- 1Key Laboratory of Pu-er Tea Science, Ministry of Education, Yunnan Agricultural University, Kunming, China
- 2Tea Research Center of Yunnan, Kunming, China
- 3College of Food Science and Technology, Yunnan Agricultural University, Kunming, China
- 4College of Pu-er Tea, Yunnan Agricultural University, Kunming, China
- 5State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Kunming, China
Galloylated catechins, as the main secondary metabolites in the tea plant, including (-)-epigallocatechin-3-gallate and (-)-epicatechin-3-gallate, comprise approximately three-quarters of all the tea plant catechins and have stronger effects than non-galloylated catechins, both on the product quality in tea processing and the pharmacological efficacy to human beings. The subcellular localization of galloylated catechins has been the primary focus of studies that assess biosynthesis and physiological functions. Classical histochemical localization staining reagents can not specifically detect galloylated catechins; thus, their subcellular localization remains controversial. In the present study, we generated a monoclonal antibody (mAb) against galloylated catechins, which can be used for the subcellular localization of galloylated catechins in the tea plant by immunohistochemistry. Direct ELISA and ForteBio Octet Red 96 System assay indicated the mAb could recognize the galloylated catechins with high specificities and affinities. In addition, tea bud was ascertained as the optimal tissue for freezing microtomic sections for immunohistochemistry. What’s more, the high quality mAbs which exhibited excellent binding capability to galloylated catechins were utilized for the visualization of them via immunohistochemistry. Our findings demonstrated that vacuoles were the primary sites of localization of galloylated catechins at the subcellular level.
Introduction
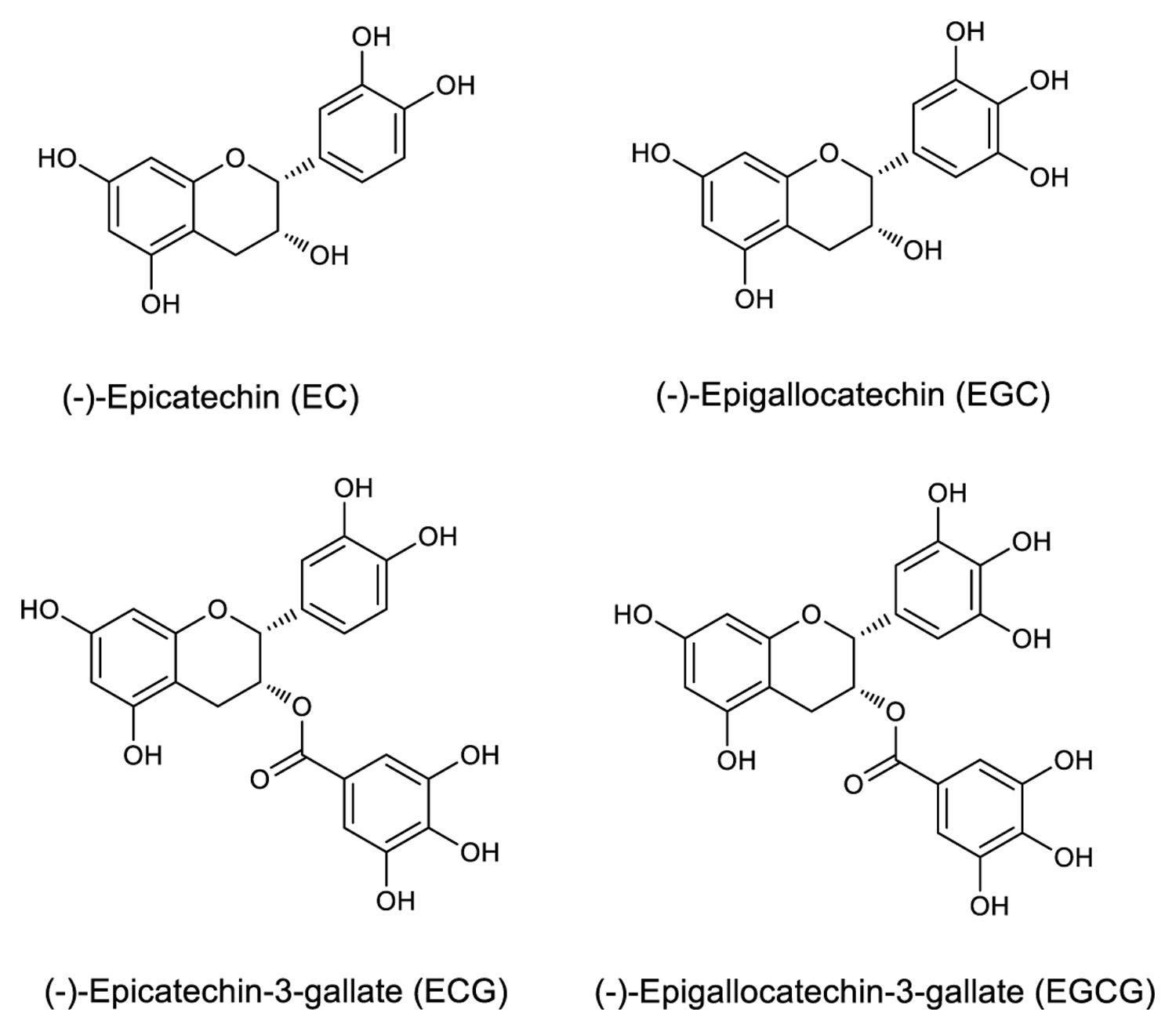
Tea is one of the most consumed beverages globally for centuries (Huang et al., 2014), second only to water. It is produced from tea plants [Camellia sinensis (L.) O. Kuntze] buds (Kim et al., 2013b; Schramm, 2013; Sonoda et al., 2014). Tea infusion contains abundant nutrients and functional ingredients, which are beneficial to the human health (El-Shahawi et al., 2012); specifically, the tea plant polyphenols have many important physiological functions (Liu et al., 2012). Catechins, consisting the most important flavonoid group, account for approximately 80% of all the polyphenols, which in turn constitute approximately 18–36% of the dry leaf weight. The main catechins are (-)-epicatechin (EC), (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG), and (-)-epigallocatechin-3-gallate (EGCG; Liu et al., 2009; Roman, 2013; Lu et al., 2014). It is notable that galloylated catechins account for approximately 76% of all catechins, of which EGCG is the most abundant, active, and thoroughly investigated, followed by ECG. Their mechanism of action indicates that the polyphenolic structure of galloylated catechins makes them good donors for hydrogen bonding, which increases the water solubility of galloylated catechins and contributes to their high affinity to proteins and nucleic acids (Yang et al., 2007).
Galloylated catechins have stronger health effects as compared to the non-galloylated catechins, with respect to the product quality in tea processing and pharmacological efficacy in humans (Bigelow and Cardelli, 2006; Tsutsumi et al., 2011). Galloylated catechins possess antioxidant, antibacterial, and anti-inflammatory effects and alleviate cardiovascular diseases (Braicu et al., 2011; Jin et al., 2013; Kim et al., 2013a; Moravcova et al., 2014); however, their subcellular localization have not been fully addressed (Suzuki et al., 2003; Liu et al., 2009, 2012). Previous studies have demonstrated that catechins were mainly located in the chloroplasts or vacuoles at the subcellular level (Suzuki et al., 2003; Liu et al., 2009). These finding were largely attributable to the methodologies applied, each having a unique set of advantages and limitations. Although, the classical histochemical localization staining reagents, including vanillin-HCl, diazotized amines, 4-dimethylaminocinnamaldehyde (DMACA), and potassium permanganate (Feucht et al., 1992; Liu et al., 2009; Bhattacharya et al., 2015), are extensively used to determine the subcellular localization of polyphenols, they do not specifically detect the galloylated catechins. It is well known that immunohistochemistry could be used to localize small molecules specifically. Moreover, this method has been successfully used to localize some secondary metabolites in plant. Immunohistochemical localization with primary anti-caffeine antibodies and conjugated secondary antibodies on tea leaf sections proved at the tissue level that caffeine was localized and accumulated within vascular bundles, mainly the precursor phloem (van Breda et al., 2013). Similarly, delta 9-tetrahydrocannabinol (THC) localization in glandular trichomes and bracteal tissues of Cannabis was examined with a mAb, which can specifically recognize THC (Kim and Mahlberg, 1997). Therefore, the subcellular localization of galloylated catechins in the tea plant by mAb as a rapid and sensitive method is necessary.
