Exploring the impact of left ventricular diastolic dysfunction on postoperative cardiac surgery outcomes, with a focus on sex disparities: a comprehensive literature review
- 1Department of Anesthesiology, Amsterdam University Medical Center, Amsterdam, Netherlands
- 2Department of Cardiology, Amsterdam University Medical Center, Amsterdam, Netherlands
Introduction: Left ventricular diastolic dysfunction (LVDD) is known to cause worse outcomes after cardiac surgery. Females have twice the risk of developing LVDD compared with males. The aim of this literature review was to evaluate the association between LVDD and early (≤30 days) outcomes after cardiac surgery, including sex disparities.
Methods: We identified 454 studies in Medline and Embase, of which 19 were included. Articles were assessed for inclusion of female patients with LVDD, ≥50 years of age undergoing cardiac surgery (on or off-pump) or transcatheter aortic valve replacement. The primary outcome was early postoperative mortality. Secondary outcomes were early postoperative complications, ICU length of stay (ICULOS), hospital length of stay (HLOS), and other sex-related postoperative outcomes.
Results: In patients with LVDD, the majority of studies showed that higher LVDD grades correlate with higher early postoperative mortality. In patients with LVDD, who underwent on-pump cardiac surgery, female sex was significantly associated with higher LVDD grades and baseline E/e' ratios. Females with LVDD, were associated with prolonged HLOS after off-pump coronary artery bypass grafting (CABG). In combined cardiac surgery, a twofold increased risk of prolonged HLOS and increased ICULOS was reported. Furthermore, increased left ventricular end-diastolic filling pressure, an increased need for postoperative inotropic support, and difficult separation from cardiopulmonary bypass were observed.
Discussion: Despite the limited number of studies focusing on sex differences, females with LVDD appear to have worse early outcomes after cardiac surgery compared to men with LVDD. Future research will need to identify sex-specific risk factors and target treatment optimization.
Introduction
Left ventricular diastolic dysfunction (LVDD) is defined as abnormal or restrictive relaxation and filling of the left ventricle (1, 2). This increases left ventricular end-diastolic pressure (LVEDP) and impairs the filling ability of the ventricle (2). Causes of LVDD include coronary artery disease, hypertension, and valvular heart disease (3). LVDD can be classified into three grades of severity based on echocardiographic parameters (Table 1) (2).
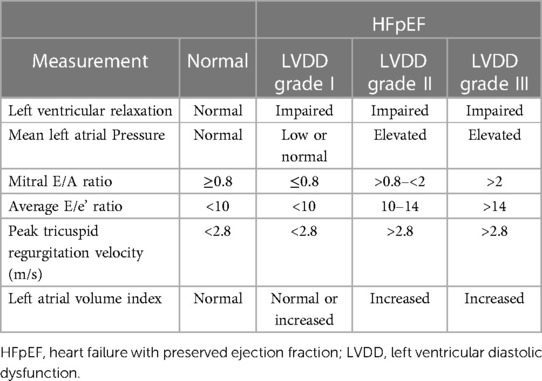
Table 1. LV relaxation, filling pressures, 2D and Doppler findings associated with different grades of LVDD, based on American society of echocardiography and European association of cardiovascular imaging standards (2).
Females are twice as likely to develop LVDD as males of the same age (4). Vascular stiffening is a major pathophysiological factor contributing to the higher prevalence of LVDD in females. With aging, ventricular elastance decreases more rapidly in females (5). In addition, comorbidities such as iron deficiency, diabetes mellitus(DM), obesity, hypertension, and pre-eclampsia are associated with the increased incidence of LVDD in females by inducing an inflammatory response (5).
If LVEDP is elevated in patients with LVDD, circumstances such as tachycardia or elevated afterload can lead to an increase in left atrial pressure(LAP), causing pulmonary congestion, which may cause subsequent symptoms such as dyspnea and hypoxia (6, 7). With further deterioration of the disease, heart failure (HF) can occur. If the left ventricle ejection fraction is preserved (LVEF >40%), this form of HF is defined as Heart Failure with Preserved Ejection Fraction (HFpEF). While HF in general affects 1%–2% of the adult population worldwide, it is a growing cause of significant morbidity and mortality (8, 9). HFpEF accounts for 30%–50% of patients with HF, and incidence increases with age (10).
In the perioperative period, surgery, inflammation and stress can induce tachycardia, hypertension, high cardiac output, or volume shift, which can separately or in combination worsen existing LVDD contributing to the development of HFpEF (11).
Several studies of non-operative patients treated conservatively have investigated whether there are sex differences in the outcomes of patients with LVDD (12–17). These studies showed that females with LVDD had similar outcomes in terms of in-hospital and all-cause mortality compared to males with LVDD. In addition, the incidence of cardiac death and hospitalization due to LVDD was lower in females.
