Comparison of continuous popliteal nerve blocks using lidocaine vs. bupivacaine infusions for ambulatory foot surgery: a randomized, double-blind, noninferiority trial
- 1Department of Anesthesiology, Perioperative and Pain Medicine, Icahn School of Medicine at Mount Sinai West, New York, NY, United States
- 2Department of Anesthesiology, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
Background and objectives: Continuous sciatic popliteal nerve block effectively manages pain after ankle and foot surgery. Most studies on continuous infusion of local anesthetics by perineural catheters have been made with bupivacaine, levobupivacaine, and ropivacaine, but lidocaine has not been used. The main objective of this study was to compare the quality of analgesia, motor function, and side effects between lidocaine and bupivacaine infusions in bilateral continuous popliteal nerve blocks for foot surgery.
Methods: This was a prospective, double-blind, randomized, parallel-group, noninferiority study. We enrolled 70 patients undergoing bilateral foot or ankle surgery that could be performed under general anesthesia and continuous regional analgesia using sciatic popliteal nerve blocks. During their postoperative care, they were randomized into 2 groups: group lidocaine (lidocaine 0.5%, 5 ml/h) or group bupivacaine (bupivacaine 0.1%, 5 ml/h), administered through elastomeric pumps. The primary outcome was pain at 24 h after surgery, assessed by a verbal numeric rating scale (ranging from 0 to 10). A pre-specified non-inferiority margin of 1.5 was defined. Secondary outcomes were the degree of motor and sensitive block, total opioid use, and difficulties with pump or catheter management.
Results: The mean postoperative pain at 24 h of surgery was 2.06 (95% bootstrap confidence interval bCI 1.29, 2.83) and 1.82 (95% bCI 1.02, 2.62) in the lidocaine and bupivacaine group, respectively. The upper limit of the 95% bCI for the mean difference between lidocaine and bupivacaine was 0.82, declaring non-inferiority. No differences in the postoperative rescue analgesia use and satisfaction with care were found. No differences in postoperative NRS, sensory block and motor block were seen between groups.
Conclusions: Lidocaine 0.5% and bupivacaine 0.1% provide similar postoperative analgesia through a sciatic popliteal catheter in ambulatory bilateral foot surgery patients.
Clinical Trial Registration: https://clinicaltrials.gov/, identifier: NCT02121119
Introduction
Continuous sciatic popliteal nerve blocks are an effective method of pain management after foot surgery. Continuous infusions of short-acting anesthetic drugs have been a trend in clinical practice, increasing the flexibility of many anesthetic techniques.
However, in the case of regional anesthesia and acute post-operative analgesia, mostly long-acting agents are used. This practice has no clear reason, particularly when continuous perineural infusions are employed.
While the use of short-acting anesthetic drugs (shorter latencies, more titratable) is common in modern anesthesia (e.g., the use of remifentanil, propofol, and desflurane, to name a few), especially when administered as continuous infusions, the duration of the drug given in bolus form is not a significant factor in maintaining its effect. The intensity of this effect depends on the dose administered over time. In this regard, the use of an intermediate-duration local anesthetic like lidocaine, which is safe and whose efficacy in regional neuraxial and peripheral anesthesia is well recognized, may be an option that is at least not inferior to the current standard, which is to use long-acting local anesthetics in continuous infusion.
From a clinical utility perspective, using lidocaine, a local anesthetic that has a higher safety profile in terms of systemic toxicity (1) and is widely available while demonstrating a similar clinical effect [faster onset/earlier offset, more titratable (2)], at a fraction of the cost of the other drugs (3), appears clinically relevant.
We hypothesized that lidocaine solutions are as efficacious in providing postoperative analgesia as bupivacaine in continuous ambulatory sciatic popliteal nerve blocks. Therefore, we designed a non-inferiority trial to compare the quality of analgesia, motor function, and side effects between lidocaine and bupivacaine infusions in bilateral continuous popliteal nerve blocks for foot surgery.
Methods
We designed a prospective, double-blind, randomized, parallel-group, noninferiority study (ClinicalTrials.gov Identifier: NCT02121119, April, 2014). This study was approved by the Institutional Review Board of the School of Medicine, Pontificia Universidad Católica de Chile (IRB identifier: 13-289).
After written informed consent, subjects were enrolled at Hospital Clínico, Pontificia Universidad Católica de Chile, Santiago, Chile.
