Case Report: The management of hemorrhagic shock of different origins by target-controlled coagulation and extracorporeal organ support (continuous renal replacement therapy)
- Department of Anesthesiology and Intensive Care, Kiskunhalas Semmelweis Hospital, The Teaching Hospital of University of Szeged, Kiskunhalas, Hungary
Introduction: Trauma-related severe bleeding and peripartum hemorrhage complicated by shock state is challenging for acute care physicians. Management steps relating to each other include damage control surgery, maintenance of optimal clotting preconditions, point-of-care and targeted supplementation of coagulation factors, control of hyperfibrinolysis, and supplementation of thrombocytes. Extensive tissue damage and surgical management of bleeding activate the proinflammatory process, leading to a dysregulated immune response. The originating systemic inflammation produces further damage, harmfully altering clot formation through the activation of immunothrombosis.
Clinical findings: The case report presents one obstetric, massive bleeding patient and one politraumatized patient with severe hemorrhage. Both underwent extended surgery regarding bleeding control intervention.
Interventions and outcome: Elimination of coagulation disorder was driven by point-of-care viscoelastometry and targeted clotting factor supplementation. Continuous renal replacement therapy and the hemadsorption technique augmented the well-established, up-to-date recommendations-guided care throughout the treatment line. The patients could leave the intensive care unit 4 and 8 days following the initial injury.
Conclusion: Prompt administration of extracorporeal organ support enhanced the recovery from hemorrhagic shock.
Introduction
Severe bleeding and the vicious cycle of worsening circulatory shock and hemostatic disorders complicate the care of many surgical, trauma, and obstetric patients and significantly increase the mortality rate. This poses a complex set of challenges for clinicians in the care of massively bleeding patients. The course involves the management of hemodynamics, hemostasis, and, in various cases, a disproportionate systemic immune response related to severe tissue damage (1).
Bleeding-associated deaths account for around a third of injury-related mortality and more than 80% of preventable postinjury deaths (2). Severe bleeding related to multiple traumas and obstetric hemorrhage differs. In the latter case, numerous physiological changes occur, including the emergence of a hypercoagulable state due to the preparation for delivery and physiological blood loss (3). Despite the aforementioned changes, significant bleeding can still be expected in relation to childbirth. Various sources allocate the incidence of postpartum hemorrhage (blood loss of more than 500 mL) to around 5%–10%, whereas severe postpartum hemorrhage (blood loss of more than 1,000 mL) occurs in about 2% of cases (4). Disorders of placental implantation, such as placenta accreta, significantly increase the incidence of severe postpartum hemorrhage terminating in hysterectomy (5). Furthermore, severe postdelivery hemorrhage is the leading cause of maternal mortality worldwide (6).
Under severe bleeding, three areas need timely intervention—first, preventing the deterioration of circulatory shock by stabilizing hemodynamics; second, preventing, diagnosing, and treating disorders of hemostasis—and lastly, parallel confining of surgical bleeding to avoid further blood loss.
Managing circulatory shock is a similar approach in hemodynamically unstable patients. Care rests on intravenous fluid, vasopressors, inotrope supports, and blood products. Individualization of treatment is essential for each patient based on the etiology of the shock and the monitored clinical and vital parameters. If bleeding is the culprit behind hemodynamic instability, the goals are restoring the circulating volume, ensuring adequate perfusion and oxygen delivery to organs, and terminating further blood loss. Accordingly, fluid therapy, vasopressor support, and blood product supplementation are most important.
Management of bleeding requires a tailored approach based on the etiology. However, reviewing well-described and standardized steps is necessary to ensure the best possible outcome. Whereas distinct guidelines exist for different clinical scenarios, like perioperative and trauma-induced bleeding (7, 8), overall, each follows a similar hierarchical approach. These are actions to treat and prevent coagulopathy based on the pyramid-like treatment algorithm described by Görlinger (9).
