Effects of different anesthetic depths monitored by processed EEG analysis on glandular secretion in patients undergoing laparoscopic total hysterectomy
- 1First Clinical Medical College, Gannan Medical University, Ganzhou, China
- 2Department of Anesthesiology, The First Affiliated Hospital of Gannan Medical University, Ganzhou, China
Background: The objective of this study was to find ways to reduce intraoperative glandular secretion in patients by observing the effects of different depths of anesthesia on glandular secretion under Narcotrend monitoring (NT), to reduce the use of unnecessary anticholinergic drugs, and to improve the quality of anesthesia and patient comfort.
Methods: Ninety patients who underwent total laparoscopic hysterectomy were randomly divided into three groups. Group L: intraoperative maintenance of anesthesia depth D0–D2, group M: intraoperative maintenance of anesthesia depth E0–E1, group H: long tocopherol 0.01 mg/kg was administered intravenously 10 min before surgery without monitoring the depth of anesthesia, and the mean arterial pressure (MAP), heart rate (HR), and adverse effects were compared in each group at six time points. The salivary secretion and respiratory gland secretion were compared among the groups.
Results: Salivary secretion under general anesthesia decreased in all three groups compared with the basal value (P < 0.05), with the smallest decrease in group L (P < 0.05) and the largest decrease in group H (P < 0.001). The amount of respiratory gland secretion during the whole operation was from more to less in the order of group L, group M and group H. The dry mouth visual analogue scale (VAS) scores were lower in groups L and M than in group H (P < 0.05).
Conclusion: General anesthesia suppresses the patient's glandular secretion, and the suppression becomes more pronounced as the anesthesia deepens. Maintaining the depth of anesthesia E0–E1 can effectively reduce the patient's glandular secretion, more stable intraoperative vital signs, and more comfortable patient awakening period without increasing the incidence of adverse reactions.
Chinese Clinical Trial Registry: http://www.chictr.org.cn/showproj.aspx?proj=147905, identifier ChiCTR2200055422.
Background
The majority of patients undergoing surgery need general anesthesia and tracheal intubation. The stimulation of the tracheal tube, sleeve, and some inhaled anesthetics can lead to increased airway and oral secretions, and excessive secretions at extubation can increase the risk of perioperative regurgitation, aspiration, and laryngospasm in patients, leading to decreased quality of awakening (1).
The normal airway epithelium secretes a small amount of mucus, which serves to protect and lubricate the airway. When the respiratory tract is irritated with inflammatory lesions, the composition of the respiratory fluid changes, and sputum is formed (2), which is mainly secreted by the bronchial mucosal epithelial submucosal glands and goblet cell. The secretion of the glands is innervated by the vagus nerve (3), and stimulation of the vagus nerve can induce an increase in glandular secretion. In addition to being innervated by the vagus nerve, the secretory glands and cupped cells are also directly influenced by inhaled dry air and irritating gases.
Perioperative anesthesiologists often use anticholinergic drugs such as atropine and pentacyclidine hydrochloride to reduce the secretion of the patient's glands, which can be effective. However, their side effects are more frequent and patients are prone to anti-M cholinergic-like side effects such as difficulty urinating, constipation, tachycardia, and dry mouth (4, 5). The study by Elpidio et al. confirmed that anticholinergic drugs are associated with cognitive impairment and impaired mobility in patients and recommended that, where possible, clinicians physicians should avoid drugs with anticholinergic properties (6). So, is there a way to both reduce the amount of anticholinergic drugs used and reduce glandular secretion in patients?
Opioid analgesics are currently the most commonly used analgesic drugs, producing powerful analgesic effects by activating μ-receptors in vivo and altering the activity of a variety of neural pathways. Such drugs modulate both the transmission of cholinergic effects and have cough suppressant, mucosal secretion inhibitory, and neurally mediated microcirculatory permeation effects. There are opioid peptide binding sites within the airway mucosa. The consequences of opioid peptides on the respiratory system may involve inhibitory regulation of mucus secretion and the oscillatory frequency of respiratory mucus cilia. Therefore, narcotic analgesic drugs such as fentanyl, sufentanil, and remifentanil may be directly or indirectly involved in the regulation of respiratory, glandular secretion.
