Sugammadex shortens the time to extubate and discharge from PACU in patients with tracheobronchial stenosis undergoing rigid bronchoscopy procedures: A retrospective cohort study
- 1Department of Anesthesiology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
- 2Outcomes Research Consortium, Cleveland, OH, United States
Introduction: Incomplete reversal of neuromuscular blocking drugs can delay patients' rapid recovery and lead to adverse events in the postoperative period, especially in high-risk patients. Sugammadex as a reversal agent, may offer distinct advantages to the scenario where residual neuromuscular blockade may be poorly tolerated. We aimed to investigate the efficacy of sugammadex compared with neostigmine on perioperative outcomes in patients with preoperative tracheal stenosis undergoing rigid bronchoscopy.
Method: We conducted a retrospective cohort study of adults who were diagnosed with primary or secondary tracheobronchial stenosis, and scheduled for interventional therapy under rigid bronchoscopy, at Shanghai Chest Hospital between December 2016 and July 2020. The exposure was categorized into two groups according to the antagonists of muscle relaxants administered after surgery: Group neostigmine vs. Group sugammadex. The primary outcome was the time to extubate after surgery, and the second outcome was the time to discharge from PACU. Perioperative adverse events were recorded.
Results: A total of 98 patients undergoing rigid bronchoscopy procedures were included. Patients in Group sugammadex showed less time to extubate (11 [8, 17] vs. 16 [12, 22] min, P = 0.003) and discharge from PACU (27 [20, 33] vs. 32.5 [24, 44] min, P = 0.013) than in Group neostigmine. The incidence and duration of hypotension during the procedure in Group sugammadex were significantly lower than that in Group neostigmine (18.5 vs. 40.8%, P = 0.038; 0 [0, 0] vs. 0 [0, 8] min, P = 0.036 respectively).
Conclusions: Sugammadex shortens the time to extubate and discharge from PACU in patients with tracheobronchial stenosis, accelerating postoperative recovery.
Introduction
With the development of interventional pulmonology, rigid bronchoscopy, as an interventional channel, allows a variety of instruments to enter the airway, which makes it used more widely in the respiratory and thoracic fields [1]. Compared with fiberoptic bronchoscopy, the wider channel of the rigid bronchoscope lends itself to debulk benign or malignant masses in the trachea and mainstem bronchi, dilate proximal stenotic airways, and deploy tracheobronchial stents [2]. The rigid bronchoscopy procedures must be deployed under general anesthesia, while they pose many challenges to anesthesiologists, especially in the population with underlying respiratory compromise [3].
The choice of anesthesia and ventilation techniques varies greatly among anesthesiologists and institutions related to rigid bronchoscopy. Because rigid bronchoscopy is a much more invasive procedure than flexible bronchoscopy, it always requires general anesthesia under muscle relaxants which can facilitate the placement of rigid bronchoscopy, restrict patients' involuntary movement and improve operation conditions during surgery. Selecting potent muscle relaxants during anesthesia induction provides optimal conditions in placing rigid bronchoscopy, however, owing to the short duration of rigid bronchoscopy procedures in tracheobronchial diseases (usually around 30 min), the time to extubate and discharge from post-anesthesia care unit (PACU) can be delayed due to residual blockade from the muscle relaxants. Residual blockade may affect efficiency of the operating room and lead to a series of respiratory complications [4]. Complete reversal of the neuromuscular blockade at the end of the procedure is fundamental, because most patients who undergo rigid bronchoscopy may present a limited pulmonary reserve and cannot tolerate residual paralysis [5]. As the specific antagonist of rocuronium, sugammadex can effectively reverse the moderate and deep neuromuscular blockade of rocuronium, significantly shorten the time to extubate, and promote enhanced recovery after surgery [5, 6]. However, there are few reports on the application of sugammadex in rigid bronchoscopy.
Therefore, we aimed to compare the efficacy and safety of sugammadex with neostigmine on antagonizing the effects of non-depolarizing muscle relaxants in patients undergoing rigid bronchoscopy. Our primary aim was to compare the efficacy of sugammadex with neostigmine on early postoperative outcomes in PACU; Specifically, we tested the primary hypothesis that sugammadex provides less time to extubate laryngeal mask airway compared with neostigmine. Secondarily, we tested the hypothesis that sugammadex shortens the time to discharge from PACU and decreases perioperative adverse events.