In summary, the pyramid-like therapy in coagulopathy takes steps that are built on each other. The primary aim is to optimize clotting and prevent further deterioration while reassessing the actual state and efficiency of the whole hemostatic system. The foundation of all processes is the control of the bleeding, medically and surgically simultaneously, if required. When further blood loss is prevented, optimization of coagulation should involve ensuring normal physiologic parameters. The latter is necessary as the coagulation cascade contains enzymes; thus, only adequate in vivo conditions can ensure optimal function. These include establishing optimal pH (>7.2), core temperature (>35°C), and the presence of an optimal level of Ca2+ (>1 mmol/L). The infamous triad of death (10) described in trauma care, consisting of hypothermia, acidosis, and coagulopathy, is addressed in the treatment line as soon as possible in such a way as to manage the former two to prevent the latter. Further steps include preventing and treating hyperfibrinolysis and identifying specific deficits in the coagulation cascade resulting from consumption and dilution. The latter facilitates targeted replacement of coagulation factors in order of their particular deficit.
This approach necessitates frequent monitoring of the actual state of the hemostatic system. For this purpose, the most widespread clinical tests are conventional laboratory tests and point-of-care (PoC) viscoelastometry. Conventional coagulation studies such as INR, aPTT, PT, and fibrinogen concentration are well established. However, their main disadvantage is the time between blood sampling and result presentation, a crucial factor in bleeding management. Therefore, PoC viscoelastometry is becoming more prevalent in trauma-related and postpartum hemorrhage scenarios (11, 12), not only because it offers almost real-time and quantitative results but also because it grants specific tests for a wide range of hemostatic mismatches or even detects the effect of various anticoagulants.
The development of an overwhelming immune response leading to systemic consequences frequently complicates severe bleeding management. Large-scale tissue damage related to damage control surgery or just the initial trauma generates the release of damage-associated molecular patterns (DAMP), promoting the proinflammatory process (13). Activation of monocytes and neutrophils through the innate immune system can culminate in a cytokine storm (14).
A dysregulated immune response, evolving from tissue damage, harmfully alters coagulation, fibrinolysis, platelet, endothelial, and major organ function, leading to a vicious circle. In addition, vasodilation and increased vascular permeability, host response leads to microvascular thrombosis, an unnecessary activation of thrombocytes, and consumption of coagulation factors, called immunothrombosis (14). Hence, microvascular thrombus formation further deteriorates organ functions. The bulk and unspecific removal of the inflammation-maintaining mediators can regain balance in such a dysregulated immune response, thus improving shock reversal. The latter concept offers a rational use of hemadsorption techniques to regulate the large-scale tissue injury-mediated immune over-response (15) and homeostasis simultaneously. CytoSorb is one of the most applied blood purification modes, containing biocompatible, porous polymer beads, binding DAMP, pro-, and anti-inflammatory molecules under the range of 55 kDa (16).
Here, we present the management of a severe postpartum bleeding patient and a politraumatized hemorrhagic shock patient based on PoC viscoelastometry, where extracorporeal organ support and hemadsorption enhanced the treatment.
Narrative
Case 1
A 25-year-old gravidus arrived at the obstetric unit with increasing vaginal bleeding at 18 weeks of pregnancy. The patient was complaining of nausea and minor lower abdominal pain. Previously, the pregnancy was uneventful. However, 2 years earlier the patient had undergone a previous cesarean section because of uterine inertia. Otherwise, she had a silent past medical history. Admission ultrasound revealed fetal heartbeats and total placenta previa with a hematoma of 20 mm in diameter over the cervix. Fluid resuscitation was running following large-bore peripheral venous cannulation.
Following the short observation and diagnostic period, substantial bleeding commenced, and the obstetricians performed an urgent cesarean section due to placental abruption. The surgeons performed a sectio parva to terminate the pregnancy under general anesthesia. During the operation, the anesthesiologist inserted arterial lines and further large-bore peripheral venous cannulas, while invasive blood pressure monitoring was ongoing. Blood gas analysis and ClotPro (enicor GmbH, Munich, Germany) examinations (Table 1) required regular blood sampling. Following the cesarean section, the patient was transferred to the intensive care unit, intubated, ventilated, and sedated with severe hemodynamic instability and norepinephrine infusion. The estimated intraoperative blood loss was 2,200 mL. The first viscoelastometry measurements revealed a 245 s long clotting time (CT) on the extrinsic (EX) test and 256 s long on the intrinsic (IN) test, with a maximum clot firmness (MCF) of only 33 and 35 mm, respectively. At the same time, there was no clot formation on the fibrinogen (FIB) test at all.