In cardiac surgical patients, the presence of LVDD was associated with higher postoperative mortality and serious adverse cardiac events after surgery such as postoperative atrial fibrillation (POAF) (18). Female patients are more likely to develop LVDD, potentially increasing their risk of mortality and morbidity compared to males. However, there is currently no research into whether female sex exacerbates the adverse effects on postoperative outcomes in LVDD patients (19, 20).
Our intention was therefore to evaluate whether LVDD predicts worse early outcomes (≤30 days) after cardiac surgery, in female patients compared with male patients, with similar baseline characteristics. For this reason, we conducted a literature review to assess the effect of LVDD—by sex—on the early cardiac surgical outcomes (mortality and morbidity) after on- and off-pump cardiac surgery and surgical and percutaneous aortic valve procedures.
Materials and methods
We defined keywords relevant for this review: left ventricular dysfunction, diastolic dysfunction, LVDD, HFpEF, cardiac surgery, echocardiography, females, and postoperative outcomes. These keywords were used to find MeSH (Medical Subject Headings)- and related terms to build the search, which was performed in April 2023. Articles were included if written in English, Dutch, or German and published on Medline or Embase between January 2000 and April 2023. Articles were assessed for further evaluation if they included female patients with LVDD (grade I–III), aged ≥50 years that underwent cardiac surgery (on-pump or off-pump) or transcatheter aortic valve replacement (TAVR). Articles regarding pediatric cardiac surgery, congenital cardiac surgery, case reports, case series, and in vitro and animal studies were excluded.
The following data was extracted from the studies: early postoperative mortality (≤30 days) after cardiac surgery, early postoperative complications (≤30 days), ICULOS, HLOS, LVDD grades, E/e’ ratios at baseline and other perioperative outcomes with emphasis on sex differences.
All results were categorized according to the different surgical approaches (coronary artery bypass grafting(CABG) surgery, valvular surgery, combined cardiac surgery(CABG and valvular surgery), and TAVR.
After initial selection, the references of the included articles were reviewed to find additional relevant publications. Of the remaining articles, the title and abstract were screened by three independent reviewers to ensure they met the inclusion criteria. After this selection, the full-text articles were checked for quality before final inclusion.
The risk of bias was assessed using the ROBINS-1 tool (21). Articles with serious judgments were discussed separately among the independent researchers to determine their suitability for inclusion. The PRISMA guidelines were used in the writing of this review.
Results
We identified 397 articles from the primary search, an additional 57 were included after checking references, 107 resulting in a total of 454 articles. After the removal of duplicates, a total of 412 records remained for further 108 screening. After screening by title and abstract, 372 articles were excluded, the remaining 20 articles 109 underwent a full-text assessment. Finally, 19 articles were included in this review, with a total of 104.325 patients (33.0% female) (Table 2).