Study participants
We enrolled ASA I-II patients between 20 and 70 years old, scheduled for elective bilateral foot or ankle surgery. The suitability to receive a continuous sciatic nerve block was assessed before surgery. All participants provided written, informed consent.
We excluded patients with a history of substance abuse, preexisting neuropathy, psychiatric disorder, chronic pain syndromes or chronic opioid use, and the use of regular daily doses of systemic opioids for the past 48 h of the surgery, BMI ≥ 35 kg/m2, infection near the insertion site of the catheters, coagulation disorders, pregnancy, severe gastroesophageal reflux, and known allergy to the study drugs.
Randomization
Patients were randomized to receive a postoperative infusion of either lidocaine 0.5% (L group) or bupivacaine 0.1% (B group) using the sciatic nerve block catheter elastomeric pumps. A randomization sequence was generated using the website Randomization.com with a 1:1 allocation ratio, using randomly permuted blocks of 2, 4, and 6. Group allocation was concealed using consecutively numbered, sealed opaque envelopes, which were opened prior to the procedure. To keep the study blind, an anesthesiologist not involved in the patient's care or study evaluation prepared the anesthetic solutions based on the randomization list.
During the entire study period, investigators performing the postoperative assessments, medical staff (nurse, anesthesiologist, and surgeon), and patients were blinded to group allocation.
Anesthesia, surgery and postoperative analgesia
In the operating room, standard ASA monitors were applied. Before performing the peripheral nerve blocks, intravenous access was obtained, the patient was placed in the prone position, and conscious sedation with midazolam (0,03 mg/kg) IV and fentanyl (1 mcg/kg) IV was started. All patients received supplemental oxygen via facemask.
All popliteal catheter placements were performed before surgery by 1 of 4 anesthesiologists with extensive experience in ultrasound-guided nerve block procedures.
Using the HFL38, 10–5 MHz, 38 mm broadband linear array transducer (HFL38; SonoSite®, Bothell, WA, USA) covered by a sterile sleeve, the popliteal fossa was scanned, and the sciatic nerve identified in a transverse cross-sectional view. After the skin was infiltrated with 1 ml of lidocaine 2%, a 40 mm nerve block needle (Contiplex ®, B. Braun Medical, Inc., Bethlehem, PA) was inserted using an out-of-plane approach. Once the tip of the needle was close to the bifurcation, motor stimulation was used (Stimuplex HNS 12, B. Braun Medical, Inc., Bethlehem, PA) to verify the final position of the tip. Acceptable motor response was defined as dorsiflexion or plantar flexion of the foot/toes or inversion using a current amplitude between 0.30 and 0.50 mA, at a frequency of 2 Hz, and a stimulation duration of 0.1 ms. Once a proper motor response was achieved, a bolus of 1% lidocaine 20 ml was slowly injected while visualizing the spread around the nerve, followed by the insertion of a 20-gauge perineural catheter, advanced 5 cm–7 cm beyond the tip of the needle. The catheter was then tunneled and covered with a sterile dressing.
The same procedure was repeated in the contralateral leg. General anesthesia was then induced with fentanyl 1 to 2 mcg/kg and propofol 2 mg/kg, followed by inserting a laryngeal mask and maintained with sevoflurane. Additional boluses of fentanyl (1 mcg/kg) were given if the mean blood pressure was 25% or higher than the baseline (measured in the preoperative area). All patients received 60 mg of I.V. ketorolac before the inflation of tourniquets. All patients were extubated in the operating room and transferred to the post-anesthesia care unit.
Three hours after the placement of both catheters 2 elastomeric pumps (LV infusor Baxter® Elastomeric Pump, total capacity of 300 ml) containing either lidocaine 0.5% (L group) or bupivacaine 0.1% (B group) were connected based on randomization. The infusions were fixed at 5 ml/h.
All patients received oral acetaminophen, 1 g every 8 h, celecoxib, 200 mg every 12 h. Tramadol, 50 mg P.O., was given in case of breakthrough pain (NRS >4).
Primary and secondary endpoints
Pain at rest and during motion was recorded at 0, 12, 24, and 48 h after surgery using a Numeric Rating Scale (NRS; 0, no pain; 10, worst possible pain). The primary outcome was pain during motion at 24 h postoperative. If pain was different between legs, the highest number was used for the analysis.
The degree of motor block was assessed by asking the patient to move each ankle and grading them from no movement at all to full movement and normal strength (4). The sensory block was evaluated using a pinprick on the sole of the feet, where 0 = no sensory block, 1 = partial sensory block, 2 = complete sensory block. Total opioid consumption (expressed as morphine milligram equivalents) and difficulties with catheter management were also recorded.