Precise information regarding the subcellular localization of galloylated catechins in the tea plant would be helpful for understanding how their biosynthesis is controlled, thereby providing additional insights into the related metabolic pathways. Advances in the high specificity of immunohistochemistry have prompted us to probe the subcellular localization of galloylated catechins in the tea plant. In this content, the anti-galloylated catechin mAb was generated. The high galloylated catechin content tissues were subsequently assessed via high-performance liquid chromatography (HPLC), and the determination of the subcellular localization of galloylated catechins was determined using immunohistochemistry.
This study reports production of a anti-galloylated catechin mAb which can recognize the galloylated catechins with high specificities and affinities. The high quality mAbs which exhibited excellent binding capability to galloylated catechins were further used to localize them in the tea plant. Our findings demonstrated that vacuoles were the primary sites of localization of galloylated catechins at the subcellular level, which is intuitive for their underlying mechanisms of biosynthesis, transport, storage, and physiological functions. Moreover, the subcellular localization of galloylated catechins in the tea plant assessed via immunohistochemistry may contribute toward a theoretical reference for the assessment of the subcellular localization of secondary metabolites in plants.
Materials and Methods
Chemicals and Reagents
Epigallocatechin-3-gallate, ECG, EGC, EC, gallic acid (GA), and resveratrol (RSV) were of high purity grade (≥98%) and purchased from Aladdin (Shanghai, China). Culture media and serum were obtained from Thermo Fisher Scientific (Waltham, MA, USA) and Biological Industries Israel Beit Haemek Ltd., respectively. Optimal Cutting Temperature (OCT) Compound was purchased from Sakura Finetek, USA. Formalin was obtained from Jinan Biological Technology Co., Ltd. Paraformaldehyde (PFA) was obtained from the Tianjin Branch of Chemical Reagent Co., Ltd (Tianjin, China). The ABC high-HRP immunostaining kit was manufactured by Vectastain Co., Ltd. The haematoxylin stain was of high purity grade and was purchased from Sigma-Aldrich (St Louis, MO, USA). Normal mouse IgG (sc-2025) was obtained from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). The HRP-DAB Chromogenic Substrate Kit was obtained from Tiangen Biotech (Beijing, China) Co., Ltd. Neutral red was obtained from Shanghai Yuanye Biological Technology Co., Ltd (Shanghai, China). Acetonitrile and trifluoroacetic acid used in the mobile phases were of HPLC grade and procured from Tedia Co., Inc. (Fairfield, OH, USA) and Merck KGaA (Darmstadt, Germany), respectively.
Plant Materials
Tea plant [C. sinensis (L.) O. Kuntze] samples were obtained from the Yunnan Agricultural University, Kunming, China. Each sample included bud, first leaf, second leaf, third leaf, and tender stem, which were separated from the tea plant. The samples were maintained at 4°C to inhibit air contact prior to analysis.
Animals
Healthy male BALB/c mice (6–8 weeks old) were purchased from Nanjing Peng-sheng Biotechnology Co., Ltd, China. All mice were housed in polypropylene cages with sterile paddy husk; the animals were maintained under ambient conditions of temperature and humidity with a 12 h light/dark cycle. Food and water were provided ad libitum. All mice experiments were performed in the animal facility according to the institutional guidelines and were approved by the Institutional Animal Care and Use Committee of Yunnan Agricultural University. The physical condition of the animals was monitored daily, and any adverse events were not observed. The animals were anesthetized prior to immunization, and spleen cells were obtained from the immunized mice, which were euthanized via cervical dislocation following CO2 anesthesia.
Production of Monoclonal Antibodies
Two BALB/c mice were immunized with oxidized tea polyphenols (OTP), which contains EGCG as the major group (Huang et al., 2014). Briefly, each mouse was vaccinated five times with 40 μg of OTP at a 2-week interval. The first immunization was performed using Freund’s complete adjuvant; the incomplete adjuvant was used for the subsequent immunizations. Following the immunization procedure, spleen cells from the immunized mice were mixed with myeloma SP2/0 cells at a ratio of 5:1. The mixture was washed twice with pre-warmed RPMI-1640 (37°C) and pre-warmed 50% polyethylene glycol (PEG) 1500 (Sigma–Aldrich, Germany) was used for fusion. A HAT selective medium (Sigma–Aldrich, Germany) was consecutively used for the selection of hybridoma cells. Direct Enzyme-linked immunosorbent assay (ELISA) was performed to screen the EGCG positive hybridoma cells, with the plates coated with EGCG during the cloning of EGCG hybridomas. One hybridoma clone, P6C2, was selected from 326 clones raised against EGCG. Positive hybridomas were cultured for 2 weeks. The anti-galloylated catechin antibody was purified from the culture supernatants via affinity chromatography using a PierceTM protein A/G column (Bayat et al., 2013). The eluted antibody was dialyzed against phosphate buffered saline (PBS) at pH = 7.2. SDS-PAGE analysis was performed following the Laemmli method, with a 12.0% separating gel to assess the purity and molecular weight of the mAb. The gel was stained with 0.25% Coomassie Brilliant Blue G-250.
Amino Acid Sequence of the Light Chain Variable Region Gene in the P6C2 Monoclonal Antibody
RNA was extracted from P6C2 hybridoma cells by RNeasy (Qiagen, Inc.). Reverse transcription was performed using SuperscriptTM III (Invitrogen, Inc.). cDNA fragments of the P6C2 light chain variable region gene were amplified via PCR using Fast-Pfu (Transgene, Beijing, China) with universal primers (Wang et al., 2000) as follows:
F-Kc: 5′-ACAAATGCCTATGCAGAYATTGTGMTSACMCARWCTMCA-3′, R-Kc: 5′-GGATACAGTTGGTGCAGCATC-3′.
The amplified PCR fragment was cloned into the pBR322 vector with an infusion kit (Clontech Inc., Mountain View, CA, USA) according to the manufacturer’s protocol. The DNA insert was sequenced by Invitrogen (Shanghai, China), and the primary structure of the kappa chain variable region was deduced.
Antibody Specificity Analysis
Epicatechin, EGC, ECG, and EGCG have a basic 2-phenylchromone structure (Figure 1), and non-galloylated catechins (EC and EGC) and GA are the precursor substances of galloylated catechins (ECG and EGCG). These compounds and RSV were used to evaluate the mAb’s specificity. Direct ELISA was performed on 96-well immunoplate (Nunc Polysorp; Thermo Fisher Scientific), which were coated with EGCG, ECG, EGC, EC, GA, and RSV (at the same concentration, 50 μg mL-1) overnight at 4°C and then blocked by incubation with 1% bovine serum albumin (BSA). The mAbs were applied to duplicate wells, followed by HRP-conjugated goat anti-mouse IgG (Thermo Fisher Scientific, Waltham, MA, USA) as the secondary antibody. Tetramethylbenzidine (TMB) high sensitivity substrate solution (BioLegend, San Diego, CA, USA) was added subsequently and the absorbance was recorded at 450 nm using an ELISA reader (Thermo Fisher Scientific, Waltham, MA, USA).