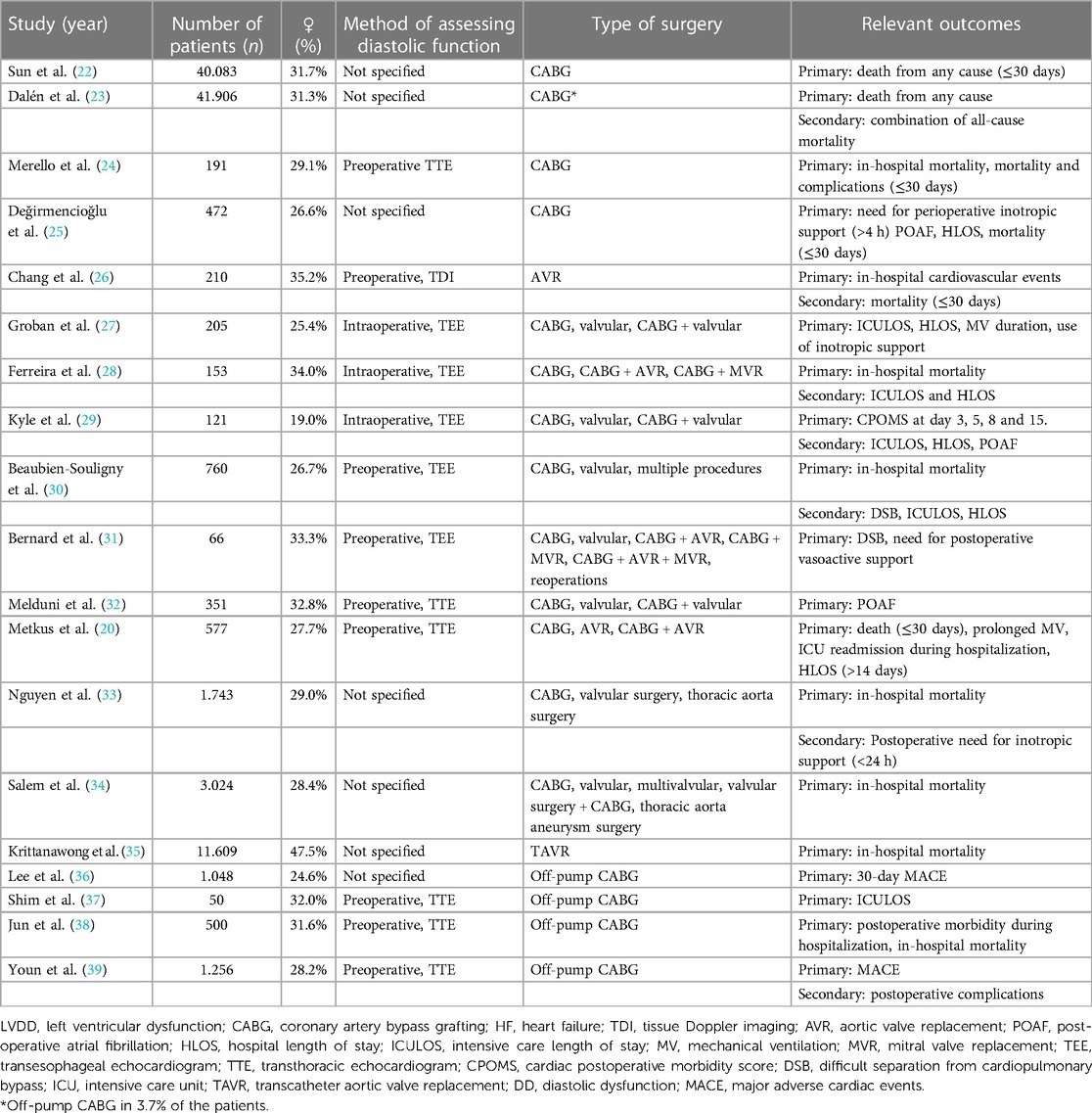
Table 2. Overview of the included articles with their relevant outcomes ordered by the procedure performed.
Early (<30 day) mortality
CABG surgery
Three articles (Sun et al., Dalén et al., and Merello et al.,) evaluated 30-day mortality after on-pump CABG in 82.180 (31.5% female) patients (22–24). Dalén et al., showed that patients with LVDD grade I/II/III had a higher risk of early mortality, compared to patients with LVDD grade 0[HR 1.83, 95% CI (1.26, 2.66)]. Additionally, Merello et al., confirmed this finding: patients with LVDD grade II/III had a higher risk of 30-day mortality compared to patients with LVDD grade 0/I/II[OR 20.9, 95% CI (5.5, 78.9), p = .018].
Sun et al. did not include study patients with LVDD, but focused on patients with HFpEF(EF >50%) and found this group had higher 30-day mortality after on-pump CABG, than patients with Heart Failure with Reduced Ejection Fraction(HFrEF) (Hazard Ratio(HR) 2.57, 95% CI [1.96, 3.36] (22).
Only Sun et al., and Merello et al., analyzed data on sex differences. Sun et al., showed that females with HFpEF had a higher 30-day mortality compared to males with HFpEF(HR 2.89, 95% CI [1.91, 4.37] vs. HR 2.32, 95% CI [1.62, 2.34]) (22). In contrast, Merello et al., observed that female sex was not an independent predictor of 30-day mortality in patients with LVDD undergoing CABG[OR 1.7, 95% CI (0.5, 5.4), p = .376] (24). However, the latter study was substantially smaller than the previous two studies.
Valvular surgery
Chang et al., investigated 30-day mortality in 210 patients with LVDD grade III, who underwent surgical aortic valve replacement (AVR) (26). None of the patients died within 30 days, so sex differences in mortality were not analyzed.
Combined cardiac surgery
Five studies reported on in-hospital mortality in patients undergoing combined cardiac surgery (20, 28, 30, 33, 34). Salem et al., showed that a LVEDP ≥19 mmHg was associated with a significantly higher risk of in-hospital mortality and independent of LVEF [OR 1.19, 95% CI (1.05, 1.35), p = .0062] (34).
Nguyen et al., demonstrated that LVDD grade I/II/III was predictive of in-hospital mortality after cardiac surgery, independent of the EuroSCORE II [HRadj: 1.6, 95% CI (1.0, 2.6), p = .049] (33). This was also the conclusion of Metkus et al., and Beaubien-Souligny et al., In the results of Metkus et al., LVDD grades II/III were significantly predictive of in-hospital mortality(p = .004), as were those of Beaubien-Souligny et al. In the latter study, LVDD grade III was significantly associated with an increased risk of in-hospital mortality after cardiac surgery, compared to patients without LVDD [OR 19.39, 95% CI (2.37, 158.48), p = .006] (20, 30).
A solitary study demonstrated the exact opposite. Ferreira et al., reported that none of the following were significant predictors of in-hospital mortality after cardiac surgery: female sex, LVDD or E/e’ levels (p = .62, p = .11, and p = .24, respectively) (28).