Materials and methods
With institutional review board approval (IRB# IS 2146) and waived informed consent, we conducted a retrospective cohort study in adults who underwent rigid bronchoscopic procedure under general anesthesia between December 2016 and July 2020. Data were extracted from Anesthesia Institute's electronic health record system (Yifei Huatong, Beijing, China) and Chart review.
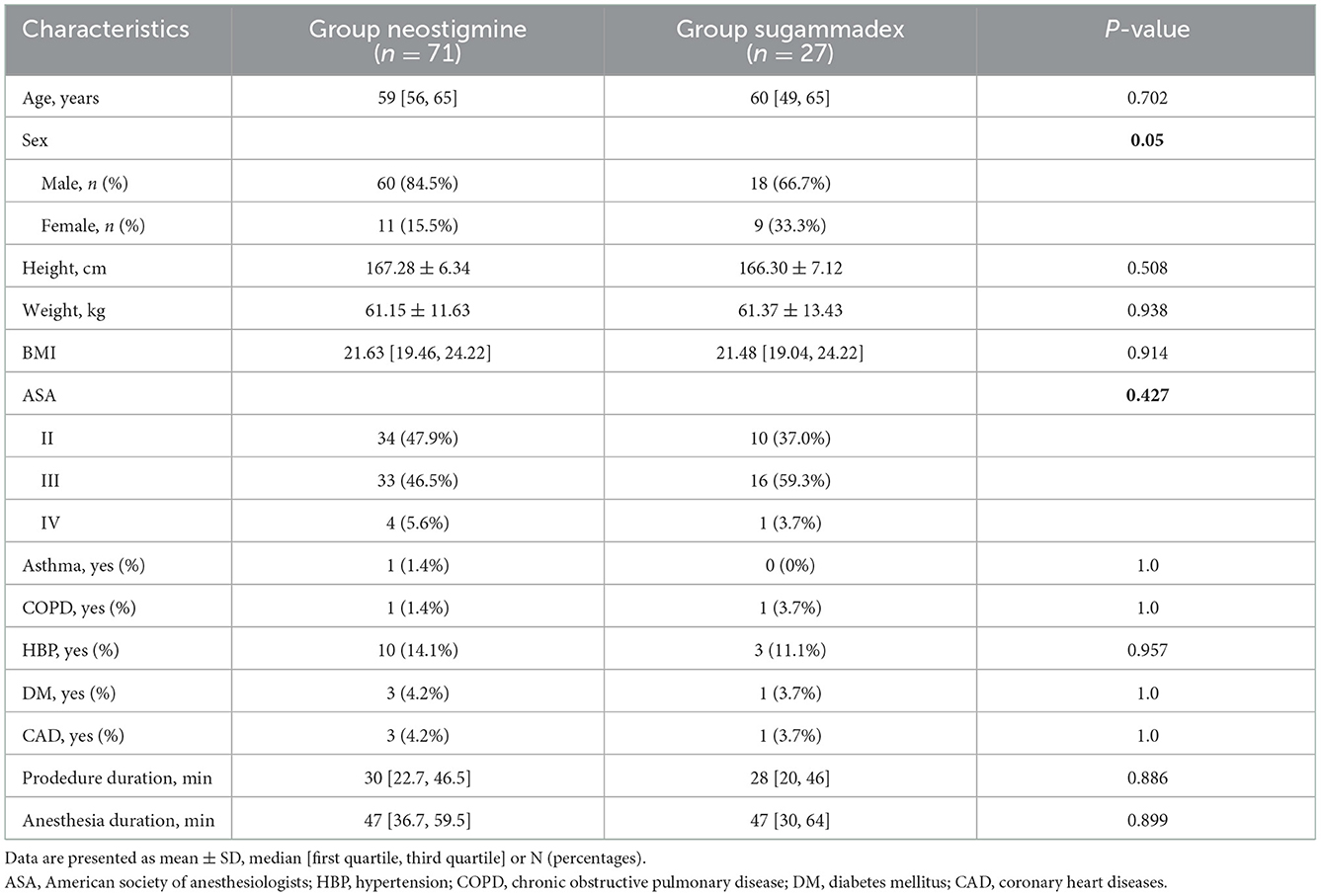
We included adult patients of American Society of Anesthesiologists (ASA) classification I–IV, diagnosed with tracheobronchial stenosis by computed tomography, scheduled for rigid bronchoscopic procedures, using muscle relaxants at anesthesia induction and reversal agents of the neuromuscular blockade at the end of the procedure, and were directly admitted to PACU after the procedure. Rigid bronchoscopic procedure included various methods for controlling the airway, mainly consisting of electrocoagulation, electrocautery, cryotherapy, balloon and rigid dilation, and silicone or covered metal stent placement or removal. We excluded patients when they undergone urgent surgery, had pre-existing severe cardiovascular diseases, and were directly transferred to ICU.