Furthermore, the interactions between the anti-galloylated catechin antibody and EGCG, ECG, EGC, EC, and GA, were determined using Super Streptavidin (SSA) biosensors in an Octet Red 96 instrument (ForteBio Inc., Menlo Park, CA, USA). Interactions were assessed at 30°C in PBS (pH = 6.5) unless otherwise stated. Briefly, the antibody was biotinylated at a 1:1 stoichiometry for 1-h at room temperature (RT). The biotinylated antibody was subsequently desalinated by a PD SpinTrapTM G-25 column (GE Healthcare, UK). SSA tips were pre-wet for 10 min in buffer immediately prior to use, and the 96-well black plates utilized in the Octet were filled with 200 μL of sample or buffer per well and agitated at 1000 rpm. The antibody was loaded to the SSA biosensors, which were washed with the buffer for 120 s and transferred to the wells that contained the compounds.
Antibody Binding Affinity Determined by Octet
The binding affinity of anti-galloylated catechin antibody with EGCG or ECG was determined using SSA biosensors in the Octet Red 96 instrument. The biotinylated antibody and EGCG or ECG at different concentrations were prepared in the assay buffer (PBS, pH = 6.5) as described above. We measured EGCG and ECG association and dissociation for 10 min, respectively. The kinetic parameters and affinities were calculated from a non-linear global fit of the data between the galloylated catechins and the antibody, using Octet Data Analysis software version 7.0 (ForteBio Inc., Menlo Park, CA, USA).
HPLC Analysis
The required sample amounts were crushed under liquid N2 to increase the extraction yield. An 80% methanol–water mixture was used as the extraction solvent (El-Shahawi et al., 2012; Otles and Yalcin, 2012). HPLC analysis was performed using a linear gradient system with mobile phase A (100% acetonitrile) and mobile phase B (0.03% trifluoroacetic acid), which were filtered through a 0.45 μm filter. The mobile phase A was increased from 10 to 60% in 36 min (Ning et al., 2009). A 10 μL sample was analyzed using an auto sampler (G1329B, 1260ALS, Agilent, USA), an ultraviolet detector (G1314F, 1260VWD, Agilent, USA) at 280 nm, and a HPLC pump (G1311B, 1260Quat Pump, Agilent, USA) at 1.0 mL min-1 flow at 40°C (G1316A, 1260TCC, Agilent, USA) through a C18 ODS column (ZORBAX SB-C18 4.6 mm × 250 mm, 5 microns, Agilent, USA).
Tissue Slicing and Immunohistochemistry
Fixation, embedding, sectioning, and staining of tea tissues were performed using the standard methods. Briefly, the tea tissues were fixed for 24 h at RT in 10% neutral buffered formalin. The OCT embedded tissues were cut into 20 μm sections using a Cryostat Microtome (Leica, Germany). The slides were fixed with 4% PFA for 10 min at 4°C and blocked with Blocking Solution (SP-6000, Vector Laboratories, Inc.) for 20 min at 37°C. After blocking for 30 min at 37°C with normal serum, histological sections of the tea tissues were incubated overnight at 4°C with the anti-galloylated catechin antibody. The sections were then sequentially incubated with biotinylated antibody and the ABC reagent for 30 min at 37°C. The reaction was visualized with DAB, and counterstaining was conducted with haematoxylin for 5 min. The sections were subsequently analyzed via fluorescence microscopy (Leica). Images of the tea tissue sections were acquired before and after staining.
Statistical Analyses
All values were presented as mean ± standard errors of the mean (SEM). All analyses were performed using SPSS 17.0 (Chicago, IL, USA), Graphpad Prism 5 (Graphpad Software, Inc., La Jolla, CA, USA) and Octet Data Analysis software version 7.0 (ForteBio Inc., Menlo Park, CA, USA).
Results
Antibody Expression
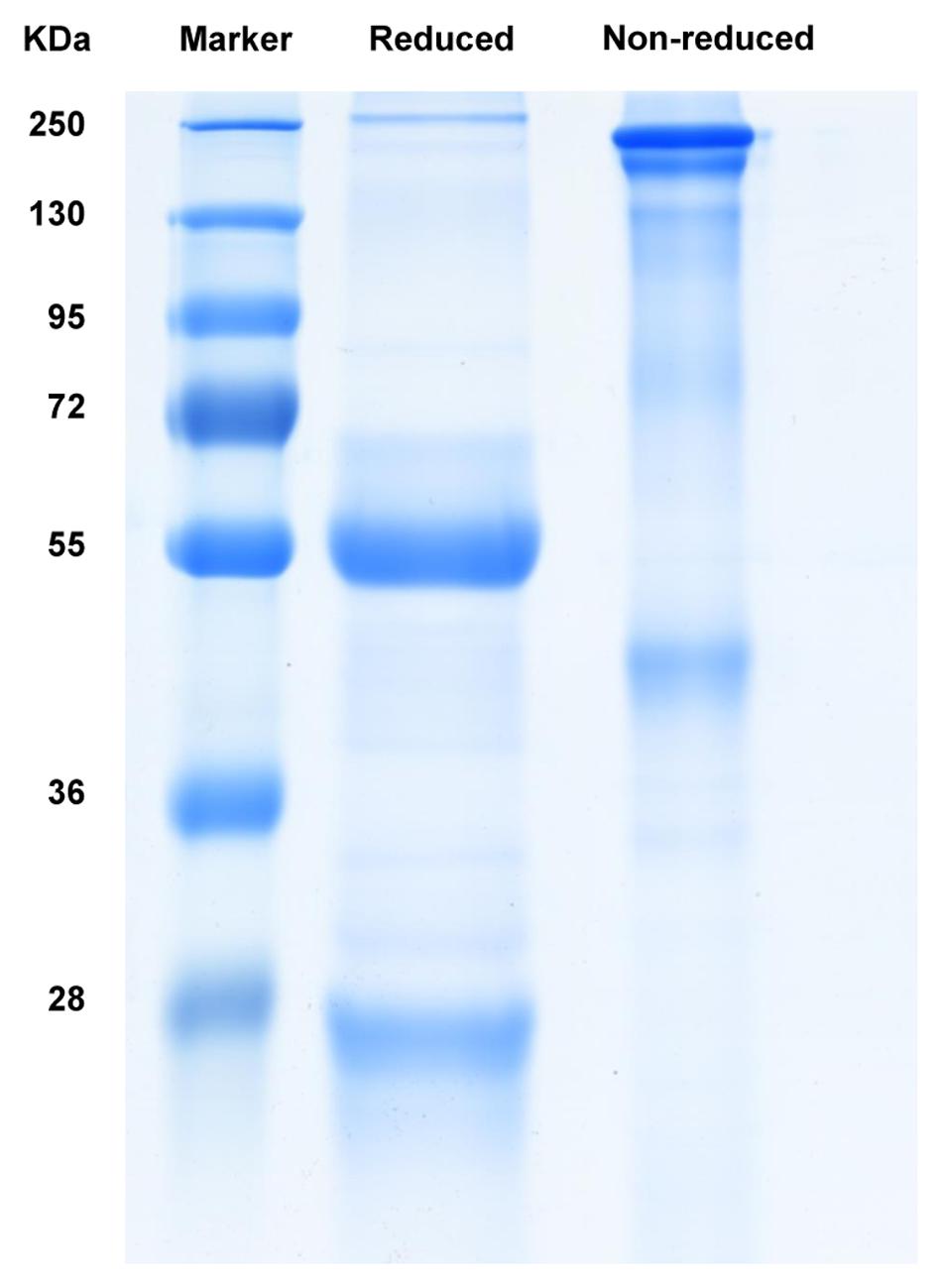
The anti-galloylated catechin antibody was expressed in hybridoma cells and purified by affinity chromatography using a PierceTM protein A/G column. The SDS-PAGE analysis of the purified mAb demonstrated the whole protein in the non-reducing conditions (~150 kDa) and 50 and 25 kDa heavy and light chains, respectively, in the reducing conditions (Figure 2).