There are no studies to show whether there is an effect between different depths of anesthesia on glandular secretion. Whether there is a correlation between depth of anesthesia and glandular secretion needs to be further investigated. The objective of this study is to investigate whether the depth of anesthesia can regulate the secretion of respiratory glands, to provide anesthesiologists with a reference for the appropriate depth of anesthesia in clinical practice, to reduce the use of anticholinergic drugs and the incidence of related complications, to improve the patient experience, and to accelerate patient recovery.
Methods
The Ethics Committee approved the protocol of this prospective randomized controlled study of the First Affiliated Hospital of Gannan Medical College on June 25, 2021. The trial was registered with the Chinese Clinical Trials Registry on January 9, 2022 (registration No. ChiCTR2200055422) and comply with the CONSORT statement. Written informed consent was obtained from all patients in accordance with local regulations and the principles of the Declaration of Helsinki.
Ninety patients who underwent total hysterectomy in the First Affiliated Hospital of Gannan Medical College from June 2021 to October 2021 were selected. A randomized numerical table method was used to randomly divide the 90 patients into three groups. Group L: intraoperative maintenance of anesthesia depth D0–D2, group M: intraoperative maintenance of anesthesia depth E0–E1, group H: long tocopherol 0.01 mg/kg was administered intravenously 10 min before surgery without monitoring the depth of anesthesia. Patients were routinely fasted and fasted from food and drink. Patients were triangulated upon admission and routinely monitored for HR, pulse oxygen saturation (SpO2), non-invasive blood pressure (NIBP) in the upper extremities, and electrocardiogram (ECG). The basal salivary secretion was measured with specific test strips before anesthesia, and NT single-channel monitoring was started in groups L and M by cleaning the patient's head skin with alcohol gauze and placing three special blue-cored electrodes pads on the forehead. Group H was administered with 0.01 mg/kg of pentoxifylline hydrochloride intravenously 10 min before the start of anesthesia. After adequate preoxygenation, midazolam 0.05 mg/kg, fentanyl 4 μg/kg, and propofol 1.5–4.0 mg/kg were injected sequentially, and rocuronium 0.8 mg/kg was slowly injected after the patient lost consciousness. After the muscle relaxants were fully effective and the appropriate depth of anesthesia was reached, a tracheal tube was inserted under visual laryngoscopy. All three groups maintained anesthesia by continuous pumping of propofol and remifentanil, with the former pumping rate adjusted in the range of 4–12 mg kg- 1 h- 1 and the latter controlled in the range of 0.2–1.0 μg kg- 1 min- 1. Narcotrend Index (NI) values were maintained between 64 and 37 and 36–20 in groups L and M, respectively, by adjusting the pumping rate of propofol and remifentanil, while in Group H, the anesthesiologist adjusted the pumping rate according to the patient's vital signs to maintain the depth of anesthesia. In group H, the anesthesiologist adjusted the pumping rate according to the patient's vital signs to maintain the depth of anesthesia and added rocuronium bromide at 0.3 mg/kg every 45 min. When the suture was started, flurbiprofen 50 mg was given as a sedative, and propofol and remifentanil were stopped. No antagonist was used during resuscitation. After the patient has reached the indication for tracheal extubation, a disposable sputum culture bottle is used to aspirate the secretions from the airway and mouth, and the tracheal tube is removed.
Sample size calculation method
This study is a randomized controlled trial, the observation group is the conventional anesthesia group and the deep anesthesia group, and the control group is the positive control group of pentoxifylline hydrochloride, the salivary secretion of the study subjects is the observed outcome index, according to the review of literature and the results of the pre-experiment, the salivary secretion of the positive control group is 51.27 ± 17.34 ml, and the salivary secretion of the deep anesthesia group is expected to be reduced by 15.86 ml, set bilateral α = 0.05, and the degree of certainty was 90%. The sample size was calculated according to the following sample size calculation formula, and n = 26 cases were calculated. Considering the 1:1 randomization grouping, i.e., 26 cases each in the observation group and positive control group, and considering 15% of missed visits and refusals, at least 30 cases each in the observation group and control group were required, for a total of at least 90 study subjects.
Inclusion and exclusion criteria
Inclusion Criteria: (1) No significant abnormalities in preoperative pulmonary function tests and no respiratory and pulmonary infections; (2) No bronchial asthma, chronic obstructive pulmonary disease, tuberculosis or other pulmonary disorders; (3) American Society of Anesthesiologists (ASA) grade I–II, no serious cardiovascular or cerebrovascular disease in all patients before surgery, and no significant abnormalities in liver or kidney function; (4) No recent use of cholinergic receptor antagonists; (5) All voluntarily participated in this study and signed the informed consent form.