After being discharged home, all patients were contacted daily by phone, and data regarding pain scores (static, dynamic, worst pain), rescue analgesia (use of oral opioids “yes/no”), and user satisfaction were registered on day 1, 2, after elastomeric pump removal and on postoperative day 7.
Statistics
The sample size calculation was based on the findings by White et al. (5) that compared the analgesic effect of a bupivacaine infusion vs. placebo for a continuous nerve block after foot and ankle surgery. The approximate mean effect size (ɛ) reported was 3.5 (numeric rating scale) for pain at 24 h. Using a non-inferiority limit [delta] of 1.5 (less than 0.5 of ɛ) (6) and a standard deviation of 1.84 points (institutional pilot data), 32 patients per group were needed to show noninferiority of postoperative lidocaine infusion in comparison with bupivacaine infusion on the primary outcome with a power of 0.9 and an α level of 0.025. We enrolled 35 subjects per arm to allow for possible dropouts. Normality was tested using the shapiro-wilk test and Q-Q plots.
Noninferiority of postoperative lidocaine infusion to bupivacaine infusion on the primary outcome—pain during motion at 24 h postoperative—was assessed using a 1-tailed t-test at the 0.025 significance level and noninferiority delta of 1.5 points. For a more robust analysis, we also calculated the 95% confidence interval (CI) of the mean differences between lidocaine and bupivacaine using bootstrapping (95% bCI, based on 10,000 replicates) and the upper limit of the CI was compared to the prespecified delta.
Continuous demographic and secondary outcomes data were analyzed using paired or unpaired Student t-test, and Wilcoxon rank-sum test for between-group comparisons, as appropriate. Chi-squared test was used for inferences on proportions.
Data are expressed as mean (standard deviation) or median (interquartile range), unless otherwise stated. All reported P values, except for the noninferiority analysis, are based on two-sided tests with an alpha level of 0.05. All analyses were performed with STATA/SE 14.2 following the intention-to-treat principle.
Results
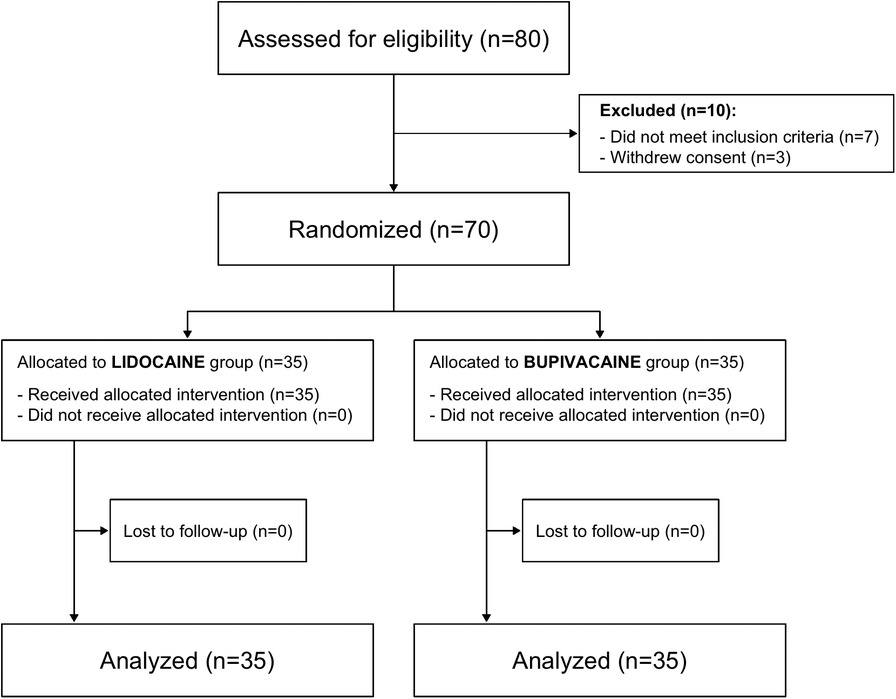
Seventy patients were enrolled. All received their assigned study intervention. In four cases, the catheter was dislodged and replaced on admission to the recovery room (3 in the lidocaine and 1 in the bupivacaine group). In 2 cases, the catheter was removed inadvertently at 24 h (bupivacaine group), and in 3 cases at 48 h (lidocaine group). Figure 1 shows the CONSORT diagram.