Exposure
According to the type of reversal muscle relaxants utilized: we classified patients into two groups: (1) In Group neostigmine, neostigmine 0.04 mg/kg combined with atropine 0.5 mg was administrated to reverse the effect of muscle relaxants at the end of procedure; (2) In Group sugammadex, sugammadex 2~4 mg/kg was injected to reverse the effect of muscle relaxants at the end of procedure.
In included patients, all patients received general anesthesia induction composed of 2–4 μg/ml of propofol and 2–4 ng/ml of remifentanil with target-controlled infusion. Anesthesia was maintained with propofol and remifentanil and adjusted according to the hemodynamics during the procedure by anesthesiologists on site. The rigid bronchoscopy was placed after muscle relaxants injected with rocuronium or cisatracurium. According to the dose of cisatracurium during anesthesia induction, the Group neostigmine was divided into two subgroups. The group cisatracurium N1 received 0.1 mg/kg of cisatracurium (2 × ED95 of cisatracurium) during anesthesia induction, and the group cisatracurium N2 received 0.15 mg/kg of cisatracurium (3 × ED95 of cisatracurium) during anesthesia induction. Rocuronium was administered at 0.6 mg/kg during anesthesia induction.
At the end of the procedure, reversal drugs were utilized to antagonize the effect of muscle relaxants, and a laryngeal mask airway (LMA) was inserted to assist mechanical ventilation until the patient woke up. All patients were transferred to PACU to extubate the laryngeal mask. Patients were observed in the PACU by an attending anesthesiologist.
Outcome
The primary outcome was the time to LMA removal which was defined as the period from the end of procedure to remove the laryngeal mask. The laryngeal mask was removed when criteria were met:(i) clear consciousness: hearing verbal instructions (e.g., open eyes); (ii) active reflection: obvious swallowing and coughing reflections; (iii) muscle strength recovery: holding firmly, looking up persistently; (iv) respiratory frequency: 14–25 times/min, tidal volume > 5 ml/kg, SpO2 > 95%.
The secondary outcomes included: (i) The time to discharge from PACU when patients met the criteria (stable respiration and hemodynamics, clear consciousness, Steward awakening score ≥6). (ii) Perioperative adverse events consisting of hypotension (MAP < 65 mmHg), hypoxemia (SpO2 < 85% or SpO2 < 90% for a duration exceeding 1 min), hypertension (the systolic pressure > 160 mmHg), severe arrhythmia and other perioperative adverse events.
The time interval was automatically retrieved from Anesthesia Institute's electronic health record system, including the time to extubate and discharge from PACU.
Statistical analysis
SPSS 20.0 statistical software was used to analyze the data. The continuous data meeting normality assumption were presented with mean ± SD and analyzed by Student's T-test. The data with non-normal distribution were expressed with median [25th percentile, 75th percentile] and compared with non-parametric test. The counting data were expressed by number (%), and chi-square test was used for comparison. P < 0.05 was considered statistically significant.
Results
From December 2016 and July 2020, we identified 112 qualified patients, of whom 98 patients had complete data and were included in the final analysis.
Among them, 71 patients were assigned to Group neostigmine and 27 patients were assigned to Group sugammadex. There was no statistically significant difference between the two groups regarding age, sex, height, weight, body mass index (BMI), ASA physical status, procedure time, anesthesia time and comorbidities (Table 1). All patients were given a single dose of muscle relaxant at anesthesia induction without additional doses added. In both groups, the procedure was accomplished uneventfully.