Exclusion Criteria: (1) Severe cardiovascular and cerebrovascular diseases, severe liver, kidney, endocrine and immune system diseases, etc.; (2) preoperative application of cholinergic receptor antagonists; (3) Preoperative detection of pulmonary infection and symptoms of respiratory tract infection; (4) Having respiratory diseases such as bronchial asthma and chronic obstructive pulmonary disease; (5) Those who are unable to cooperate with this study for various other reasons; (6) A history of allergy to anesthetic drugs or input fluids; (7) Patients with long-term smoking.
Rejection Criteria: (1) Patients with ≥2 intubations due to difficulty in exposing the vocal cords or other reasons; (2) Patients with intraoperative use of atropine due to slow heart rate. (3) Patients already included who have changed their anesthesia surgery plan due to anesthesia modality or surgical reasons.
Observed indexes
Main observation indexes: (1) Recording of basal saliva secretion and intraoperative saliva secretion. (2) Collecting oral and tracheal secretions with a disposable sputum culture bottle before extubation and recording the volume.
Secondary observation indexes: (1) Basic conditions of patients in the three groups were recorded: age, body mass index (BMI), and ASA classification; (2) Recording MAP and HR values at six time points: before induction of anesthesia (T0), at the time of intubation (T1), immediately after the establishment of pneumoperitoneum (T2), 60 min after tracheal intubation (T3), at the end of surgery (T4) and 10 min after extubation (T5); (3) Recording the duration of surgery, postoperative eye-opening time, tracheal extubation time, and hospitalization time; (4) Recording intraoperative fluid intake and output: bleeding volume, fluid volume, urine volume; (5) Record the use of propofol, remifentanil and vasoactive drugs; (6) Recording the occurrence of complications such as PONV, intraoperative awareness, and postoperative agitation; (7) Recording the VAS score of dry mouth 10 min after extubation: a ruler marked with 0–10 cm was used to assess the degree of dry mouth, with “0” representing no dry mouth and “10” representing the unbearable dry mouth.
Salivary gland secretion measurement method
Special round standard blotting paper was placed under the patient's tongue for 1 min and timed using a stopwatch. The diameter of the absorbent paper was 2.0 cm. The absorbent paper was weighed with an electronic balance before and after the measurement, and the salivary secretion was calculated as follows: salivary secretion (mg) = wet weight of absorbent paper—dry weight of absorbent paper.