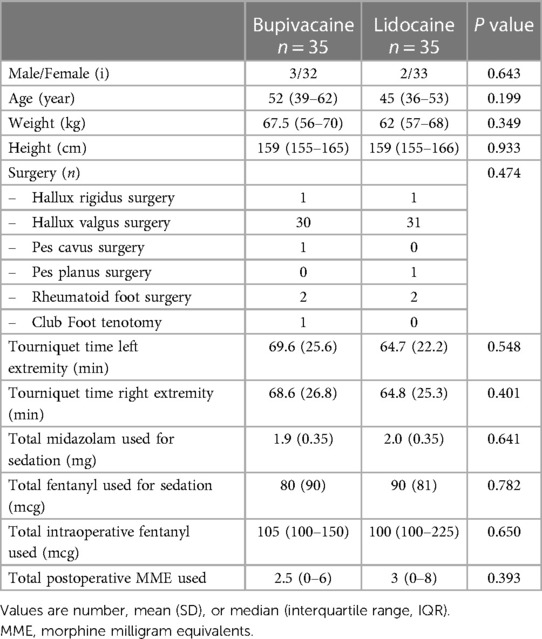
Both groups were similar with respect to patient characteristics and intraoperative data (Table 1).
The mean postoperative pain at 24 h of surgery was 2.06 (95% bCI 1.29, 2.83) and 1.82 (95% bCI 1.02, 2.62) in the lidocaine and bupivacaine group, respectively. The upper limit of the 95% bCI for the mean difference between lidocaine and bupivacaine was 0.82. Since 0.82 was below the pre-specified non-inferiority margin of 1.5, non-inferiority was declared (Figure 2).
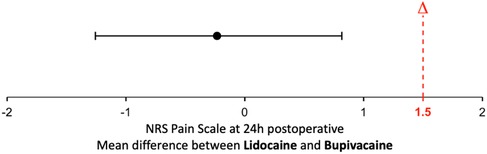
Figure 2. Noninferiority diagram of numerical rating scale (NRS) pain difference of lidocaine-bupivacaine. The dotted red line designates the noninferiority margin (Δ) of 1,5 on a 0 to 10 pain scale. The error bar designates the 95% bootstrap Confidence Interval (bCI) of the difference between the groups.
No differences in the postoperative rescue analgesia use and satisfaction with care were found. No differences in postoperative NRS, sensory block and motor block were seen between groups (Figure 3).
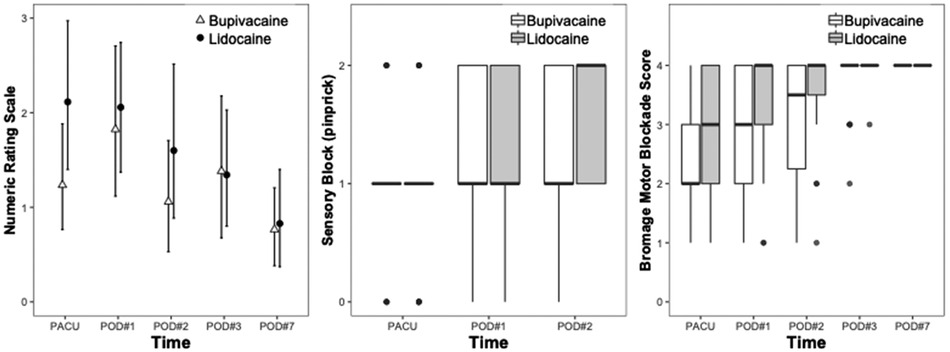
Figure 3. Clinical outcome differences for lidocaine and bupivacaine sciatic infusions after foot surgery. Postoperative Numeric Rating Scale (NRS), sensory block, and motor block were evaluated in the post-anesthesia care unit (PACU), on postoperative day (POD) 1, 2, 3, and 7.
Discussion
The main finding of this study is that Lidocaine was non-inferior to Bupivacaine in providing postoperative analgesia through sciatic popliteal catheters in patients undergoing bilateral foot surgery.