Comparison of the time to extubate LMA and discharge from PACU
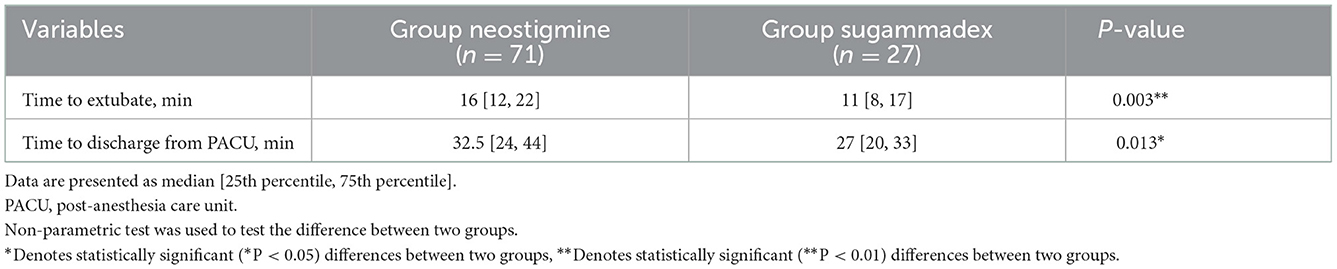
The laryngeal mask was removed when extubation criteria were fully met in PACU. Time to LMA removal was significantly shorter in Group sugammadex than that in Group neostigmine (11 [8, 17] vs. 16 [12, 22] min, P < 0.05). Also, the time to discharge from PACU was significantly shorter in Group sugammadex than that in Group neostigmine (27 [20, 33] vs. 32.5 [24, 44] min, P < 0.05) (Table 2 and Figure 1).
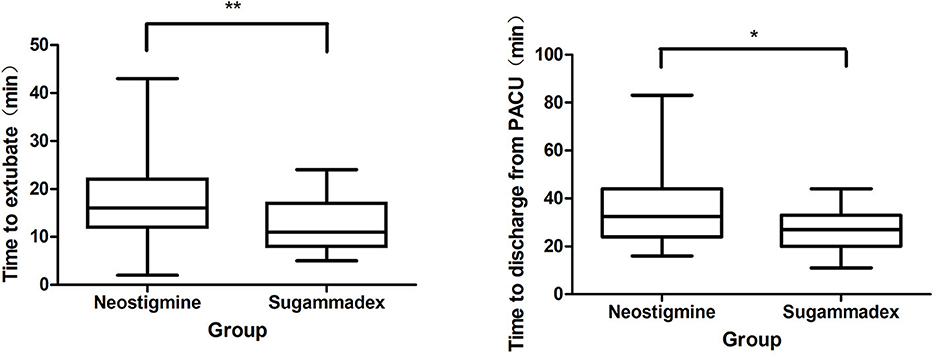
Figure 1. Comparison of the time to extubate and to discharge from PACU. PACU, postanesthesia care unit. *Denotes statistically significant (*P < 0.05) differences between two groups. **Denotes statistically significant (**P < 0.01) differences between two groups.
In Group neostigmine, 53.5% (38/71) of patients stayed over 30 min in PACU compared to 20.8% (10/17) of patients in Group sugammadex, even without reaching a statistical significance (P > 0.05). 5.6% (4/71) of patients in Group neostigmine stayed over 1 h in PACU compared to none of patients in Group sugammadex.
Comparison of adverse events during the perioperative period
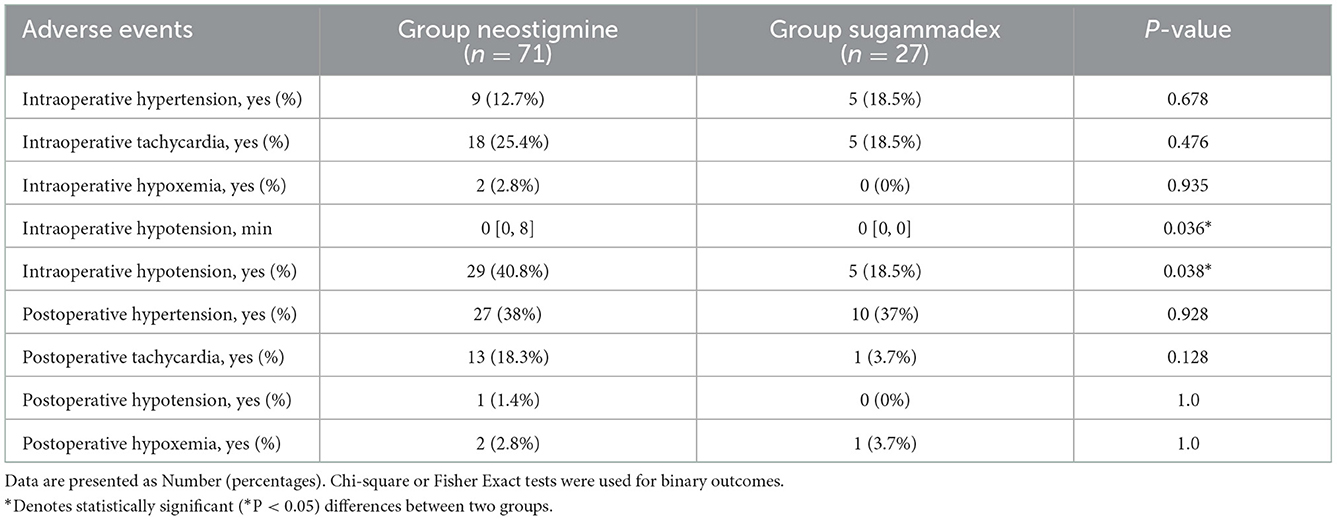
The incidence of hypotension during the procedure in Group sugammadex was significantly lower than in Group neostigmine (18.5 vs. 40.8%, P < 0.05), and the duration of intraoperative hypotension was also significantly shorter in Group sugammadex (Table 3). There was no significant difference between the two groups in the incidence of hypertension, tachycardia and hypoxemia during the perioperative period.