Statistical analysis
SPSS 26.0 statistical software was used to analyze and process the data, and the measures were tested for normality and expressed as mean ± standard deviation () if they conformed to a normal distribution, and one-way ANOVA was used for comparison between groups, and Bonferroni correction was used to determine differences between groups. If they did not conform to a normal distribution, they were expressed using median and interquartile spacing, and non-parametric tests were performed. Observed indicators at different time points were analyzed using repeated-measures ANOVA. Count data were expressed as n (%) using Fisher's exact probability method, and normality was determined using the S–W test for paired samples or the rank-sum test for paired samples if they did not conform to a normal distribution, with differences considered statistically significant at r < 0.05.
Results
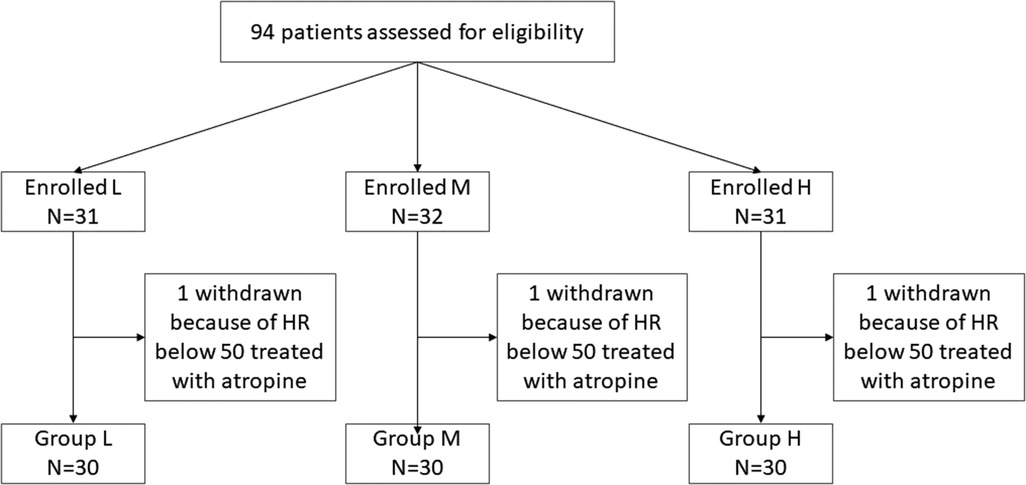
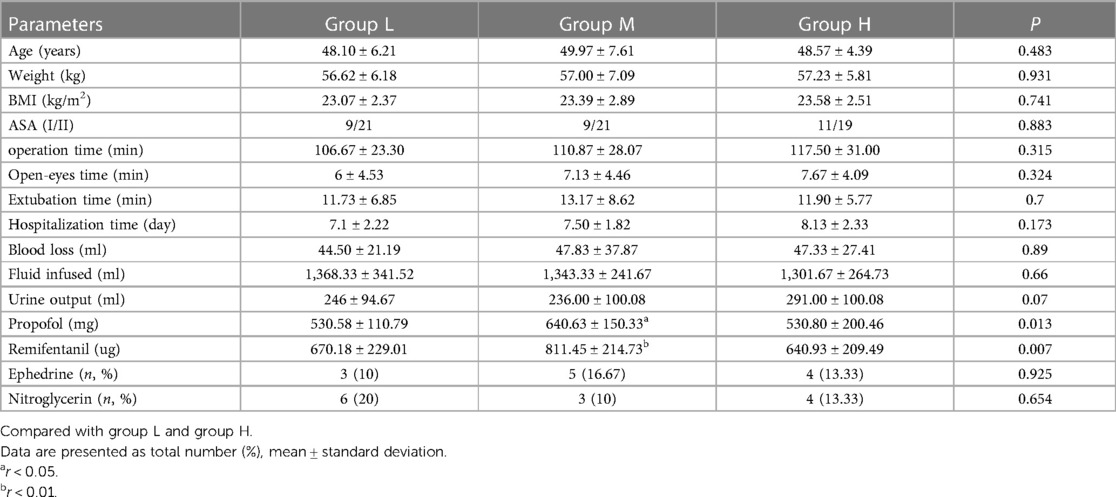
A total of 94 patients were enrolled in the protocol. Using a randomized numerical method of grouping, three patients had atropine used for HR below 50 beats/min, and one patient had an intraoperative change of procedure, excluding a total of 4 cases. Finally, each group contained 30 cases (Figure 1). Patient demographics and surgical characteristics are shown in Table 1.
MAP and HR comparison
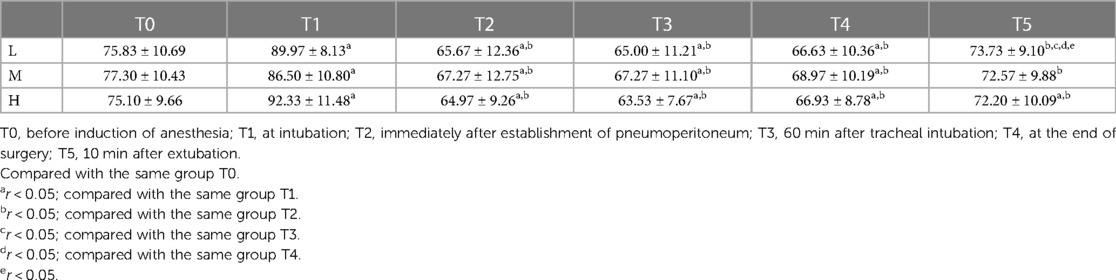
At T1, MAP in group L, group M and group H was significantly higher than that at the moment of T0 (r < 0.05); comparison between groups: at T1, group L and group H with MAP was significantly higher than that of group M (r < 0.05), and there was no statistically significant difference compared with the moment of T0, T2, T3, T4 and T5 in each group (r > 0.05) (Table 2). Compared with T0, HR was increased in all three groups at T1 (r < 0.001), at T2, T3, and T4, HR was lower in all three groups than at T0 (r < 0.01), and there was no statistically significant difference between T5 and T0 (r > 0.05) (Table 3).
Comparison of saliva production
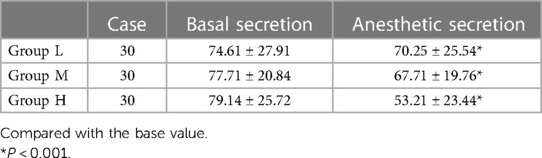
As can be seen from the Table 4, salivary secretion decreased in all three groups after anesthesia compared to the basal values, (r < 0.05), and decreased more in groups H and M compared to the basal values in group L, (r < 0.001).