Previous studies have reported the use of lidocaine in continuous perineural infusions, comparing it against long-acting local anesthetics (2, 3, 7). The existence of these prior studies attests to the rationale behind our approach, and all of them were conceived as superiority-designed studies, hypothesizing that using lidocaine infusions instead of long-acting local anesthetic choices could provide benefits in terms of faster onset time, quicker titration, or less toxicity. Unfortunately, the superiority design, statistically, given the sample sizes used, prevents ruling out whether the lack of difference is due to non-inferiority or a statistical power issue (type II error). Interestingly, from the clinical standpoint, none of those studies found any differences in analgesic effectiveness. Four studies were performed on truncal blocks and one on the upper extremity. All the studies performed on truncal blocks found no differences in analgesic effectiveness. Casati et al. found that lidocaine 1% had significantly more pain compared to ropivacaine 0.2% in the first 8 h, using a 0–100 mm pain scale (median 20 [range 0–60] vs. 0 [range 0–50]) but no differences were observed beyond this point. The use of rescue tramadol was also higher in the lidocaine group. Maybe the fact that we used a dual-guided (US and PNS) technique, ensuring a more precise location of the catheters, instead of electric peripheral nerve stimulation (PNS) alone as neuro-localization technique, could explain the different outcomes. Consequently, patients in our study had good pain control and clinically significant sensory block using 0.5% lidocaine instead of 1% lidocaine.
To the best of our knowledge, this is the first study evaluating the effectiveness of lidocaine, comparing it against long-acting local anesthetics such as bupivacaine, assuming a non-inferiority approach. A non-inferiority design assuming a margin that is clinically acceptable allows us to demonstrate that lidocaine is not significantly less effective than bupivacaine but is safer and more affordable than bupivacaine.
There are clinical and practical implications to the results of the study. The findings of non-inferior analgesia using lidocaine offer a cost-effective and cost-efficient alternative to long-acting local anesthetics, such as bupivacaine or ropivacaine.
One advantage of using lidocaine for continuous peripheral nerve blocks could be lower toxicity as compared to other agents (1).
Previous studies using lidocaine in epidural analgesia 5 have reported adequate pain relief, without any effect on lower extremity motor function when 1% lidocaine is used. In the setting of open shoulder surgery, Casati et al. (7) reported that patients receiving 1% lidocaine infusion through an interscalene catheter had higher pain scores and required rescue analgesic medications more frequently than patients receiving 0.2% ropivacaine infusions. Maybe the fact that we used a dual-guided (US and PNS) technique, ensuring a more precise location of the catheters, instead of electric peripheral nerve stimulation (PNS) alone as neuro-localization technique, could explain the different outcomes. As a consequence, patients in our study had good pain control and clinically significant sensory block using 0.5% lidocaine instead of 1% lidocaine.
A pilot study conducted by Ghisi et al. (3) reported adequate pain control after abdominal surgeries when 0.25% lidocaine was used in paravertebral blocks. This study emphasizes a cost-containment effect of using lidocaine for continuous infusions when it is compared with ropivacaine infusions (60% reduction in costs). This consideration is an important driver in terms of the adoption of any continuous regional analgesic technique.
Additionally, a critical shortage of both bupivacaine and ropivacaine had occurred in United States and Canada due to an apparent increase in drug demand and back orders from the suppliers. The Society of Obstetric Anesthesia and Perinatology (SOAP) raised a clinical practice advisory and safety alert, providing suggestions to all anesthesia providers who work in labor and delivery (8). This shortage also affects other regional anesthesia/analgesia areas, including continuous peripheral nerve blocks. In this context, lidocaine can be a reliable option to perineural infusions using more traditional drugs such as ropivacaine or levobupivacaine. Furthermore, demonstrating a similar clinical effect at a fraction of the cost of the other drugs, appears clinically relevant. This is particularly true in middle and low-income countries, where healthcare-associated costs are a significant concern and directly influence the type of care patients receive.
Our study has several limitations: Patients in both groups received a combination of regional analgesia and systemic oral analgesics: Acetaminophen and celecoxib. The analgesic effect of this combination arguably can affect our capacity to assess the effectiveness of our intervention. In modern Anesthesiology however, it is nearly impossible not to employ a multimodal analgesic approach in the perioperative period. There is an extensive body of literature and practical guidelines supporting this practice. While we are aware that the use of a non-steroidal anti-inflammatory (NSAID) drug such as celecoxib on a scheduled basis should influence reported pain scales (and the same can be said about the use of acetaminophen), our study aimed to establish comparisons between the two solutions of local anesthetics in the context of a pragmatic analgesic scheme. This would allow us to understand our findings with regular clinical practice (external validity of our results). On the other hand, if we consider that the subjects were randomly assigned to the groups, it is possible to assume that, since all characteristics were similar, there are no differences between the groups in their pain scales attributable to the assigned local anesthetic solution, and that the intensity of the reported pain would be what a patient would report under actual clinical conditions.