Amino Acid Sequence of the Kappa Chain Variable Region of the mAb
The sequence of the P6C2 kappa chain variable region was deduced from the sequence of DNA cloned into the pBR322 vector, as shown in Supplementary Figure S1. According to NCBI-blast, it was closely associated with the kappa chain variable region of the immunoglobulin raised under chloramphenicol selection.
Specificity of Antibody
The specificity of the purified mAb was assessed by direct ELISA on EGCG, ECG, EGC, EC, GA (tea polyphenols), and RSV (non-tea polyphenol; Figure 3). Our finding showed that the mAb specifically recognized EGCG and ECG, rather than EGC, EC, GA, and RSV. Besides, the specificity of the mAb was identified by Bio-Layer Interferometry (BLI) technique. The real-time interactions between the mAb and precursor substances of galloylated catechins, including the main non-galloylated catechins (EC and EGC) and GA, were determined using the ForteBio Octet system (ForteBio Inc., Menlo Park, CA, USA; Figure 4). The curves correspond to the phases of association and dissociation. In the association steps, as anticipated, EGCG and ECG could significantly bind to the anti-galloylated catechin antibody (Figures 4A,B), the binding signal values were gradually increased and finally achieved 0.40 and 0.28 nm, respectively. Whereas there were scarcely any signals in the interactions between the mAb and EGC, EC, and GA (Figures 4C–E), which means that the mAb could not recognize them. In consideration of catechin structures, EGCG and ECG contain a GA moiety at position 3 on the C ring, whereas other compounds (EGC, EC, and GA) do not contain this functional group (Figure 1), that may be the reason why the binding curves for EGCG and ECG are so different from the binding curves for EGC, EC, and GA. It indicates that the GA moiety functional group is necessary for anti-galloylated catechin antibody binding. The results above showed that the mAbs were capable of recognizing galloylated catechins with high specificity.
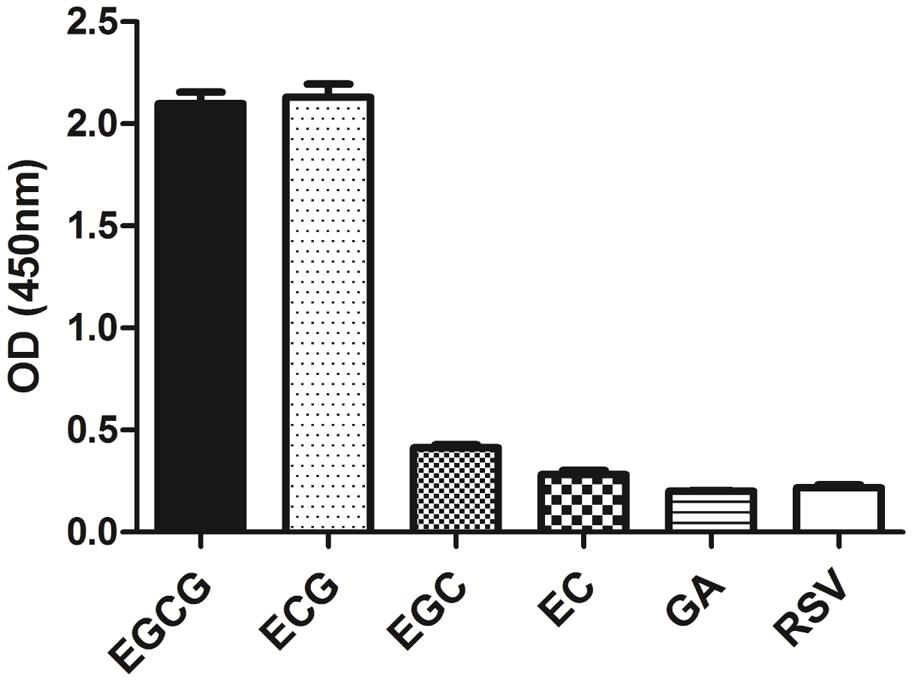
FIGURE 3. Determination of specificity of the anti-galloylated catechin mAb by direct ELISA. The anti-galloylated catechin mAb was tested against epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG), epigallocatechin (EGC), epicatechin (EC), GA (gallic acid), and resveratrol (RSV) at the same concentration (50 μg mL-1). Data were presented as mean ± SEM of three independent experiments.
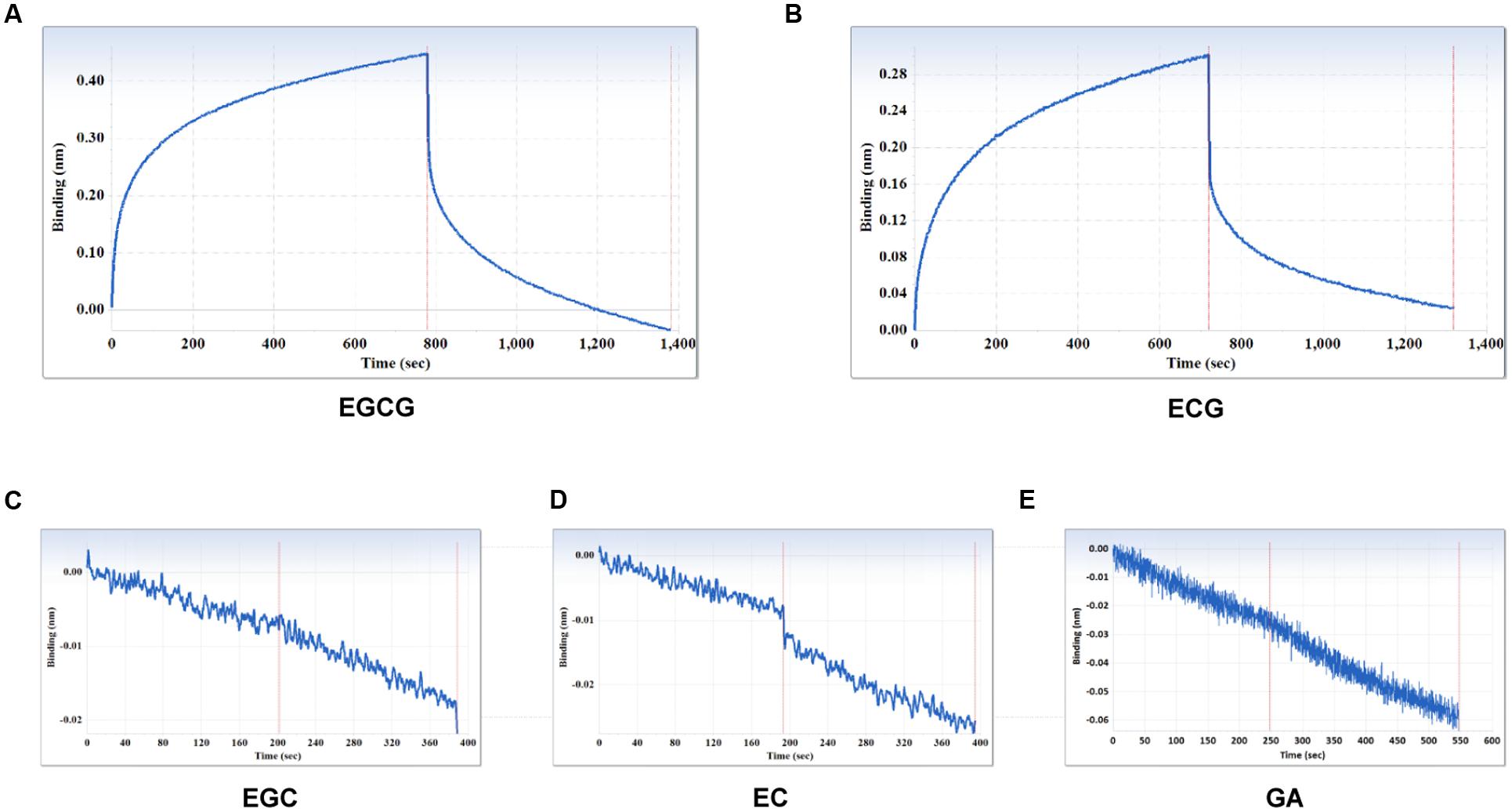
FIGURE 4. Interactions between the main compounds and anti-galloylated catechin antibody assessed via the label-free ForteBio Octet Red 96 System assay. (A) EGCG; (B) ECG; (C) EGC; (D) EC; (E) GA. Concentration, 50 μM. Curves correspond to the phases of association and dissociation.