Comparison of respiratory gland secretion
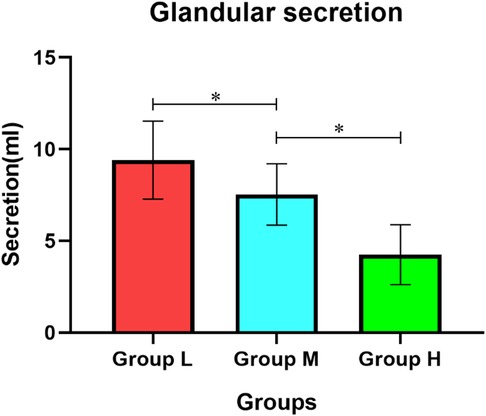
The amount of glandular secretion in group L was (9.41 ± 2.13) ml, in group M was (7.53 ± 1.67) ml and in group H was (4.25 ± 1.63) ml. The difference between the three groups was statistically significant (r < 0.001) (Figure 2).
Comparison of dry mouth VAS scores
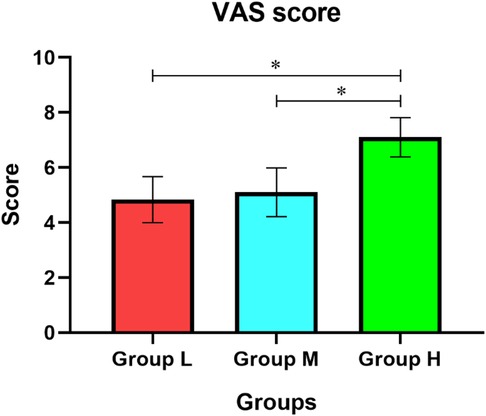
The dry mouth VAS score was (4.83 ± 0.83) in group L, (5.10 ± 0.89) in group M and (7.10 ± 0.71) in group H (Figure 3). The degree of dry mouth was significantly higher in group H than in group L and group M (P < 0.05), and there was no statistically significant difference between group L and group M (P > 0.05).
Discussion
A large amount of oral and airway secretions during the awakening period is likely to put the patient at risk of asphyxiation, laryngospasm and aspiration and greatly affect the patient's experience and comfort (7). Cholinergic receptor antagonists, including atropine, scopolamine, and pentoxifylline hydrochloride, are commonly used in clinical practice to inhibit glandular secretion, but these drugs may have adverse effects such as increased cardiac burden, difficulty urinating, and dryness of the mouth and nose. Therefore, this experiment aimed to explore other methods that could effectively reduce glandular secretion. This study showed that the secretory activity of both salivary and respiratory glands was suppressed under anesthesia, and this effect was more pronounced as anesthesia deepened.
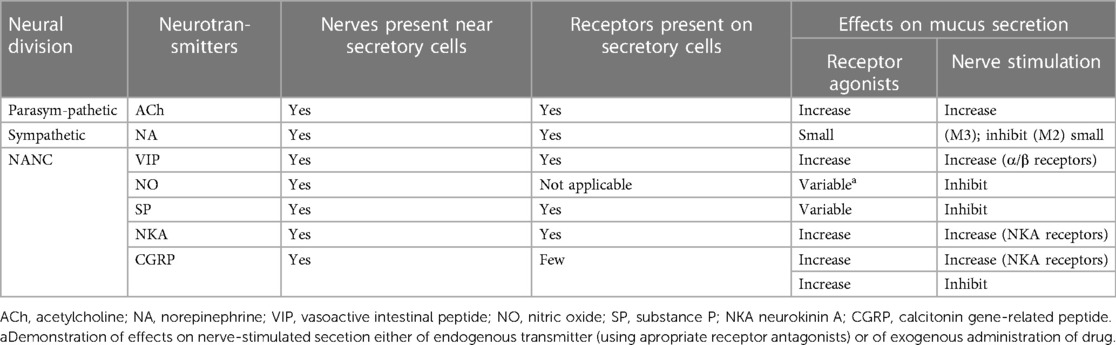
In the human respiratory tract, 5% of the respiratory mucus is secreted by cupped cells, the tips of which are rich in mucin-secreting granules, and the remaining 95% is released by submucosal glands located in the submucosal layer of the respiratory hypodermis. This mucus can resist the invasion of foreign particles, and the bactericidal enzymes in them can also reduce the risk of infection (8). However, various stimulation of the respiratory tract leads to increased mucus secretion from the glands, which may be fatal for patients who need to complete surgery under general anesthesia, and large amounts of oral and airway secretions may cause violent choking, laryngospasm, or even respiratory obstruction during extubation, which will result in serious consequences if not detected in time. The secretion of respiratory glands is regulated by nerves, and the nerves at the airway are closely connected to mucus-secreting cells, which have corresponding neurotransmitter receptors, and stimulating the nerves will increase the rate of glandular secretion. There are three signaling pathways in the airway: adrenergic, cholinergic, non-adrenergic, non-cholinergic (non-adrenergic, non-cholinergic NANC) systems (9), and their regulatory mechanisms are shown in Table 5. In addition, smoking is a strong respiratory irritant (10), and some studies have shown that smoking can lead to epithelial and cupped cell hyperplasia and mucosal gland hypertrophy, which can cause respiratory glands to secrete large amounts of mucus and cause airflow obstruction (11). Acute and chronic respiratory diseases such as bronchial asthma, chronic obstructive pulmonary disease, bronchiectasis, or lung infections can lead to airway hypersensitivity, increased inflammatory response, and increased mucus exudation (12, 13). Therefore, patients should be advised to quit smoking and actively control lung infections before surgery so that they can maintain a good lung condition to undergo surgery and reduce the incidence of pulmonary complications.
