.2009.03410.x
Chen, P., and Yuan, Y. Z. (2007). Advance of thrombomodulin-activated protein C-endothelial cell protein C receptor system for anti-inflammation. Int. J. Intern. Med. 34, 347–354.
Chen, X. D., Tian, L., Li, M., Jin, W., Zhang, H. K., and Zheng, C. F. (2011). Relationship between endothelial cell protein C receptor gene 6936A/G polymorphisms and deep venous thrombosis. Chin. Med. J. 124, 72–75. doi: 10.3760/cma.j.issn.0366-6999.2011.01.014
Cochran, W. G. (1968). The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics 24, 295–313. doi: 10.2307/2528036
Dennis, J., Johnson, C. Y., Adediran, A. S., de Andrade, M., Heit, J. A., Morange, P. E., et al. (2012). The endothelial protein C receptor (PROCR) Ser219Gly variant and risk of common thrombotic disorders: a HuGE review and meta-analysis of evidence from observational studies. Blood 119, 2392–2400. doi: 10.1182/blood-2011-10-383448
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634. doi: 10.1136/bmj.315.7109.629
Iverson, R. E., and Gomez, J. L. (2013). Deep venous thrombosis: prevention and management. Clin. Plast. Surg. 40, 389–398. doi: 10.1016/j.cps.2013.04.002
Joyce, D. E., Nelson, D. R., and Grinnell, B. W. (2004). Leukocyte and endothelial cell interactions in sepsis: relevance of the protein C pathway. Crit. Care Med. 32, S280–S286. doi: 10.1097/01.CCM.0000128037.72072.22
Karabıyık, A., Yılmaz, E., Eğin, Y., and Akar, N. (2012). The effects of endothelial protein C receptor gene polymorphisms on the plasma sEPCR level in venous thrombosis patients. Turk. J. Haematol. 29, 55–62. doi: 10.5152/tjh.2011.40
Li, J. Q., Zhang, H. R., Du, H. C., and Shi, X. M. (2015). Clinical significance and correlation between gene polymorphism of endothelial protein C receptor and deep venous thrombosis of lower limbs. Hebei Med. J. 37, 660–662.
Liaw, P. C., Neuenschwander, P. F., Smirnov, M. D., and Esmon, C. T. (2000). Mechanisms by which soluble endothelial cell protein C receptor modulates protein C and activated protein C function. J. Biol. Chem. 275, 5447–5452. doi: 10.1074/jbc.275.8.5447
Mantel, N., and Haenszel, W. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22, 719–748.
Medina, P., Navarro, S., Estellés, A., Vayá, A., Bertina, R. M., and Espa-a, F. (2005). Influence of the 4600A/G and 4678G/C polymorphisms in the endothelial protein C receptor (EPCR) gene on the risk of venous thromboembolism in carriers of factor V Leiden. Thromb. Haemost. 94, 389–394. doi: 10.1160/th05-02-0089
Medina, P., Navarro, S., Estellés, A., Vayá, A., Woodhams, B., Mira, Y., et al. (2004). Contribution of polymorphisms in the endothelial protein C receptor gene to soluble endothelial protein C receptor and circulating activated protein C levels, and thrombotic risk. Thromb. Haemost. 91, 905–911. doi: 10.1160/th03-10-0657
Naess, I. A., Christiansen, S. C., Romundstad, P., Cannegieter, S. C., Rosendaal, F. R., and Hammerstrøm, J. (2007). Incidence and mortality of venous thrombosis: a population-based study. J. Thromb. Haemost. 5, 692–699. doi: 10.1111/j.1538-7836.2007.02450.x
Navarro, S., Medina, P., Mira, Y., Estellés, A., Villa, P., Ferrando, F., et al. (2008). Haplotypes of the EPCR gene, prothrombin levels, and the risk of venous thrombosis in carriers of the prothrombin G20210A mutation. Haematologica 93, 885–891. doi: 10.3324/haematol.12448
Oganesyan, V., Oganesyan, N., Terzyan, S., Qu, D., Dauter, Z., Esmon, N. L., et al. (2002). The crystal structure of the endothelial protein C receptor and a bound phospholipid. J. Biol. Chem. 277, 24851–24854. doi: 10.1074/jbc.C200163200
Pecheniuk, N. M., Elias, D. J., Xu, X., and Griffin, J. H. (2008). Failure to validate association of gene polymorphisms in EPCR, PAR-1, FSAP and protein S Tokushima with venous thromboembolism among Californians of European ancestry. Thromb. Haemost. 99, 453–455. doi: 10.1160/th07-10-0607