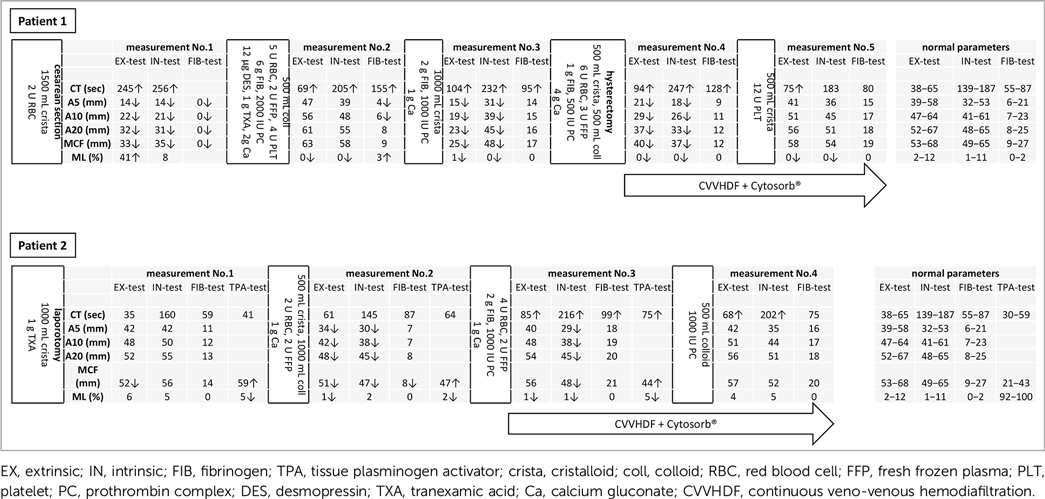
Table 1. Timelines with viscoelastic measurements, bleeding history, fluid resuscitation, and targeted hemostatic correction integrating the initiation of extracorporeal organ support.
A central venous catheter was inserted in the critical care unit, with propofol plus sufentanil analgosedation, and invasive mechanical ventilation proceeded. Continuous argipressin infusion augmented the norepinephrine vasoconstriction. The patient was heated actively by convective temperature management and warmed infusions; core temperature measurement commenced. Conventional laboratory tests, viscoelastic control measurements, and blood gas analysis took place regularly to follow up on the patient's hemostasis and metabolic state (Table 1). Viscoelastometry revealed a persistent increment in clotting time and reduced clot firmness until proper interventions and correction. Owing to severe atonic bleeding, 500 µg of sulproston was given intravenously (IV) under slow infusion. Meanwhile, an intrauterine gauze tamponade was applied, and the obstetricians performed fundal massage.
However, hemostasis optimized at all front bleeding was ongoing, and vasopressor support increased. A pelvic ultrasound revealed a large-scale intrauterine clot around the gauze tamponade, so the patient underwent an urgent hysterectomy in the intensive care unit. The surgeons explored 150 mL of blood in the abdomen and a further 500 mL in the uterine cavity. The operation revealed uterine atony, with a cervical tear as a background of severe postpartum hemorrhage. The latter evolved when the placenta, presumably accreted, was removed during the cesarean section. At this timepoint, CT was 94, 247, and 128 s long and MCF was reduced to 40, 37, and 12 mm on EX-, IN-, and FIB-tests, respectively.