General anesthesia is a complex state in which the anesthesiologist uses drugs to bring the patient to a state of a reversible loss of consciousness, including memory loss, loss of generalized pain, inhibition of stress response, weakened glandular secretion, and relaxation of skeletal muscles (14). At different depths of anesthesia (DoA), the patient's organ functions are inhibited differently, and the signs and symptoms displayed clinically vary. However, the lack of significant intraoperative arousal is one of the common problems in modern anesthesia, which may have serious negative effects on the patient's postoperative recovery. Therefore, the monitoring of anesthesia depth is particularly important (15). Bispectral Index (BIS) and NT are the more mature methods of anesthesia depth monitoring used in clinical practice today (16, 17). NI was developed by Hannover University School of Medicine, Germany, which divides the depth of anesthesia into six stages. A for the awake state, B and C for the sedated state, D and E for the anesthetized state, and F for the burst suppression. In this study, we chose to classify patients into conventional anesthesia (D0–D2) and deep anesthesia (E0–E1) groups and did not include the depth of anesthesia E2 level in the selected criteria because it is not within the recommended DoA, and a recent meta-analysis showed that too deep anesthesia might increase mortality in patients more than 90 days after surgery (18), and intraoperative too deep anesthesia or even eruptive suppression should be avoided, so careful consideration of patient safety factors ruled out this depth.
The discovery of the role of ether anesthesia made possible surgical procedures, which initially required only the absence of pain, without any expectation of a loss of consciousness. It was not until Guedel proposed the classical staging of ether anesthesia during World War II that people gradually began to focus on the concept of depth of anesthesia. The depth of anesthesia for ether anesthesia can be roughly divided into four stages and four levels, with general surgery performed in the second and third levels of the third stage (surgical anesthesia stage). In the early days, anesthesiologists could only judge the depth of anesthesia by all the clinical signs they could observe. Nowadays, with all kinds of advanced monitors, anesthesiologists can quickly obtain all the physiological data of patients, and primary conditions such as pupils, urine volume, mucosal color, and glandular secretion are often overlooked. Glandular secretion is mainly regulated by parasympathetic nerves, and the neurotransmitters released by them, such as Acetylcholine (Ach), can cause the glands to secrete mucus (19). Anticholinergic drugs are effective in reducing the secretion of these glands, but there may be a risk of causing other side effects (20). In this study, valacyclovir hydrochloride was used as a positive control group, and the results of the study showed that the respiratory gland secretion and salivary secretion were less in group H than in groups L and M, while the postoperative dry mouth VAS score was higher than in the other two groups, and the patients' postoperative dry mouth was more pronounced, and their comfort during the awakening period was significantly reduced. Compared with the basal values, salivary secretion was reduced in both groups L and M. The salivary glands are innervated by sympathetic and parasympathetic nerves, both of which can lead to increased salivary secretion after stimulation. As for the respiratory glands, the secretion in group M was less than that in group L. This indicates that the secretion of salivary glands and respiratory glands was inhibited to some extent under anesthesia, and the inhibitory effect of anesthetic drugs may have achieved the effect in the study, which became more pronounced with the deepening of anesthesia.