Subgroup analysis
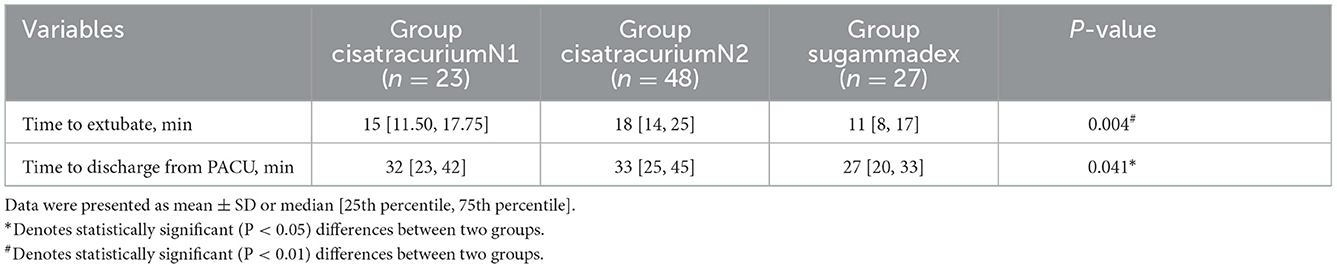
Twenty three patients receive 0.1 mg/kg cisatracurium during anesthesia induction in Group cisatracurium N1 and 48 patients receive 0.15 mg/kg cisatracurium during anesthesia induction in Group cisatracurium N2. In subgroup analysis, the time to extubate LMA was significantly longer in Group cisatracurium N2 than that in Group sugammadex (18.83 ± 9.29 vs. 12.48 ± 5.33 min, P < 0.05), while there was no significant difference between Group cisatracurium N1 and the other groups. Also, the time to discharge from PACU was significantly longer in Group cisatracurium N2 than that in Group sugammadex: 33 [25, 45] vs. 27 [20, 33] min, P < 0.05. The time to discharge from PACU in Group sugammadex was shorter than that in Group cisatracurium N1: 27 [20, 33] vs. 32 [23, 42] min, and there was no significant difference between Group cisatracurium N1 and Group cisatracurium N2 (Table 4 and Figure 2).
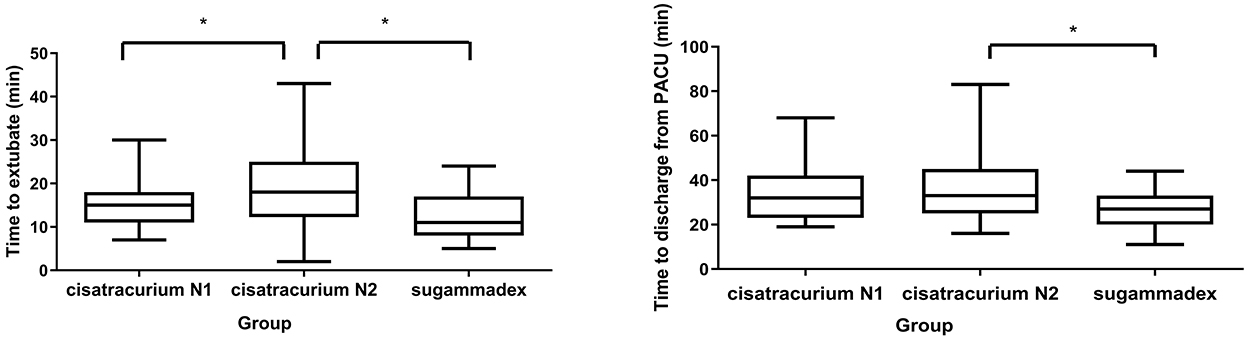
Figure 2. Subgroup analysis for extubation time and PACU stay. *Denotes statistically significant (P < 0.05) differences between two groups.