This is a single center randomized controlled trial and further investigations are needed to systematically assess lidocaine efficacy in this setting. Additionally, in terms of safety, the absence of any significant complications should be considered with caution, due to the limited number of subjects participating in the study.
Conclusions
In conclusion, our results show that lidocaine 0.5% and bupivacaine 0.1% provided similar postoperative analgesia through a sciatic popliteal catheter in patients undergoing bilateral foot surgery. This represents an option with lower costs and potentially, less risk of systemic toxicity complications in the ambulatory setting.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comité Ético Científico de Ciencias de la Salud, Pontificia Universidad Católica de Chile. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GE: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing, Visualization. FA: Conceptualization, Data curation, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing, Formal Analysis, Funding acquisition, Project administration, Resources. PM: Conceptualization, Project administration, Writing – review & editing. AA: Investigation, Project administration, Writing – review & editing. MC: Project administration, Writing – review & editing. JD: Project administration, Supervision, Writing – review & editing. Rd: Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Financial support for the publication of this article was provided by the Department of Anesthesiology, Escuela de Medicina, Pontificia Universidad Católica de Chile. No other financial support was received for the research and/or authorship of this article.
Acknowledgments
The authors would like to acknowledge: Sebastian Paredes, anesthesiologist, Department of Anesthesiology, Escuela de Medicina, Pontificia Universidad Católica de Chile, for assisting with randomization, screening and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brown DL, Ransom DM, Hall JA, Leicht CH, Schroeder DR, Offord KP. Regional anesthesia and local anesthetic-induced systemic toxicity: seizure frequency and accompanying cardiovascular changes. Anesthesia Analg. (1995) 81:321. doi: 10.1213/00000539-199508000-00020
2. Rygnestad T, Zahlsen K, Bergslien O, Dale O. Focus on mobilisation after lower abdominal surgery. A double-blind randomised comparison of epidural bupivacaine with morphine vs. lidocaine with morphine for postoperative analgesia. Acta Anaesthesiol Scand. (1999) 43:380–7. doi: 10.1034/j.1399-6576.1999.430403.x
3. Ghisi D, Fanelli A, Jouguelet-Lacoste J, Colla LL, Auroux A-S, Chelly JE. Lidocaine versus ropivacaine for postoperative continuous paravertebral nerve blocks in patients undergoing laparoscopic bowel surgery: a randomized, controlled, double-blinded, pilot study. Local Reg Anesthesia. (2015) 8:71–7. doi: 10.2147/lra.s84476
4. Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiol Scand. (1965) 9:55–69. doi: 10.1111/j.1399-6576.1965.tb00523.x
5. White PF, Issioui T, Skrivanek GD, Early JS, Wakefield C. The use of a continuous popliteal sciatic nerve block after surgery involving the foot and ankle: does it improve the quality of recovery? Anesthesia Analg. (2003) 97:1303–9. doi: 10.1213/01.ane.0000082242.84015.d4
6. Ng T-H. “Noninferiority testing in clinical trials, issues and challenges.,” In: Hall/C and, CRC. New York: Taylor & Francis (2019). p. 76–91. doi: 10.1201/b17792-8
Keywords: lidocaine, ambulatory regional analgesia, ultrasound guided regional block, popliteal nerve block, bupivacaine, non-inferiority analysis
Citation: Echevarría G, Altermatt FR, Miranda P, Araneda A, Corvetto M, de la Fuente R and De la Cuadra JC (2023) Comparison of continuous popliteal nerve blocks using lidocaine vs. bupivacaine infusions for ambulatory foot surgery: a randomized, double-blind, noninferiority trial. Front. Anesthesiol. 2:1266270. doi: 10.3389/fanes.2023.1266270
Received: 24 July 2023; Accepted: 23 October 2023;
Published: 29 November 2023.
Edited by:
Antonello Penna, University of Chile, ChileReviewed by:
Abhijit Nair, Ministry of Health, OmanDeepak Thapa, Government Medical College and Hospital, India
© 2023 Echevarría, Altermatt, Miranda, Araneda, Corvetto, de la Fuente and De la Cuadra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernando R. Altermatt falterma@uc.cl
†These authors have contributed equally to this work and share first authorship
‡ORCID Fernando R. Altermatt orcid.org/0000-0002-0464-8643
 Ghislaine Echevarría1,†
Ghislaine Echevarría1,†  Fernando R. Altermatt
Fernando R. Altermatt Marcia Corvetto
Marcia Corvetto Juan C. De la Cuadra
Juan C. De la Cuadra