Muscarinic acetylcholine receptors on the airway are G protein-coupled receptors that regulate the release of various neurotransmitters, including gamma-aminobutyric acid (GABA), glutamate, and acetylcholine. One study reported that propofol could inhibit M1-type acetylcholine receptors on GABAergic neurons in the brain and induce anesthetic effects (21). The DoA of propofol anesthesia was correlated with the GABA content for the canine brain, and the GABA concentrations in all brain regions were significantly higher in the deep anesthesia group than in the light anesthesia group. Another study showed that propofol and midazolam significantly inhibited cortical Ach release at sedative doses and that Ach returned to normal levels when the infusion was stopped (22). Therefore, under anesthesia, various anesthetic drugs may inhibit the secretory effects of various glands by suppressing autonomic tone, enhancing or inhibiting certain neurotransmitters and corresponding receptors. However, more animal experiments are still needed to prove this idea. However, in our study design, drugs that affect glandular secretion were avoided, such as dexmedetomidine and ketamine, which have been shown to decrease or increase salivary secretion. The standardized use of anesthetic drugs made our study more convincing.
There are some limitations to this study. First, the type of surgery selected for this study lasted of moderate duration, and it cannot be determined whether maintaining deep anesthesia for a more extended period would affect the patient's hemodynamics and other adverse effects; second, this study has some reference the significance for surgical patients without significant pulmonary disease, but for some specific positions and surgical procedures, the anesthesiologist should weigh the need for anticholinergic drugs based on the patient's actual condition.
Conclusions
In summary, general anesthesia suppresses the patient's glandular secretion, and the suppression becomes more pronounced as the anesthesia deepens. Maintaining the depth of anesthesia E0–E1 can effectively reduce the patient's glandular secretion, more stable intraoperative vital signs, and more comfortable patient awakening period without increasing the incidence of adverse reactions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The Ethics Committee of the First Affiliated Hospital of Gannan Medical College approved the protocol of this prospective randomized controlled study on June 25, 2021. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conception and Study design: L-yX and M-lZ; Execution, acquisition of data: K-nH and Z-hD; Analysis and Interpretation: QZ and L-yX; Took part in drafting: MZ. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the First Affiliated Hospital of Gannan Medical College (YJYB202150).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Collins S, Schedler P, Veasey B, Kristofy A, McDowell M. Prevention and treatment of laryngospasm in the pediatric patient: a literature review. AANA J. (2019) 87(2):145–51.31587728
2. Whitsett JA. Airway epithelial differentiation and mucociliary clearance. Ann Am Thorac Soc. (2018) 15(Suppl 3):S143–8. doi: 10.1513/AnnalsATS.201802-128AW
3. Proctor GB. The physiology of salivary secretion. Periodontol 2000. (2016) 70(1):11–25. doi: 10.1111/prd.12116
4. Rachana PB, Sequeira J. Effect of intramuscular atropine sulphate and glycopyrrolate on heart rate and salivary secretion in patients undergoing minor oral surgical procedure. Cureus. (2020) 12(11):e11780. doi: 10.7759/cureus.11780
5. Collamati A, Martone AM, Poscia A, Brandi V, Celi M, Marzetti E, et al. Anticholinergic drugs and negative outcomes in the older population: from biological plausibility to clinical evidence. Aging Clin Exp Res. (2016) 28(1):25–35. doi: 10.1007/s40520-015-0359-7
6. Attoh-Mensah E, Loggia G, Schumann-Bard P, Morello R, Descatoire P, Marcelli C, et al. Adverse effects of anticholinergic drugs on cognition and mobility: cutoff for impairment in a cross-sectional study in young-old and old-old adults. Drugs Aging. (2020) 37(4):301–10. doi: 10.1007/s40266-019-00743-z