Evaluation of Binding Affinity of mAbs
Furthermore, kinetic studies were performed to determine the binding affinities of the anti-galloylated catechin mAbs to serially diluted galloylated catechins from 32 to 1 μM (Figure 5; Table 1). The on-rate kinetic (Kon) values were displayed by EGCG at 2.27E + 02 Ms-1 and ECG at 2.65E + 03 Ms-1, respectively; and the off-rate kinetic (Koff) values were displayed by EGCG at 2.10E – 03 s-1 and ECG at 5.14E – 04 s-1, respectively. To understand the binding affinity value, the equilibrium dissociation constant (KD) was calculated by Koff/Kon. This showed that the mAb had strong affinity to EGCG and ECG, with the KD values of 9.25E – 06 and 1.97E – 07 M, respectively. The results indicated that the galloylated catechins had strong binding abilities with the anti-galloylated catechin antibody.
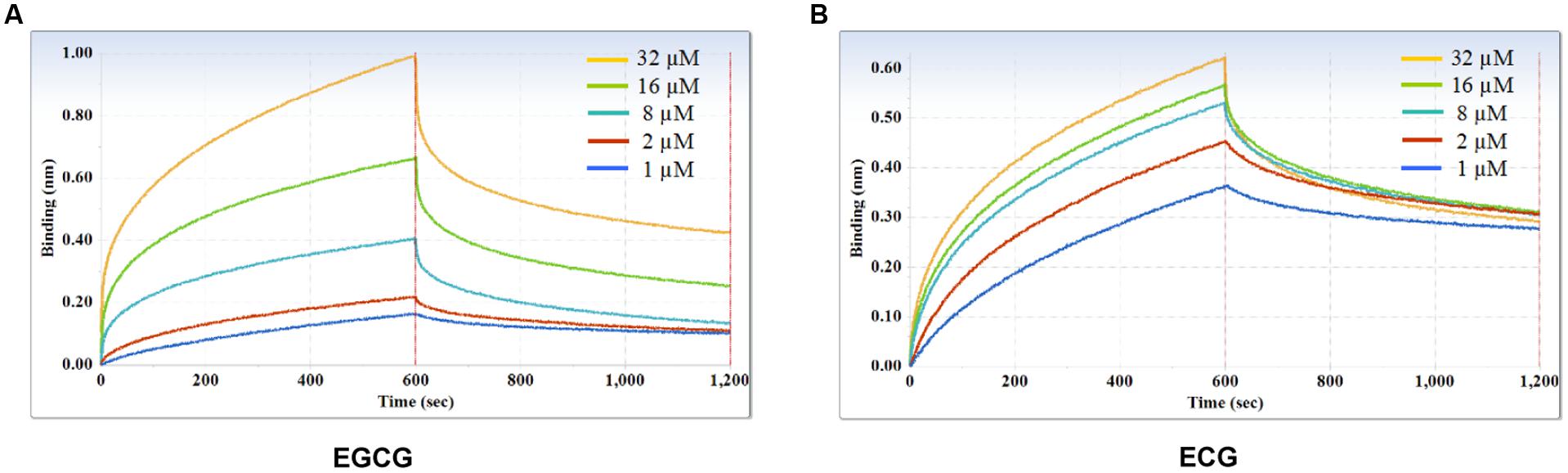
FIGURE 5. Binding affinity measurements of anti-galloylated catechin antibody with galloylated catechins assessed via the ForteBio system. (A) EGCG; (B) ECG. Curves correspond to the phases of association and dissociation of galloylated catechins at various concentrations on the monoclonal antibody anchored to the sensor chip. These curves may be used to determine the KD, Kon, and Koff.
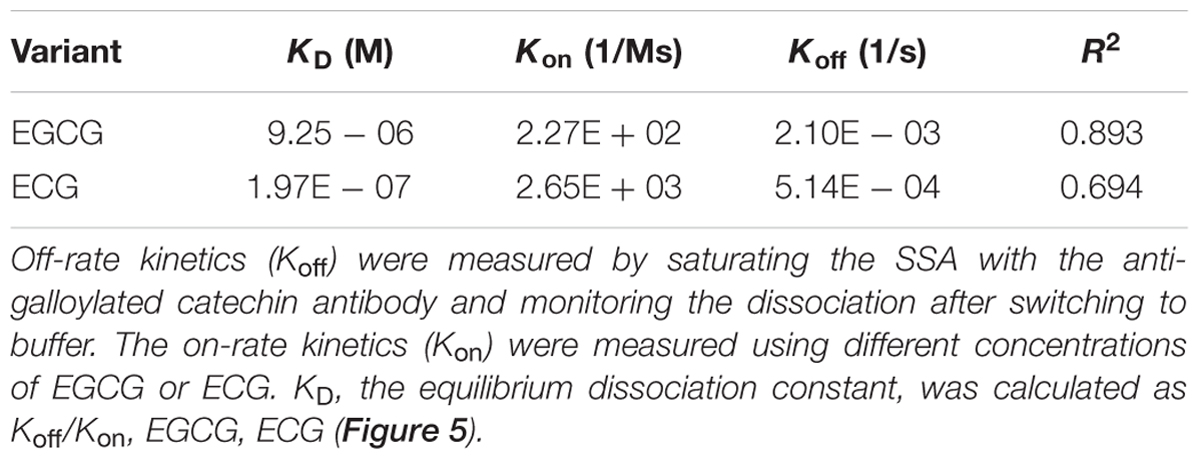
TABLE 1. Binding kinetics of the anti-galloylated catechin antibody to EGCG and ECG using the ForteBio system.