Three hours following the hysterectomy, the critical care physicians commenced continuous renal replacement therapy (CRRT) by OMNI (B. Braun, Melsungen, Germany) because of compensated metabolic and lactate acidosis and a severe systemic inflammatory reaction. The care providers began blood purification using a CytoSorb (CytoSorbents Europe GmbH, Berlin, Germany) adsorber applied in a prefilter manner in an Omniset Plus extracorporeal set (B. Braun, Melsungen, Germany). Continuous veno-venous hemodiafiltration (CVVHDF) was administered 24 h with regional citrate anticoagulation without fluid removal. We summarize the vasopressor requirements and blood gas alterations in Figure 1.
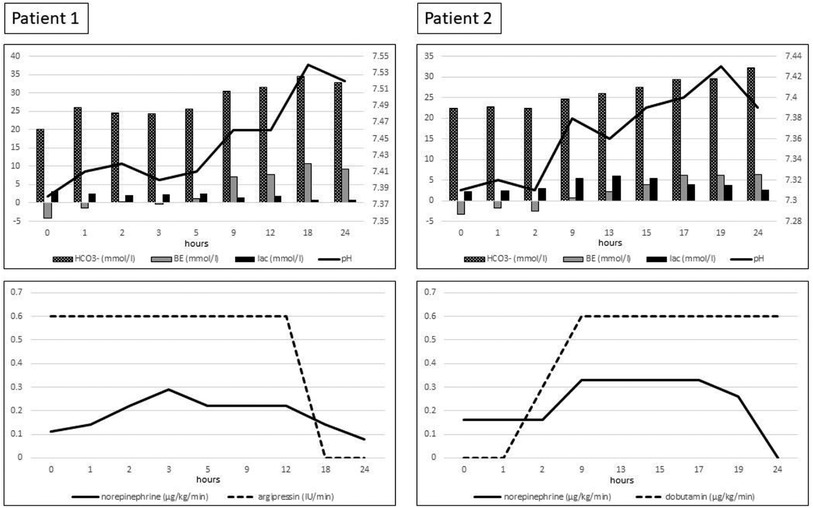
Figure 1. Blood gas results, vasopressor, and inotrope requirement during the 24 h of CytoSorb adsorption combined with continuous renal replacement therapy. HCO3−, bicarbonate; BE, base excess; lac, lactate.
The patient was extubated the following morning in a hemodynamically stable state. Observation revealed no further significant blood loss following the hysterectomy. The bedside viscoelastometry follow-up presented almost normal parameters (Table 1). The patient was discharged to the obstetric unit on day 4 with normal vital parameters and left the hospital in 3 days.
Case 2
The 59-year-old female was admitted to the emergency department by ambulance, presenting with blunt chest and abdominal trauma following being pressed to a wall by a bull. The patient had just hypertension in her past medical history. An initial advanced trauma life support assessment revealed a free airway and no sign of hemo- or pneumothorax. Because of hemodynamic instability, fluid resuscitation was started after two large-bore venous cannulas were inserted and continuous norepinephrine infusion was began via IV. Focused assessment was done with sonography for trauma-assessed free abdominal fluid; hence, the patient was transferred immediately to the operating room for laparotomy. To follow up on the patient's hemostasis and metabolic state, regular blood gas analysis, conventional laboratory tests, and ClotPro PoC control were undertaken (Table 1). First, viscoelastometry measured normal parameters. Following the severe intraoperative bleeding, the clot firmness reduced. MCF was reduced to 51, 47, and 8 mm on the EX-, IN-, and FIB-tests, respectively. When proper interventions and corrections occurred, MCF on the same tests was increased to 56, 48, and 21 mm.
The immediate preoperative assessment revealed a right-sided hemo- and pneumothorax that underwent drainage acutely. Following rapid sequence induction, under general anesthesia, the surgeons performed laparotomy. During the operation, the anesthesiologist inserted arterial lines and two further large-bore peripheral venous cannulas, and invasive blood pressure monitoring was commenced. During the operation, a right-sided nephrectomy was performed because of severe retroperitoneal bleeding originating from the kidney, and liver injuries required a packing. The estimated blood loss was 1,500 mL. Convective temperature management was ongoing under core temperature monitoring.