Qu, D., Wang, Y., Song, Y., Esmon, N. L., and Esmon, C. T. (2006). The Ser219–>Gly dimorphism of the endothelial protein C receptor contributes to the higher soluble protein levels observed in individuals with the A3 haplotype. J. Thromb. Haemost. 4, 229–235. doi: 10.1111/j.1538-7836.2005.01676.x
Riewald, M., Petrovan, R. J., Donner, A., Mueller, B. M., and Ruf, W. (2002). Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 296, 1880–1882. doi: 10.1126/science.1071699
Saposnik, B., Reny, J. L., Gaussem, P., Emmerich, J., Aiach, M., and Gandrille, S. (2004). A haplotype of the EPCR gene is associated with increased plasma levels of sEPCR and is a candidate risk factor for thrombosis. Blood 103, 1311–1318. doi: 10.1182/blood-2003-07-2520
Sun, X. L., and Yin, G. C. (2015). Screening EPCR gene polymorphism on patients of lower extremity venous thrombosis with Denaturing HPLC and PCR-RFLP technology. J Taishan Med. Coll. 36, 137–139. doi: 10.3969/j.issn.1004-7115.2015.02.006
Trégouët, D. A., Heath, S., Saut, N., Biron-Andreani, C., Schved, J. F., Pernod, G., et al. (2009). Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood 113, 5298–5303. doi: 10.1182/blood-2008-11-190389
Uitte de Willige, S., Van Marion, V., Rosendaal, F. R., Vos, H. L., de Visser, M. C., and Bertina, R. M. (2004). Haplotypes of the EPCR gene, plasma sEPCR levels and the risk of deep venous thrombosis. J. Thromb. Haemost. 2, 1305–1310. doi: 10.1046/j.1538-7836.2004.00855.x
Wang, X. B., and Hu, L. H. (2008). Association between gene polymorphism deep venous thrombosis in Chinese of endothelial protein C receptor and Han population of Hubei district. Acta Med. Univ. Sci Technol. Huazhong 37, 490–492. doi: 10.3870/j.issn.1672-0741.2008.04.019
Xu, J., Qu, D., Esmon, N. L., and Esmon, C. T. (2000). Metalloproteolytic release of endothelial cell protein C receptor. J. Biol. Chem. 275, 6038–6044. doi: 10.1074/jbc.275.8.6038
Yamagishi, K., Cushman, M., Heckbert, S. R., Tsai, M. Y., and Folsom, A. R. (2009). Lack of association of soluble endothelial protein C receptor and PROCR 6936A/G polymorphism with the risk of venous thromboembolism in a prospective study. Br. J. Haematol. 145, 221–226. doi: 10.1111/j.1365-2141.2009.07612.x
Yin, G. C., and Jin, X. (2013). The Association of Methylenetetrahydropholate Reductase Gene C677T Polymorphism and Endothelial Cell Protein C Receptor Gene A6936G Polymorphism with Deep Venous Thrombosis as Risk Factors. Shandong University Doctoral Thesis, Shandong University Press, Jinan.
Yin, G., Jin, X., Ming, H., Zheng, X., and Zhang, D. (2012). Endothelial cell protein C receptor gene 6936A/G polymorphism is associated with venous thromboembolism. Exp. Ther. Med. 3, 989–992. doi: 10.3892/etm.2012.510
Zhang, J. M., Ma, X. T., Ma, J. X., Wang, T., Sun, X. L., Tian, A. X., et al. (2016). The correlation between the polymorphism of coagulation factor XI gene rs2289252 and susceptibility of rs2036914 and venous thromboembolism:a meta-analysis. Chin. J. TCM WM Crit. Care 23, 31–35. doi: 10.3969/j.issn.1008-9691.2016.01.009
Keywords: EPCR, Ser219Gly, polymorphism, venous thromboembolism, genetic
Citation: Li Y-y, Wu J-j, Yang X-x, Geng H-y, Gong G and Kim HJ (2017) EPCR Gene Ser219Gly Polymorphism and Venous Thromboembolism: A Meta-Analysis of 9,494 Subjects. Front. Physiol. 8:339. doi: 10.3389/fphys.2017.00339
Received: 10 February 2017; Accepted: 09 May 2017;
Published: 26 May 2017.
Edited by:
John D. Imig, Medical College of Wisconsin, United StatesReviewed by:
Martin Franz Reiner, Cantonal Hospital Baden, SwitzerlandMinoli Perera, Northwestern University, United States
Copyright © 2017 Li, Wu, Yang, Geng, Gong and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-yan Li, lyynjmu123@126.com
 Yan-yan Li
Yan-yan Li Jing-jing Wu2
Jing-jing Wu2