7. Wine JJ. Parasympathetic control of airway submucosal glands: central reflexes and the airway intrinsic nervous system. Auton Neurosci. (2007) 133(1):35–54. doi: 10.1016/j.autneu.2007.01.008
8. Jaramillo AM, Azzegagh Z, Tuvim MJ, Dickey BF. Airway mucin secretion. Ann Am Thorac Soc. (2018) 15(Suppl 3):S164–70. doi: 10.1513/AnnalsATS.201806-371AW
9. Rogers DF. Motor control of airway goblet cells and glands. Respir Physiol. (2001) 125(1–2):129–44. doi: 10.1016/S0034-5687(00)00209-7
10. Lange P, Ahmed E, Lahmar ZM, Martinez FJ, Bourdin A. Natural history and mechanisms of COPD. Respirology. (2021) 26(4):298–321. doi: 10.1111/resp.14007
11. Maestrelli P, Saetta M, Mapp CE, Fabbri LM. Remodeling in response to infection and injury. Airway inflammation and hypersecretion of mucus in smoking subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2001) 164(10 Pt 2):S76–80. doi: 10.1164/ajrccm.164.supplement_2.2106067
12. Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. (2018) 15(5):1033. doi: 10.3390/ijerph15051033.29883409
13. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. (2016) 138(1):16–27. doi: 10.1016/j.jaci.2016.05.011
14. Rule E, Reddy S. Awareness under general anaesthesia. Br J Hosp Med (Lond). (2014) 75(10):573–7. doi: 10.12968/hmed.2014.75.10.573
15. Daunderer M, Schwender D. Depth of anesthesia, awareness and EEG. Anaesthesist. (2001) 50(4):231–41. doi: 10.1007/s001010050997
16. Li Z, Cai J, Li J, Xu X, Zheng L. Comparative evaluation of the bispectral index (BIS) and BISpro during propofol anaesthesia. J Int Med Res. (2021) 49(4):675904663. doi: 10.1177/03000605211001705
17. Kreuer S, Wilhelm W. The narcotrend monitor. Best Pract Res Clin Anaesthesiol. (2006) 20(1):111–9. doi: 10.1016/j.bpa.2005.08.010
18. Liu YH, Qiu DJ, Jia L, Tan JT, Kang JM, Xie T, et al. Depth of anesthesia measured by bispectral index and postoperative mortality: a meta-analysis of observational studies. J Clin Anesth. (2019) 56:119–25. doi: 10.1016/j.jclinane.2019.01.046
19. Ballard ST, Inglis SK. Liquid secretion properties of airway submucosal glands. J Physiol (2004) 556(Pt 1):1–110. doi: 10.1113/jphysiol.2003.052779
20. Reiter L, Stenberg-Nilsen H, Okland HG. Use of anticholinergic drugs in older patients. Tidsskr Nor Laegeforen. (2021) 141(6). doi: 10.4045/tidsskr.20.0775
21. Kim JJ, Gharpure A, Teng J, Zhuang Y, Howard RJ, Zhu S, et al. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature. (2020) 585(7824):303–8. doi: 10.1038/s41586-020-2654-5
Keywords: Narcotrend, depth of anesthesia, total hysterectomy, glandular secrete, anesthesia
Citation: Xia L-y, Zhang Q, Zhuo M, Deng Z-h, Huang K-n and Zhong M-l (2023) Effects of different anesthetic depths monitored by processed EEG analysis on glandular secretion in patients undergoing laparoscopic total hysterectomy. Front. Anesthesiol. 2:1237970. doi: 10.3389/fanes.2023.1237970
Received: 10 June 2023; Accepted: 16 October 2023;
Published: 9 November 2023.
Edited by:
Hong Liu, UC Davis Health, United StatesReviewed by:
Etrusca Brogi, University of Pisa, ItalyChristian Bohringer, UC Davis Medical Center, United States
© 2023 Xia, Zhang, Zhuo, Deng, Huang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mao-lin Zhong 958765351@qq.com
†Present Address: Qian Zhang, Department of Anesthesiology, Nanjing Gulou Hospital Group Suqian Hospital Co., Ltd., Suqian, China
‡These authors have contributed equally to this work
Abbreviations NT, Narcotrend; NI, narcotrend index; VAS, visual analogue scale; ASA, American Society of Anesthesiologists; MAP, mean arterial pressure; HR, heart rate; DoA, depth of anesthesia; ECG, electrocardiogram; SpO2, pulse oxygen saturation; NIBP, non-invasive blood pressure; Bispectral Index, bispectral index; Ach, acetylcholine; NANC, non-adrenergic, non-cholinergic; NA, norepinephrine; VIP, vasoactive intestinal peptide; NO, nitric oxide; SP, substance P; NKA, neurokinin A; CGRP, calcitonin gene-related peptide.
 Ling-yi Xia
Ling-yi Xia Qian Zhang
Qian Zhang Ming Zhuo2
Ming Zhuo2