Discussion
The intervention of rigid bronchoscopy in tracheobronchial stenosis disease are high-risk procedures for anesthesiologists. During the procedure, use of muscle relaxants facilitates the placement of rigid bronchoscopes and prevents life-threatening patient' movement or coughing. Nevertheless, residual muscle paralysis poses many challenges, especially for patients with pre-existing tracheal stenosis. The choice of skeletal muscle relaxants and antagonists in short-duration procedures is a significant issue for anesthesiologists. In our study, we found that the sugammadex can quickly reverse the residual muscle relaxation in patients undergoing rigid bronchoscopy, shorten the time to LMA removal and PACU stay, and the combination of rocuronium and sugammadex may reduce intraoperative hypotension.
The use of muscle relaxants during anesthesia is associated with postoperative residual neuromuscular blockade and respiratory complications. Hanowell et al. [7] proved 10% of patients undergoing rigid tracheoscopic surgery had significant postoperative residual neuromuscular blockade, which was associated with longer PACU length of stay [4, 8]. Neostigmine, a traditional neuromuscular block antagonist, has been proved ineffective in producing adequate reversal of block at deep degrees of neuromuscular block [9, 10]. Sugammadex, a modified gamma-cyclodextrin, reduces the concentration of rocuronium at the neuromuscular junction by selectively encapsulating free rocuronium molecules [11]. Clinical studies have shown that sugammadex can quickly reverse the muscle relaxing effect of rocuronium (or vecuronium) and promote the rapid recovery of spontaneous respiration [9, 12, 13]. For patients with pulmonary disease, 2 mg/kg of sugammadex could completely antagonize the muscle relaxation of rocuronium within 2.1 min when the TOF counts reach 2 [14].
Our data showed that sugammadex could reverse the muscle relaxation induced by rocuronium more quickly than neostigmine after rigid bronchoscopy. The time to LMA removal and PACU stay in sugammadex group were significantly shorter than neostigmine group. In subgroup analysis, time to LMA removal and PACU stay were significantly longer in Group N2 (patients received 3 × ED95 of cisatracurium) than in Group sugammadex (patients received 2 mg/kg of sugammadex). To shorten the extubation time for short-duration procedure, some anesthesiologists tend to use sub-intubation dose of non-depolarizing muscle relaxants for anesthesia induction. Our study found that although the use of sub-intubational dose of non-depolarizing muscle relaxants (2 × ED95 of cisatracurium) can shorten the extubation time and PACU stay, but whether it affects the intubation condition and increase the incidence of laryngospasm or bronchospasm need further clinical studies. Therefore, our study believes that the use of sugammadex can effectively shorten the time to LMA removal in patients undergoing rigid bronchoscopy, resulting in improved operating room turnover and better patients' prognosis on overall safety and recovery. Although without reaching a statistical significance, we noted that 53.5% of patients in group neostigmine stayed over 30 min in PACU compared to 20.8% of patients in group sugammadex. Moreover, 5.6% of patients in group neostigmine stayed over 1 h in PACU compared to none of patients in group sugammadex. For the limitation of small sample size, we failed to detect the difference between groups and should be more cautious to interpret the results. Since patients with tracheobronchial stenosis disease were likely to develop airway obstruction requiring intervention and post-procedural hypoxemia in PACU, residual neuromuscular blockade might aggravate this risk so that anesthesiologists should be more prudent. Previous literature demonstrated a decrease in upper airway obstruction requiring intervention and desaturation in patients administered rocuronium-sugammadex compared with cisatracurium-neostigmine (2.3 vs.17.4%) [15].