TAVR
The only study concerning TAVR, Krittanawong et al., found no difference in in-hospital mortality between patients with HFpEF vs. patients with HFrEF (3.17% vs. 3.66%; p = .35) (35). No analysis was done on the comparison LVDD vs. no LVDD regarding in-hospital mortality. Sex was not significantly associated with mortality in the HF cohorts (HFpEF and HFrEF).
Early(≤30-day) postoperative complications and morbidity
CABG surgery
Merello et al., reported on the early complication rate among 191 patients undergoing on-pump CABG (24). They found a significantly higher incidence of the following complications in patients with LVDD grade III, compared to patients with LVDD grade 0/I/II: low cardiac output (p = .009), acute renal failure (p < .001), longer duration of mechanical ventilation (p < .001), and overall complication rate (p = .01). No data were available on the association between female sex and these early complications.
Lee et al., and Youn et al., examined the early complication rate in 2,304 patients undergoing off-pump CABG (36, 39). Lee et al., reported that patients with LVDD grade III were independently associated with a higher percentage of 30-day Major Adverse Cardiovascular Events (MACE) [OR 2.4, 95% CI (1.4–3.9), p = .001], while Youn et al., stated that no significant difference was found in 30-day MACE (p = .23) among patients with different LVDD grades. However, in the same study, multivariate analysis revealed that LVDD grade II/III independently predicted respiratory complications [OR 3.68, 95% CI (1.84–20.36), p = .01]. Early morbidity was also studied by Jun et al., in 500 patients undergoing off-pump CABG (38). They showed that a LVDD grade III, before off-pump CABG, was significantly associated with a higher risk of postoperative morbidity, compared to patients without LVDD[OR 2.42, 95% CI (1.42, 4.14), p < .005].
None of the studies reported on sex-differences regarding early postoperative morbidity and complications.
Combined cardiac surgery
Two studies evaluated early complications in 472 patients during combined cardiac surgeries (CABG, valvular, or CABG + valvular combined) (29, 32). Kyle et al., showed in 121 patients that LVDD (grade unspecified) correlated with new-onset POAF, compared to patients without LVDD [OR 4.50, 95% CI (1.22, 25.17), p = .016]. Thus, each grade of LVDD was associated with a higher Cardiac Postoperative Morbidity Score (CPOMS) on day 5(4.73 ± 7.18 vs. 3.04 ± 2.31; p = .009) and day 8(5.23 ± 8.62 vs. 2.83 ± 7.76; p = .009) compared to patients without LVDD. In addition, the overall CPOMS was 1.14 points higher (p = .01) after adjustment for potential confounders. Similarly, Melduni et al., demonstrated that higher grades of LVDD were significantly associated with an increased incidence of POAF, compared to patients without LVDD [grade I: OR 9.9, 95% CI (3.41, 29.8), p < .001], grade II: [OR 22.2, 95% CI (7.69, 63.8), p < .001], grade III: [OR 45.5, 95% CI (12.0, 173.0), p < .001] (32).
There were no studies that provided information about sex-based disparities in terms of early postoperative morbidity and complications.
HLOS and ICULOS
CABG surgery
Merello et al., demonstrated that HLOS for patients undergoing on-pump CABG was significantly longer in patients with LVDD grade III, compared with patients with LVDD grade 0/I/II(13 vs. 8 days, respectively) (24). In contrast, Değirmencioğlu et al., reported no significant difference in HLOS after on-pump CABG in patients with mild LVDD (grade I), compared with patients without LVDD (6.57 ± 0.14d vs. 7.19 ± 0.45d) (25). There was also no significant difference in ICULOS (26.2 ± 1.9 h vs. 24.1 ± 1.4 h).
No research findings indicated any distinctions between sexes concerning regarding HLOS and ICU.
In off-pump CABG, Lee et al., and Shim et al., showed that patients with LVDD grade III underwent prolonged ICULOS compared to patients with LVDD grade I/II, and without LVDD (p = 0.003 and p = 0.004, respectively) (36, 37). In addition, Lee et al., reported that LVDD grade III was also predictive of prolonged HLOS, compared to LVDD grade I/II (p < .001). No data on sex-differences regarding HLOS and ICULOS were reported in these two studies. A third study by Youn et al., noted that patients with LVDD grade III had a higher risk of HLOS >12 days, compared with patients without LVDD [OR 5.75, 95% CI (1.81, 13.23), p < .01] (39). Multivariate logistic regression showed that female sex was predictive of HLOS >12 days after off-pump CABG (p < .01).