Identification of High Galloylated Catechin Content Tissues
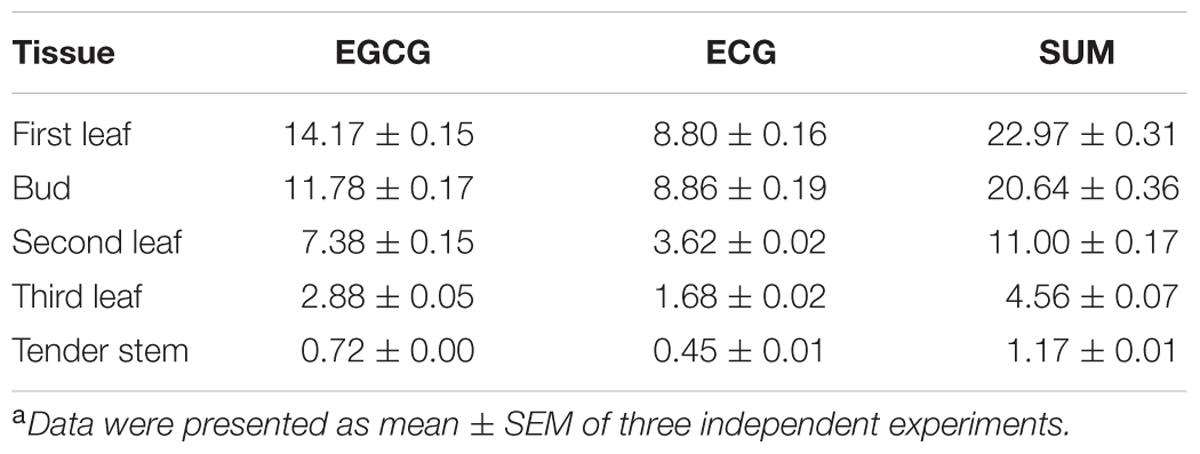
To identify the optimal tissue for the assessment of the subcellular localization of galloylated catechins via immunohistochemistry, high galloylated catechin content tissues were ascertained using HPLC. Peaks were identified by retention time compared with EGCG and ECG standards, and calibration curves were performed using serially diluted standards (Supplementary Figure S2). The total galloylated catechin amounts were highest in the first tea leaf, followed by the tea bud, second leaf, and third leaf, with only small quantities of galloylated catechins in the tender stem (Supplementary Figure S3; Table 2). The EGCG content was higher as compared to the ECG, which is consistent with previous studies. Furthermore, the galloylated catechins were mainly distributed in the tea plant’s tender shoots.
Subcellular Localization of Galloylated Catechins via Immunohistochemistry
The observation that anti-galloylated catechin antibody specifically recognized galloylated catechins prompted us to probe the subcellular localization of these compounds in the tea plant. The tea bud is a galloylated catechin-rich tissue and is suitable for frozen section preparation. Thus, it was selected for neutral red staining and immunohistochemistry. Using both the methods, sharp microphotographs were acquired (Figure 6). Vacuole localization was typically examined by neutral red staining since it specifically reacted with the plant vacuoles to yield a red complex. Following neutral red staining, the red-stained vacuoles in the tea bud were easily detected at the subcellular level, and the staining was not observed in the plastids (Figures 6A,B). The anti-galloylated catechin antibody was successively used to detect the subcellular localization of the galloylated catechins via immunohistochemistry. Interestingly, sepia-stained galloylated catechins in the tea bud were easily detected in vacuoles, whereas the chloroplasts were not stained (Figure 6C). This finding indicated that the galloylated catechins accumulated mainly in the vacuoles.
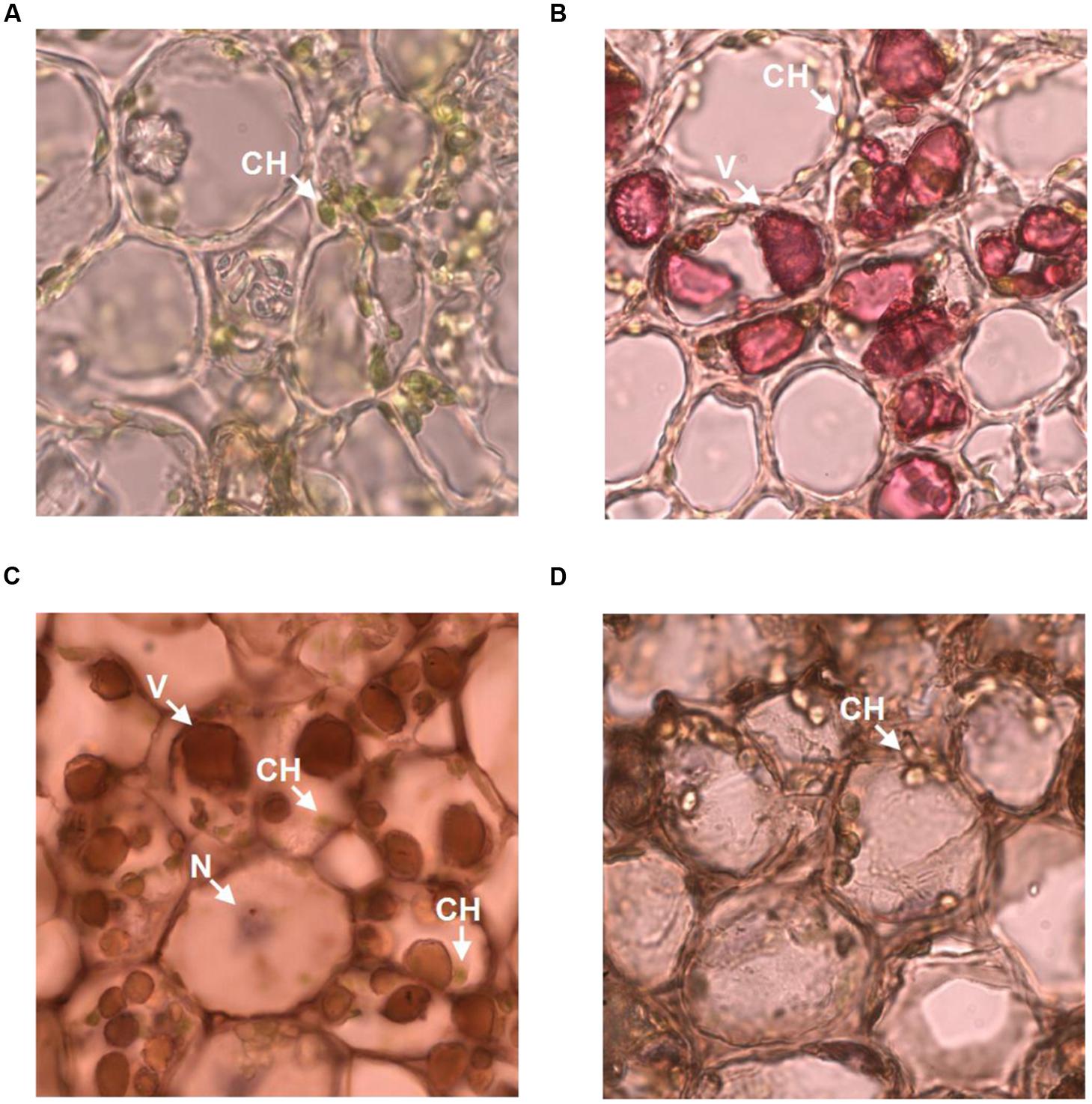
FIGURE 6. Subcellular localization of galloylated catechins in the tea bud. (A,B) Transverse bud sections before and after 0.5% neutral red staining, with red indicating the vacuoles. (C,D) Transverse bud sections after anti-galloylated catechin antibody and normal IgG antibody staining, respectively. Sepia represents galloylated catechin-accumulation areas. CH, chloroplast; V, vacuole; N, nucleus. Slice thickness, 20 μm. Oil microscopy.
To validate the immunohistochemistry data, a normal IgG antibody was selected as a control. Any general or plastid staining was not identified (Figure 6D). Similarly, no staining was identified in plastids. Taken together, these findings suggested that vacuoles are the main sites for galloylated catechins at the subcellular level. Finally, these data supported immunohistochemistry as a novel, specific, and sensitive method for the assessment of the subcellular localization of galloylated catechins in tea plant samples.