The reason why sugammadex is still limited in availability is its higher cost. Sugammadex is still under patent and expensive compared to neostigmine. However, sugammadex offers a significantly faster and more predictable recovery profile than neostigmine. Theoretically, patients may get better prognosis for the quicker and more complete reversal of neuromuscular blockade, then be moved from PACU to ward instead of intensive care unit. The use of sugammadex thus improved efficiency, reduced complications and reduced associated costs [16–18]. Conflict existed on the routine use of sugammadex. One analysis showed sugammadex potentially lead to cost savings for the reversal of rocuronium-induced moderate or profound NMB compared to no reversal and reversal with neostigmine in the Spanish health care setting [19]. But other studies didn't support routine use of sugammadex because they didn't find significant difference in time to readiness to discharge from PACU in routine patients [20]. They recommended continued use of either agent at the anesthesiologist's discretion. In our center, we didn't use sugammadex in our routine anesthesia for bronchoscopic procedures because of the cost. But in patients with tracheobronchial stenosis undergoing rigid bronchoscopy procedures, we preferred sugammadex for its safety. Complete and quick reversal of the neuromuscular blockade was fundamental since most patients who undergo rigid bronchoscopy had a significantly decreased pulmonary reserve and could not tolerate residual paralysis.
Our data showed that in group sugammadex, the incidence and duration of intraoperative hypotension, was reduced from 40.8 to 18.5%, which may be due to the fact that rocuronium provided deep status of neuromuscular blockade to facilitate the insertion of rigid bronchoscope, therefore dose of propofol and remifentanil may be decreased. The favorable pharmacokinetic profile and quick metabolism of remifentanil and propofol make them agents of choice for rigid bronchoscopy. However, when muscle relaxation was not sufficient, anesthesiologists tended to increase the dosage of propofol and remifentanil to prevent patients from moving or coughing. High-dose of propofol might cause a marked drop in blood pressure, especially for elderly and weak patients. Compared with the neostigmine group, we also found that the incidence of intraoperative tachycardia in group sugammadex showed a downward trend, from 18.3 to 3.7%, although not reaching to statistical significance. The reversing process of sugammadex itself was not the primary reason for this difference in hemodynamics, but with sugammadex, anesthesiologists had more freedom to use rocuronium and provided stable anesthetic agents.
However, there are some shortcomings in this study that may limit generalization of these findings. First, it was a single-center retrospective study with small sample size and thus the results should be interpreted with caution. Randomized controlled trials with large samples are still needed in the future to verify our results. Furthermore, there was no muscle relaxation monitoring, and the treatment generally depends on the anesthesiologists' preferences owing to the retrospective nature of this study. Finally, studies are required to determine the effect on long-term clinical outcomes.
In conclusion, sugammadex can quickly reverse the residual muscle relaxation, shorten the time to LMA removal and PACU stay. These pharmacologic strategies may provide more safety in patients undergoing rigid bronchoscopy with preoperative tracheobronchial stenosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Chest Hospital (IRB# IS2146). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conception and design: YQ and JW. Administrative support: YQ and MX. Provision of study materials or patients: YQ and XL. Collection and assembly of data and data analysis and interpretation: YQ, XL, TL, and XC. Manuscript writing and final approval of manuscript: All authors.
Funding
This work was supported by Shanghai Municipal Commission of Health (202040200) and National Natural Science Foundation of China (82071233).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Diaz-Mendoza J, Peralta AR, Debiane L, Simoff MJ. Rigid bronchoscopy. Semin Respir Crit Care Med. (2018) 39:674–84. doi: 10.1055/s-0038-1676647
2. Batra H, Yarmus L. Indications and complications of rigid bronchoscopy. Expert Rev Respir Med. (2018) 12:509–20. doi: 10.1080/17476348.2018.1473037
3. Pathak V, Welsby I, Mahmood K, Wahidi M, MacIntyre N, Shofer S. Ventilation and anesthetic approaches for rigid bronchoscopy. Ann Am Thorac Soc. (2014) 11:628–34. doi: 10.1513/AnnalsATS.201309-302FR
4. Butterly A, Bittner EA, George E, Sandberg WS, Eikermann M, Schmidt U. Postoperative residual curarization from intermediate-acting neuromuscular blocking agents delays recovery room discharge. Br J Anaesth. (2010) 105:304–9. doi: 10.1093/bja/aeq157
5. Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. (2010) 111:120–8. doi: 10.1213/ANE.0b013e3181da832d
6. de Lima A, Kheir F, Majid A, Pawlowski J. Anesthesia for interventional pulmonology procedures: a review of advanced diagnostic and therapeutic bronchoscopy. Can J Anaesth. (2018) 65:822–36. doi: 10.1007/s12630-018-1121-3
7. Hanowell LH, Martin WR, Savelle JE, Foppiano LE. Complications of general anesthesia for Nd:YAG laser resection of endobronchial tumors. Chest. (1991) 99:72–6.