Combined cardiac surgery
Five studies evaluated ICULOS and HLOS after combined cardiac surgery. Groban et al., found LVDD grade I-III were independently associated with prolonged ICULOS in patients undergoing CABG, valvular, or CABG with valvular surgery(p = .037) (27). This association was also confirmed by Ferreira et al., who reported that higher E/e' ratios were predictive of prolonged ICULOS (p = .009) but not HLOS (p = .086) (28). Kyle et al., demonstrated that any grade of LVDD was significantly associated with longer ICULOS compared to patients without LVDD (p = .019) (29). Furthermore, Beaubien-Souligny et al., showed that LVDD grade II/III was significantly associated with longer ICULOS compared to patients without LVDD, or grade I(p = .002 and p < .001) and additionally that LVDD grade III was related to longer HLOS, compared to patients without LVDD (p = .02) (30). Metkus et al., added that higher grades of LVDD were predictive of longer HLOS (grade 0: 9 (7–13) days vs. grade I: 10(8–14) days vs. grade II: 11(7–17) days vs. grade III: 20.5(12.5–24) days) (p = .0001) (20).
One study reported on sex differences in ICULOS and HLOS after combined cardiac surgery. Ferreira et al., reported that female sex was independently associated with a two-fold higher risk of prolonged HLOS[IHR 2.27, 95% CI (1.55, 3.32), p < .001] and increased ICULOS[IHR 1.73, 95% CI (1.20, 2.49), p < .017]. However, they found no significant association between LVDD and prolonged HLOS or ICULOS (p = .232 and p = .318) (28).
Other sex-related perioperative outcomes
Ferreira et al., demonstrated that patients with elevated LV filling pressures (E/e' ≥8) were more often female (p < .01), and that females had significantly higher LVDD grades (p < .01) (28). Bernard et al., pointed out that female sex, together with LVDD, predicted an increased inotropic need [OR 8.44, 95% CI (2.09, 42.09), p = .004], and difficult separation from cardiopulmonary bypass (p = .004) (31). No subgroup analysis of LVDD grading was made.
Discussion
This review aimed to investigate the impact of LVDD on early outcomes such as mortality, morbidity, complications, HLOS, and ICULOS after cardiac surgery and percutaneous aortic valve procedures, focusing on sex disparity.
Female sex was revealed to be significantly associated with higher LVDD grades and E/e' ratios at baseline in patients undergoing cardiac surgery. Females with LVDD presented with increased LVEDP, twice the risk of prolonged HLOS, increased ICULOS, increased need for inotropic support up to 12 h after surgery, and difficult separation from cardiopulmonary bypass. In addition, after off-pump CABG, females with LVDD showed significantly longer HLOS compared to males with LVDD.
Concerning LVDD and HLOS or ICULOS, however, there is some inconsistency in the literature. While studies by Metkus et al., Kyle et al., and Beaubien-Souligny et al. (20, 29, 30) indicated significant correlations between LVDD and extended HLOS and ICULOS, Ferreira et al., reported no notable link between the same variables (28). Interestingly, the latter study revealed an independent association between female sex and an almost twofold increase in the risk of extended HLOS and ICULOS. The inconsistency in results might be due to the absence of sex-based stratification in the three studies that showed a significant connection between LVDD and prolonged HLOS and ICULOS. In contrast, Ferreira et al., analyzed results by sex and identified an independent association between female sex and prolonged ICULOS or HLOS.
Concerning mortality, no significant association was found between female sex and in-hospital mortality after on-pump cardiac surgery. Results regarding the effect of female sex on early mortality (≤30 days) after on-pump CABG surgery, however, were inconsistent; Sun et al., reported female sex was associated with higher early mortality in LVDD patients (22), while Merello et al., found that female sex was not an independent predictor of 30-day mortality (24). There are different reasons for this inconsistency. This discrepancy could be attributed to the varying baseline characteristics of the included patients. Patients in the study of Sun et al., were older (mean age difference: 7.6 years) and were more likely to have a history of hypertension(95.4% vs. 71.1%), COPD (40.5% vs. 0.4%), DM (59.9% vs. 34.6%) or obesity (32.9% vs. 10.0%), compared with patients in the study by Merello et al. These factors already confer a higher a priori risk of increased 30-day mortality in the study by Sun et al. (22). Furthermore, the study carried out by Merello et al., featured a notably smaller sample size, resulting in a decrease in statistical power. Therefore, although the study result was statistically non-significant, it is still compatible with an increased mortality risk, which would be clinically relevant.
The outcomes concerning predictors of in-hospital mortality, following on-pump cardiac surgery, were largely consistent. Four studies identified three predictors of in-hospital mortality after on-pump cardiac surgery: LVDD, LVEDP ≥19 mmHg, and higher LVDD grades (20, 30, 33, 34). In contrast, although the study by Ferreira et al., showed that females were more likely to present with higher grades of LVDD before on-pump cardiac surgery, they found no significant association between female sex, LVDD, or E/e' values and in-hospital mortality (28). It is possible that the baseline characteristics of patients in the study by Ferreira et al., might have been more favorable concerning comorbidities than those in the studies by Metkus et al., Beaubien-Souligny et al., Nguyen et al., and Salem et al. (20, 30, 33, 34). Another explanation might be that the study by Ferreira et al., has a considerably smaller study population (n = 153) vs. Beaubien-Souligny et al. (n = 760), Metkus et al. (n = 577), Nguyen et al. (n = 1,743), and Salem et al. (n = 3,024), and may thus be underpowered to detect any differences in these outcomes.