The patient was transferred to the intensive care unit, intubated, ventilated, and sedated. Following the extension of hemodynamic monitoring by inserting a pulse index contour cardiac output cannula (Getinge AB, Getinge, Sweden), the norepinephrine vasoconstriction was supplemented by dobutamine inotropic support because of reduced contractility resulting from heart contusion. The secondary survey assessed right-sided pulmonary contusion and the fractures of all spinous and right transversal processes from the ninth thoracic to the fifth lumbar vertebrae.
Two hours following the laparotomy, CRRT was started using OMNI because of compensated metabolic and lactate acidosis and severe systemic inflammatory reaction—CytoSorb blood purification was applied by an adsorber situated in a prefilter position in an Omniset Plus extracorporeal set. CVVHDF ran in the next 24 h with regional citrate anticoagulation without fluid removal. The vasopressor, the inotrop requirements, and the blood gas alterations are summarized in Figure 1.
Furthermore, viscoelastometry follow-up revealed almost normal parameters on postoperative day 1 (Table 1). Blood loss was 1,200 mL through the intraabdominal drains postoperatively during the first 24 h. On day 5, the perihepatic package was removed under a second-look laparotomy when no further bleeding was presented from the liver injuries. The patient was extubated 1 day later with no catecholamine support, and she was discharged to the surgical unit on day 8 with normal vital parameters. She left the hospital after 6 days.
Discussion
We presented two patients with hemorrhagic shock where adjuvant extracorporeal organ support promoted the successful management of coagulation disorders and injury-induced systemic inflammatory reactions. However, the cases differed in origin, postpartum bleeding, and major trauma; the pathophysiology pathways and their management share common elements.
Regardless of the initial mechanism, the most acute and life-threatening problems involved significant bleeding, leading to hemorrhagic shock and coagulopathy, requiring immediate attention and management. Without the implemented prompt and sufficient interventions, the courses could have spiraled into irreversible pathophysiologic changes with lethal consequences.
Previously implemented guidelines (7, 8) supported the elimination of circulatory shock. Management was tailored by advanced monitoring techniques and individualized, goal-directed fluid therapy, catecholamine, and vasopressor support, just like targeted blood product supplementation (Table 1). In both cases, managing the hemorrhage involved damage control surgery to eliminate the source of bleeding. For monitoring the current hemostatic status and reassessment following blood product supplementation, PoC viscoelastometry was performed since it provides a more specific correction of the underlying pathophysiology in a shorter time (11). Moreover, in trauma-related hemorrhage, PoC viscoelastometry promotes the reduction of allogeneic blood transfusion and guides actions leading to decreased mortality (12).
For a critical care team, one of the most significant challenges is to ensure optimal conditions in a hemorrhagic shock patient to reserve the balance of the substantial physiological pathways. These comprise an almost constant state of homeostatic parameters, such as core temperature, serum ion levels, and blood pH (17), despite the readily worsening condition and the exhaustion of normal physiologic responses. Therefore, the novelty of the aforementioned approach is based on re-establishing the homeostasis as soon as possible. Accordingly, extracorporeal blood purification was started in the form of CRRT supplemented by cytokine adsorption to ensure a smooth reconversion and to simultaneously obtain control over the critical physiological parameters (Figure 1).
Treatment evolved before typical acute kidney injury (AKI) symptoms figured. Correspondingly, CRRT was performed according to unconventional indications; in this particular case for the management of homeostasis and, with its support, hemostasis. The rationale behind the treatment was that coagulopathy is an active process that requires a stable milieu (18). The considerable alterations in the state of the hemostatic and the surrounding systems necessitated reconditioning into a narrow spectrum of physiological parameters for providing optimal enzyme function. Moreover, CRRT could gain control of core temperature and stabilize the acid–base state. At the same time, the management of citrate-calcium regional anticoagulation ensured frequent follow-up of serum calcium levels, thus providing the correct function of the presented and supplemented coagulation factors.