8. Unzueta MC, Casas I, Merten A, Landeira MJ. Endobronchial high-frequency jet ventilation for endobronchial laser surgery: an alternative approach. Anesth Analg. (2003) 96:298–300. doi: 10.1213/00000539-200301000-00059
9. Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. (2008) 109:816–24. doi: 10.1097/ALN.0b013e31818a3fee
10. Lemmens HJ, El-Orbany MI, Berry J, Morte JB, Martin G. Reversal of profound vecuronium-induced neuromuscular block under sevoflurane anesthesia: sugammadex vs. neostigmine. BMC Anesthesiol. (2010) 10:15. doi: 10.1186/1471-2253-10-15
11. Kaye A.D., Kaye R. J., Cornett E. M., Urits I., Orhurhu V., Viswanath O., et al. (2019). The role of sugammadex, a novel cyclodextrin compound in modern anesthesia practice: conventional neuromuscular physiology and clinical pharmacology. Expert. Rev. Clin. Pharmacol. 12:917-9. doi: 10.1080/17512433.2019.1659134
12. Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. Eur J Anaesthesiol. (2010) 27:874–81. doi: 10.1097/EJA.0b013e32833d56b7
13. Li L, Jiang Y, Zhang W. Sugammadex for fast-track surgery in children undergoing cardiac surgery: a randomized controlled study. J Cardiothorac Vasc Anesth. (2021) 35:1388–92. doi: 10.1053/j.jvca.2020.08.069
14. Amao R, Zornow MH, Cowan RM, Cheng DC, Morte JB, Allard MW. Use of sugammadex in patients with a history of pulmonary disease. J Clin Anesth. (2012) 24:289–97. doi: 10.1016/j.jclinane.2011.09.006
15. Martinez-Ubieto J., Ortega-Lucea S., Pascual-Bellosta A., Arazo-Iglesias I., Gil-Bona J., Jimenez-Bernardó T., et al. (2016). Prospective study of residual neuromuscular block and postoperative respiratory complications in patients reversed with neostigmine vs. sugammadex. Miner. Anestesiol. 82:735–42.
16. Bailey CR. Sugammadex: when should we be giving it? Anaesthesia. (2017) 72:1170–5. doi: 10.1111/anae.13960
18. Deyhim N, Beck A, Balk J, Liebl MG. Impact of sugammadex vs. neostigmine/glycopyrrolate on perioperative efficiency. Clinicoecon Outcomes Res. (2020) 12:69–79. doi: 10.2147/CEOR.S221308
19. Jiang Y, Bash LD, Saager L. A clinical and budgetary impact analysis of introducing sugammadex for routine reversal of neuromuscular blockade in a hypothetical cohort in the US. Adv Ther. (2021) 38:2689–708. doi: 10.1007/s12325-021-01701-1
Keywords: sugammadex, rigid bronchoscopy, extubation time, residual neuromuscular blockade, tracheal stenosis
Citation: Lu X, Li T, Chen X, Xu M, Wu J and Qiu Y (2023) Sugammadex shortens the time to extubate and discharge from PACU in patients with tracheobronchial stenosis undergoing rigid bronchoscopy procedures: A retrospective cohort study. Front. Anesthesiol. 1:1116271. doi: 10.3389/fanes.2022.1116271
Received: 05 December 2022; Accepted: 26 December 2022;
Published: 16 January 2023.
Edited by:
Biljana Kuzmanovska, Saints Cyril and Methodius University of Skopje, North MacedoniaReviewed by:
Marija Jovanovski-Srceva, Ss. Cyril and Methodius University in Skopje, North MacedoniaMaja Mojsova Mijovska, University Clinic of Anesthesia, Reanimation and Intensive Care -Skopje, North Macedonia
Copyright © 2023 Lu, Li, Chen, Xu, Wu and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuwei Qiu,  qiuyuweixk@163.com
qiuyuweixk@163.com
 Xiaofei Lu
Xiaofei Lu Tingting Li1
Tingting Li1  Jingxiang Wu
Jingxiang Wu Yuwei Qiu
Yuwei Qiu