Limitations
Our literature review had different limitations. Firstly, there was no uniform definition of LVDD in the included studies. Various combinations of echocardiography parameters and different cutoff values were used to determine the LVDD grade, making it more difficult to compare results directly. This is probably due to the fact that part of the included studies predate the 2016 guideline from the American Society of Echocardiography and the European Association of Cardiovascular Imaging, which shifted the focus away from volume status-dependent parameters to the consequences of diastolic impairment, such as left atrial volume. Secondly, the timing and imaging modalities used to determine LVDD differed between studies, as this was assessed either preoperatively, intraoperatively, or perioperatively and either by transesophageal echocardiography (TEE) or transthoracic echocardiography (TTE). Importantly, it should be noted that intraoperative assessment of LVDD may have been affected by factors such as anesthetic medication, inotropic and vasoactive medication, and positive pressure ventilation. By including these studies in our review, we assumed that preoperatively diagnosed LVDD has the same impact on early postoperative outcomes after cardiac surgery, as LVDD identified during the intraoperative phase. Furthermore, due to the heterogeneity of cardiac surgical procedures and the variation in study outcomes, comparing results with a conclusive statement is difficult.
Strength
The strength of our review is a systematic approach including the ROBINS-I tool we used to assess the available literature. Selection bias was minimized by including independent reviewers in identifying articles. In addition, we only included studies with higher-quality evidence, such as randomized controlled trials and observational studies. Finally, the authors of the included articles attempted to adjust for baseline characteristics. Thus, outcomes among patients with LVDD who underwent cardiac surgery were more comparable by differentiating underlying differences in baseline characteristics.
Future
Further research is essential to address the open questions surrounding LVDD. Previous studies have shown that females are at a higher risk of LVDD than males, yet the studies in this review included fewer female participants than males. To improve future study methodology, additional research should assess LVDD at a standardized moment (e.g., pre-operative phase) utilizing an uniform standard of LVDD grading, conduct subgroup analysis or stratification by sex, adjusting for baseline differences, and differentiate outcomes by cardiac surgical procedures. A follow-up study can help identify specific therapies to manage intraoperative LVDD and minimize the risk of poorer outcomes in females.
Conclusion
Despite the limited number of studies focusing on sex differences, females with LVDD appear to have worse early outcomes (≤30 days) after cardiac surgery, compared to men. Future research will need to identify sex-specific risk factors and target treatment optimization.
Author contributions
TL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. TA: Writing – review & editing. JB: Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. EK: Methodology, Supervision, Writing – review & editing. SJ: Conceptualization, Formal analysis, Methodology, Writing – original draft. SB: Writing – review & editing. JH: Writing – review & editing. HH: Writing – review & editing. SE: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
SE, JB and HH received the BJA ESAIC Research Grant 2022 FERACS01 founded by the British Journal of Anaesthesia and European Society of Anaesthesia and Intensive Care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American college of cardiology foundation/American heart association task force on practice guidelines: developed in collaboration with the international society for heart and lung transplantation. Circulation. (2009) 119(14):e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065
2. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29(4):277–314. doi: 10.1016/j.echo.2016.01.011
3. King M, Kingery J, Casey B. Diagnosis and evaluation of heart failure. Am Fam Physician. (2012) 85(12):1161–8.22962896
4. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. (2006) 355(3):251–9. doi: 10.1056/NEJMoa052256
5. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology. Circulation. (2018) 138(2):198–205. doi: 10.1161/CIRCULATIONAHA.118.034271