Discussion
Immunohistochemistry is widely used in biomedical research to localize specific epitopes of molecules in cells and tissues (Hewitt et al., 2014). In addition, immunohistochemistry is advantageous for the localization of small molecules because the technique is substantially more specific than other standard histochemical methods using stains, such as vanillin-hydrochloric acid, which are not unique for a distinct small molecule, but rather an entire class of compounds, and false positives or side reactions may occur. Furthermore, immunohistochemistry has been used to determine effectively the localization of caffeine in young tea leaves (van Breda et al., 2013). Monoclonal antibodies are a superior choice for immunohistochemistry because they detect only one epitope in a particular antigen. Small changes in the compound of interest structure as a result of fixation protocols typically do not affect the binding affinity. In the present study, OTP, which contains EGCG as the major group, was first used for production of the anti-galloylated catechin mAb. The specificities of the mAbs were checked by direct ELISA and BLI assays, and, the binding affinities of the mAbs with EGCG and ECG were determined using ForteBio Octet Red 96 System. Most importantly, the anti-galloylated catechin mAb was used for the subcellular localization of galloylated catechins in the tea plant. Our experimental results indicated that the specific binding of the mAb to galloylated catechins with high affinities could be used to investigate efficiently the subcellular localization of these compounds via immunohistochemistry. Considering catechin structures, what’s more, our findings suggest that the R1 galloyl group is necessary for anti-galloylated catechin antibody binding because both EGCG and ECG are the only catechins that contain this functional group (Figure 1). Therefore, immunohistochemistry is a useful method for assessing the subcellular localization of galloylated catechins in the tea plant. Using immunohistochemistry for galloylated catechins and neutral red staining for vacuoles, we demonstrated that the vacuoles were the primary sites for galloylated catechins at the subcellular level.
Although the galloylated catechins are the key secondary metabolites in the tea plant, the subcellular localization of galloylated catechins remains controversial. Using vanillin-HCl staining, Liu et al. (2009) demonstrated that catechins were primarily localized in the chloroplasts of mesophyll cells. However, catechins were simultaneously demonstrated to concentrate in callus vacuoles. In addition, Suzuki et al. (2003) demonstrated that catechins were primarily stored in the central vacuoles of the mesophyll cells as determined by Fe (II) staining. Till date, the subcellular localization of galloylated catechins remains unsolved, that is because none of the staining reagents could specifically detect galloylated catechins. Plant cells are distinguished from other eukaryotic cells by a rigid cellulose-based cell wall, chloroplasts, and a large central vacuole (Chi et al., 2015). The secondary metabolites may be considered chemical defense compounds against herbivores or microorganisms and are thus important for plant fitness. Several secondary metabolites are also toxic to the plant; thus, they need to be stored in a separate compartment (Wink, 1993). Polyphenol oxidase (PPO) is a ubiquitous copper metalloprotein in many plants, localized in chloroplasts, whereas flavonoid biosynthesis is speculated to occur in the cytoplasm (Roberts and Wood, 1950; Bokuchava et al., 1970; Buzun et al., 1970; Ono et al., 2006). We deduced that chloroplasts might not be the prime sites for galloylated catechin accumulation in the case of oxidization. As previously discussed, vacuoles were the primary sites for galloylated catechin accumulation at the subcellular level.
Recent studies have focused on the subcellular localization of galloylated catechins in the tea plant because these compounds possessed diverse biological activities and functions. Furthermore, multiple studies have identified promising preventative and therapeutic roles for galloylated catechins (Betts and Wareham, 2014; Chen et al., 2014; Cocci et al., 2014; Hodgson et al., 2014; Kim et al., 2014). Thus, the precise subcellular localization of galloylated catechins appears to be critical for unlocking valuable information regarding their biological roles. Our findings demonstrated that galloylated catechins accumulated in tea bud vacuoles, which indicates that vacuoles have a robust capacity to accumulate galloylated catechins. To assess the mode of transportation of synthesized galloylated catechins, sieve tube fluid of new shoots of tea plants was prepared via chelator-facilitated exudation (Dinant and Kehr, 2013), and the galloylated catechin content was determined by HPLC. Interestingly, galloylated catechins were detected in the sieve tube fluid (approximately 0.05% of the total amounts), which indicated that galloylated catechins may be transported through the sieve tube to vacuoles. The relative transportation mechanism need to be further studied.
Conclusion
This study reports production of a anti-galloylated catechin mAb which can recognize the galloylated catechins with high specificities and affinities. The high quality mAbs which exhibited excellent binding capability to galloylated catechins were further used to localize them in the tea plant. Our findings demonstrated that vacuoles were the primary sites of localization of galloylated catechins at the subcellular level.
Author Contributions
JS, XW, and YH conceived and designed the experiments. HX, YW, YC, PZ, YZ, and YH performed the experiments. HX, YH, and XW analyzed the data. JS and XW contributed reagents/materials/analysis tools. HX, XW, and YH wrote the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JPV and handling Editor declared their shared affiliation and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgment
This research was financially supported by grants from the National Natural Science Foundation of China (No.31460392).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00728
References
Bayat, A. A., Yeganeh, O., Ghods, R., Zarnani, A. H., Ardekani, R. B., Mahmoudi, A. R., et al. (2013). Production and characterization of a murine monoclonal antibody against human ferritin. Avicenna J. Med. Biotechnol. 5, 212–219.
Betts, J. W., and Wareham, D. W. (2014). In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 14:172. doi: 10.1186/1471-2180-14-172
Bhattacharya, A., Sharma, M., Gulati, A., Joshi, R., Chanda, S. K., and Ahuja, P. S. (2015). Histochemical evaluation of catechins in PEG stressed transgenic tea plants using catechin-specific-diazotized sulfanilamide reagent. Biotechnol. Histochem. 90, 45–54. doi: 10.3109/10520295.2014.942369
Bigelow, R. L., and Cardelli, J. A. (2006). The green tea catechins, (-)-Epigallocatechin-3-gallate (EGCG) and (-)-Epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene 25, 1922–1930. doi: 10.1038/sj.onc.1209227
Bokuchava, M. A., Shalamberidze, T., and Soboleva, G. A. (1970). Localization of polyphenol oxidase in the tea leaf. Dokl. Akad. Nauk SSSR. 192, 1374–1375.
Braicu, C., Pilecki, V., Balacescu, O., Irimie, A., and Neagoe, I. B. (2011). The relationships between biological activities and structure of flavan-3-ols. Int. J. Mol. Sci. 12, 9342–9353. doi: 10.3390/ijms12129342
Buzun, G. A., Dzhemukhadze, K. M., and Mileshko, L. F. (1970). Some properties of tea polyphenol oxidase. Biokhimiia 35, 1002–1006.