6. Apostolakis EE, Baikoussis NG, Parissis H, Siminelakis SN, Papadopoulos GS. Left ventricular diastolic dysfunction of the cardiac surgery patient; a point of view for the cardiac surgeon and cardio-anesthesiologist. J Cardiothorac Surg. (2009) 4:67. doi: 10.1186/1749-8090-4-67
7. Hatabu H, Barile M. Detection of pulmonary congestion in heart failure with preserved ejection fraction using quantitative chest CT. JACC Cardiovasc Imaging. (2022) 15(4):638–40. doi: 10.1016/j.jcmg.2022.01.017
8. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. (2020) 22(8):1342–56. doi: 10.1002/ejhf.1858
9. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. doi: 10.1093/cvr/cvac013
10. Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening. Circulation. (2005) 112(15):2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078
11. Ma C, Luo H, Fan L, Liu X, Gao C. Heart failure with preserved ejection fraction: an update on pathophysiology, diagnosis, treatment, and prognosis. Brazilian J Med Biol. (2020) 53(7):e9646. doi: 10.1590/1414-431x20209646
12. Goyal P, Paul T, Almarzooq ZI, Peterson JC, Krishnan U, Swaminathan R V, et al. Sex- and race-related differences in characteristics and outcomes of hospitalizations for heart failure with preserved ejection fraction. J Am Heart Assoc. (2017) 6(4):e003330. doi: 10.1161/JAHA.116.003330
13. Duca F, Zotter-Tufaro C, Kammerlander AA, Aschauer S, Binder C, Mascherbauer J, et al. Gender-related differences in heart failure with preserved ejection fraction. Sci Rep. (2018) 8(1):1080. doi: 10.1038/s41598-018-19507-7
14. Merrill M, Sweitzer NK, Lindenfeld J, Kao DP. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Fail. (2019) 7(3):228–38. doi: 10.1016/j.jchf.2019.01.003
15. Nakada Y, Kawakami R, Nakano T, Takitsume A, Nakagawa H, Ueda T, et al. Sex differences in clinical characteristics and long-term outcome in acute decompensated heart failure patients with preserved and reduced ejection fraction. Am J Physiol Circ Physiol. (2016) 310(7):H813–20. doi: 10.1152/ajpheart.00602.2015
16. Zsilinszka R, Shrader P, DeVore AD, Hardy NC, Mentz RJ, Pang PS, et al. Sex differences in the management and outcomes of heart failure with preserved ejection fraction in patients presenting to the emergency department with acute heart failure. J Card Fail. (2016) 22(10):781–8. doi: 10.1016/j.cardfail.2015.12.008
17. Hsich EM, Grau-Sepulveda M V, Hernandez AF, Peterson ED, Schwamm LH, Bhatt DL, et al. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J. (2012) 163(3):430–7.e3. doi: 10.1016/j.ahj.2011.12.013
18. Rong LQ, Di Franco A, Rahouma M, Dimagli A, Patel A, Lopes AJ, et al. Baseline intraoperative left ventricular diastolic function is associated with postoperative atrial fibrillation after cardiac surgery. Anesthesiology. (2023) 139:602–13. doi: 10.1097/ALN.0000000000004725
19. Kaw R, Hernandez A V, Pasupuleti V, Deshpande A, Nagarajan V, Bueno H, et al. Effect of diastolic dysfunction on postoperative outcomes after cardiovascular surgery: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. (2016) 152(4):1142–53. doi: 10.1016/j.jtcvs.2016.05.057
20. Metkus TS, Suarez-Pierre A, Crawford TC, Lawton JS, Goeddel L, Dodd-O J, et al. Diastolic dysfunction is common and predicts outcome after cardiac surgery. J Cardiothorac Surg. (2018) 13(1):67. doi: 10.1186/s13019-018-0744-3
21. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/bmj.i4919
22. Sun LY, Tu J V, Bader Eddeen A, Liu PP. Prevalence and long-term survival after coronary artery bypass grafting in women and men with heart failure and preserved versus reduced ejection fraction. J Am Heart Assoc. (2018) 7(12):e008902. doi: 10.1161/JAHA.118.008902
23. Dalén M, Lund LH, Ivert T, Holzmann MJ, Sartipy U. Survival after coronary artery bypass grafting in patients with preoperative heart failure and preserved vs reduced ejection fraction. JAMA Cardiol. (2016) 1(5):530. doi: 10.1001/jamacardio.2016.1465
24. Merello L, Riesle E, Alburquerque J, Torres H, Aránguiz-Santander E, Pedemonte O, et al. Risk scores do not predict high mortality after coronary artery bypass surgery in the presence of diastolic dysfunction. Ann Thorac Surg. (2008) 85(4):1247–55. doi: 10.1016/j.athoracsur.2007.12.068
25. Değirmencioğlu A, Şenay Ş, Güllü Ü, Zencirci E, Karakuş G, Ugur M, et al. The effect of mild left ventricular diastolic dysfunction on outcome after isolated coronary bypass surgery. Kardiol Pol. (2014) 72(6):541–5. doi: 10.5603/KP.a2013.0354