Chen, W. C., Hsieh, S. R., Chiu, C. H., Hsu, B. D., and Liou, Y. M. (2014). Molecular identification for epigallocatechin-3-gallate-mediated antioxidant intervention on the H2O2-induced oxidative stress in H9c2 rat cardiomyoblasts. J. Biomed. Sci. 21:56. doi: 10.1186/1423-0127-21-56
Chi, W., Feng, P., Ma, J., and Zhang, L. (2015). Metabolites and chloroplast retrograde signaling. Curr. Opin. Plant Biol. 25, 32–38. doi: 10.1016/j.pbi.2015.04.006
Cocci, P., Mosconi, G., and Palermo, F. A. (2014). Partial cloning, tissue distribution and effects of epigallocatechin gallate on hepatic 3-hydroxy-3-methylglutaryl-CoA reductase mRNA transcripts in goldfish (Carassius auratus). Gene 545, 220–225. doi: 10.1016/j.gene.2014.05.030
Dinant, S., and Kehr, J. (2013). Sampling and analysis of phloem sap. Methods Mol. Biol. 953, 185–194. doi: 10.1007/978-1-62703-152-3_12
El-Shahawi, M. S., Hamza, A., Bahaffi, S. O., Al-Sibaai, A. A., and Abduljabbar, T. N. (2012). Analysis of some selected catechins and caffeine in green tea by high performance liquid chromatography. Food Chem. 134, 2268–2275. doi: 10.1016/j.foodchem.2012.03.039
Feucht, W., Treutter, D., and Christ, E. (1992). Localization and quantitative determination of catechins and proanthocyanidins in the phloem of elm and cherry. Tree Physiol. 10, 169–177. doi: 10.1093/treephys/10.2.169
Hewitt, S. M., Baskin, D. G., Frevert, C. W., Stahl, W. L., and Rosa-Molinar, E. (2014). Controls for immunohistochemistry: the histochemical society’s standards of practice for validation of immunohistochemical assays. J. Histochem. Cytochem. 62, 693–697. doi: 10.1369/0022155414545224
Hodgson, A. B., Randell, R. K., Mahabir-Jagessar, T. K., Lotito, S., Mulder, T., Mela, D. J., et al. (2014). Acute effects of green tea extract intake on exogenous and endogenous metabolites in human plasma. J. Agric. Food Chem. 62, 1198–1208. doi: 10.1021/jf404872y
Huang, Y. W., Xu, H. H., Wang, S. M., Zhao, Y., Huang, Y. M., Li, R. B., et al. (2014). Absorption of caffeine in fermented Pu-er tea is inhibited in mice. Food Funct. 5, 1520–1528. doi: 10.1039/c4fo00051j
Jin, H., Gong, W., Zhang, C., and Wang, S. (2013). Epigallocatechin gallate inhibits the proliferation of colorectal cancer cells by regulating Notch signaling. Onco Targets Ther. 6, 145–153. doi: 10.2147/OTT.S40914
Kim, E., and Mahlberg, P. (1997). Immunochemical localization of tetrahydrocannabinol (THC) in cryofixed glandular trichomes of Cannabis (Cannabaceae). Am. J. Bot. 84, 336. doi: 10.2307/2446007
Kim, H. S., Montana, V., Jang, H. J., Parpura, V., and Kim, J. A. (2013a). Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: a potential role for reducing lipid accumulation. J. Biol. Chem. 288, 22693–22705. doi: 10.1074/jbc.M113.477505
Kim, H. S., Quon, M. J., and Kim, J. A. (2014). New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2, 187–195. doi: 10.1016/j.redox.2013.12.022
Kim, J. J., Tan, Y., Xiao, L., Sun, Y. L., and Qu, X. (2013b). Green tea polyphenol epigallocatechin-3-gallate enhance glycogen synthesis and inhibit lipogenesis in hepatocytes. Biomed Res. Int. 2013, 920128. doi: 10.1155/2013/920128
Liu, Y., Gao, L., Liu, L., Yang, Q., Lu, Z., Nie, Z., et al. (2012). Purification and characterization of a novel galloyltransferase involved in catechin galloylation in the tea plant (Camellia sinensis). J. Biol. Chem. 287, 44406–44417. doi: 10.1074/jbc.M112.403071
Liu, Y., Gao, L., Xia, T., and Zhao, L. (2009). Investigation of the site-specific accumulation of catechins in the tea plant (Camellia sinensis (L.) O. Kuntze) via vanillin-HCl staining. J. Agric. Food Chem. 57, 10371–10376. doi: 10.1021/jf902614n
Lu, Z., Liu, Y., Zhao, L., Jiang, X., Li, M., Wang, Y., et al. (2014). Effect of low-intensity white light mediated de-etiolation on the biosynthesis of polyphenols in tea seedlings. Plant Physiol. Biochem. 80, 328–336. doi: 10.1016/j.plaphy.2014.04.016
Moravcova, A., Cervinkova, Z., Kucera, O., Mezera, V., and Lotkova, H. (2014). Antioxidative effect of epigallocatechin gallate against D-galactosamine-induced injury in primary culture of rat hepatocytes. Acta Med. (Hradec Kralove). 57, 3–8. doi: 10.14712/18059694.2014.1
Ning, J. M., Zhang, Z. Z., Gu, X. G., Wan, X. C., and Sun, W. T. (2009). Study on HPLC fingerprint of Pu-erh crude tea in Yunnan. Food Ferment. Ind. 35, 36–40.
Ono, E., Hatayama, M., Isono, Y., Sato, T., Watanabe, R., Yonekura-Sakakibara, K., et al. (2006). Localization of a flavonoid biosynthetic polyphenol oxidase in vacuoles. Plant J. 45, 133–143. doi: 10.1111/j.1365-313X.2005.02625.x
Otles, S., and Yalcin, B. (2012). Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci. World J. 2012:564367. doi: 10.1100/2012/564367
Roberts, E. A., and Wood, D. J. (1950). Oxidation of catechol by tea-oxidase. Nature 165:32. doi: 10.1038/165032b0
Roman, M. C. (2013). Determination of catechins and caffeine in camillia sinensis raw materials, extracts, and dietary supplements by HPLC-uv: single-laboratory validation. J. AOAC Int. 96, 933–941. doi: 10.5740/jaoacint.10-488
Schramm, L. (2013). Going green: the role of the green tea component EGCG in chemoprevention. J. Carcinog Mutagen. 4:1000142. doi: 10.4172/2157-2518.1000142
Sonoda, J. I., Ikeda, R., Baba, Y., Narumi, K., Kawachi, A., Tomishige, E., et al. (2014). Green tea catechin, epigallocatechin-3-gallate, attenuates the cell viability of human non-small-cell lung cancer A549 cells via reducing Bcl-xL expression. Exp. Ther. Med. 8, 59–63. doi: 10.3892/etm.2014.1719
Suzuki, T., Yamazaki, N., Sada, Y., Oguni, I., and Moriyasu, Y. (2003). Tissue distribution and intracellular localization of catechins in tea leaves. Biosci. Biotechnol. Biochem. 67, 2683–2686. doi: 10.1271/bbb.67.2683
Tsutsumi, H., Kinoshita, Y., Sato, T., and Ishizu, T. (2011). Configurational studies of complexes of various tea catechins and caffeine in crystal state. Chem. Pharm. Bull. (Tokyo). 59, 1008–1015. doi: 10.1248/cpb.59.1008
van Breda, S. V., van der Merwe, C. F., Robbertse, H., and Apostolides, Z. (2013). Immunohistochemical localization of caffeine in young Camellia sinensis (L.) O. Kuntze (tea) leaves. Planta. 237, 849–858. doi: 10.1007/s00425-012-1804-x
Wang, Z., Raifu, M., Howard, M., Smith, L., Hansen, D., Goldsby, R., et al. (2000). Universal PCR amplification of mouse immunoglobulin gene variable regions: the design of degenerate primers and an assessment of the effect of DNA polymerase 3’ to 5’ exonuclease activity. J. Immunol. Methods 233, 167–177. doi: 10.1016/S0022-1759(99)00184-2
Keywords: Camellia sinensis, catechins, galloylated catechins, subcellular localization, immunohistochemistry, HPLC
Citation: Xu H, Wang Y, Chen Y, Zhang P, Zhao Y, Huang Y, Wang X and Sheng J (2016) Subcellular Localization of Galloylated Catechins in Tea Plants [Camellia sinensis (L.) O. Kuntze] Assessed via Immunohistochemistry. Front. Plant Sci. 7:728. doi: 10.3389/fpls.2016.00728
Received: 25 February 2016; Accepted: 12 May 2016;
Published: 26 May 2016.
Edited by:
Marisa Otegui, University of Wisconsin–Madison, USAReviewed by:
Biswapriya Biswavas Misra, University of Florida, USAJulio Paez Valencia, University of Wisconsin–Madison, USA
Copyright © 2016 Xu, Wang, Chen, Zhang, Zhao, Huang, Wang and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Sheng, shengj@ynau.edu.cn; Xuanjun Wang, wangxuanjun@gmail.com; Yewei Huang, lichuangyewei100@163.com
†These authors have contributed equally to this work.
 Huanhuan Xu
Huanhuan Xu Ya Wang1,2,3†
Ya Wang1,2,3† Yewei Huang
Yewei Huang Jun Sheng
Jun Sheng