26. Chang S-A, Park P-W, Sung K, Lee S-C, Park SW, Lee YT, et al. Noninvasive estimate of left ventricular filling pressure correlated with early and midterm postoperative cardiovascular events after isolated aortic valve replacement in patients with severe aortic stenosis. J Thorac Cardiovasc Surg. (2010) 140(6):1361–6. doi: 10.1016/j.jtcvs.2010.02.022
27. Groban L, Sanders DM, Houle TT, Antonio BL, Ntuen EC, Zvara DA, et al. Prognostic value of tissue Doppler-derived E/e’ on early morbid events after cardiac surgery. Echocardiography. (2010) 27(2):131–8. doi: 10.1111/j.1540-8175.2009.01076.x
28. Ferreira RG, Worthington A, Huang C-C, Aranki SF, Muehlschlegel JD. Sex differences in the prevalence of diastolic dysfunction in cardiac surgical patients. J Card Surg. (2015) 30(3):238–45. doi: 10.1111/jocs.12506
29. Kyle B, Zawadka M, Shanahan H, Cooper J, Rogers A, Hamarneh A, et al. Consensus defined diastolic dysfunction and cardiac postoperative morbidity score: a prospective observational study. J Clin Med. (2021) 10(21):5198. doi: 10.3390/jcm10215198
30. Beaubien-Souligny W, Brand FZA, Lenoir M, Amsallem M, Haddad F, Denault AY. Assessment of left ventricular diastolic function by transesophageal echocardiography before cardiopulmonary bypass: clinical implications of a restrictive profile. J Cardiothorac Vasc Anesth. (2019) 33(9):2394–401. doi: 10.1053/j.jvca.2019.05.014
31. Bernard F, Denault A, Babin D, Goyer C, Couture P, Couturier A, et al. Diastolic dysfunction is predictive of difficult weaning from cardiopulmonary bypass. Anesth Analg. (2001) 92(2):291–8. doi: 10.1213/00000539-200102000-00002
32. Melduni RM, Suri RM, Seward JB, Bailey KR, Ammash NM, Oh JK, et al. Diastolic dysfunction in patients undergoing cardiac surgery. J Am Coll Cardiol. (2011) 58(9):953–61. doi: 10.1016/j.jacc.2011.05.021
33. Nguyen LS, Baudinaud P, Brusset A, Nicot F, Pechmajou L, Salem J-E, et al. Heart failure with preserved ejection fraction as an independent risk factor of mortality after cardiothoracic surgery. J Thorac Cardiovasc Surg. (2018) 156(1):188–93.e2. doi: 10.1016/j.jtcvs.2018.02.011
34. Salem R, Denault AY, Couture P, Bélisle S, Fortier A, Guertin M-C, et al. Left ventricular end-diastolic pressure is a predictor of mortality in cardiac surgery independently of left ventricular ejection fraction. Br J Anaesth. (2006) 97(3):292–7. doi: 10.1093/bja/ael140
35. Krittanawong C, Kumar A, Wang Z, Johnson KW, Rastogi U, Narasimhan B, et al. Predictors of in-hospital mortality after transcatheter aortic valve implantation. Am J Cardiol. (2020) 125(2):251–7. doi: 10.1016/j.amjcard.2019.10.014
36. Lee E-H, Yun S-C, Chin J-H, Choi D-K, Son H-J, Kim W-C, et al. Prognostic implications of preoperative E/e′ ratio in patients with off-pump coronary artery surgery. Anesthesiology. (2012) 116(2):362–71. doi: 10.1097/ALN.0b013e3182426ed6
37. Shim JK, Choi YS, Chun DH, Hong SW, Kim DH, Kwak YL. Relationship between echocardiographic index of ventricular filling pressure and intraoperative haemodynamic changes during off-pump coronary bypass surgery. Br J Anaesth. (2009) 102(3):316–21. doi: 10.1093/bja/aep005
38. Jun NH, Shim JK, Kim JC, Kwak YL. Prognostic value of a tissue Doppler-derived index of left ventricular filling pressure on composite morbidity after off-pump coronary artery bypass surgery. Br J Anaesth. (2011) 107(4):519–24. doi: 10.1093/bja/aer188
Keywords: HFpEF, LVDD, sex difference, heart disease, echocardiography, cardiopulmonary bypass, postoperative outcomes
Citation: Ludden T, Alberts TAM, Breel JS, de Klerk ES, Javaid SK, Boekholdt SM, Hermanides J, Hermanns H and Eberl S (2023) Exploring the impact of left ventricular diastolic dysfunction on postoperative cardiac surgery outcomes, with a focus on sex disparities: a comprehensive literature review. Front. Anesthesiol. 2:1280189. doi: 10.3389/fanes.2023.1280189
Received: 19 August 2023; Accepted: 25 October 2023;
Published: 8 November 2023.
Edited by:
Hong Liu, UC Davis Health, United StatesReviewed by:
Aveek Jayant, Homi Bhabha Cancer Hospital and Research Centre, India© 2023 Ludden, Alberts, Breel, de Klerk, Javaid, Boekholdt, Hermanides, Hermanns and Eberl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: H. Hermanns h.hermanns@amsterdamumc.nl
 T. Ludden
T. Ludden T. A. M. Alberts1
T. A. M. Alberts1  J. S. Breel
J. S. Breel E. S. de Klerk
E. S. de Klerk S. K. Javaid
S. K. Javaid S. M. Boekholdt
S. M. Boekholdt H. Hermanns
H. Hermanns S. Eberl
S. Eberl