Further challenges could be the dysregulated immune response emerging from the large-scale tissue lesion of the initial trauma and the damage control. Injury and surgery-related DAMP release triggers proinflammatory processes through the innate immune system (13). However, activation of the innate immune mechanisms through the pathogen-associated molecular pattern (PAMP) should play a significant role in host defense (18). Nevertheless, the DAMP and PAMP emerging signal transductions share common pathways, hence leading to similar responses evolving into systemic inflammatory responses (SIRS) (19).
Cytokines released by DAMP- and PAMP-activated monocytes and neutrophils trigger intravascular microthrombus formation, also known as immunothrombosis (20). The SIRS-altered endothelium dysfunction and dysregulated procoagulation activity lead to unnecessary consumption of clotting factors, thrombocytes, and multiorgan failure, which develops through microthrombus formation (14). Furthermore, uncontrolled proinflammation, also activated by the release of molecular patterns of the intracellular space, plays a significant role in developing multiple organ dysfunction (21).
Hemadsorption techniques can unselectively reduce cytokines that maintain the dysregulated immune response, regaining balance over the immune storm. CytoSorb is one of the most common blood purification devices containing biocompatible, porous polymer beads (16). Adsorption occurs in the cartridge when an extracorporeal system, like CRRT, perfuses blood. Purification is unselective under the 55 kDa molecular range; as a result, not only DAMPs but also cytokines and myoglobin are adsorbed (22). The elimination of the shock state and control of the dysregulated immune response could be followed by a significant reduction in vasopressor requirements and normalization of the lactate level (23) (Figure 1).
However, heart rate, blood pressure, or mean arterial pressure could demonstrate circulatory changes thanks to the advanced control of inflammation; in our second case, the hemodynamics were significantly affected by the heart contusion. Therefore, only the variation of catecholamine requirements along with the blood gas results are presented over time. The significant and rapid decline of vasopressor requirements, the stabilization of the acid–base state, and the normalization of the lactate level can all be signs of terminating the chock state (23) (Figure 1). Hence, the control over the inflammation and immunothrombosis.
However, we can only prove the latter by measuring cytokines (24) and assessing the long-term outcome of organ functions (25). This would be the task of further, well-designed investigations with homogenous cohorts. Follow-up of microthrombus formation can be deliberately challenging (14), but recurrently, assessing the rate and length of organ dysfunction can give a possible answer.
Regaining balance over the shock and disproportionate coagulation took place within 24 h in both cases. The other crucial advantage of the presented concept is the elimination time frame. According to former investigations, the shorter the period of imbalanced inflammation and hence shock, the better the organ failure and the possible survival rate that can be expected (25). The latter should again be the task of future trials. Currently, no controlled, randomized trials are available to strengthen the concept of managing hemorrhagic shock of different origins by extracorporeal organ support. Conversely, this also demonstrates the novelty of the approach.
Under both cases, the rationale behind the timely set up of CVVHDF amended by hemadsorption was to simultaneously regain control over the homeostasis and the dysregulated immune response. Hence, the extracorporeal technique probably improved coagulation and reduced the overwhelming deleterious effects of SIRS. The adjuvant, extracorporeal therapeutic modality was applied in both cases to mitigate the adverse effects of the hemorrhagic shock and damage control surgery, thereby stabilizing the hemostatic function throughout the intervention steps of the therapeutic pyramid of severe bleeding management.
One of the significant limitations is the low number of cases. The other limitation is that, to the best of our knowledge, no randomized controlled trials exist where extracorporeal organ support is investigated in trauma-related or peripartum bleeding–associated inflammation and homeostasis management. At the same time, this is the novelty of our approach, which is one of the major strengths of this case report.
Conclusion
Optimizing homeostasis during peripartum and trauma-induced hemorrhagic shock is essential in maintaining optimal coagulation preconditions. Simultaneously, regaining control over the dysregulated immune response provoked by the large-scale traumatic or damage control surgery facilitates beneficial coagulation processes and eliminates SIRS-induced multiorgan failure. Extracorporeal organ support techniques like CVVHDF supplemented by hemoperfusion can achieve these goals, hence regaining balance in the exaggerated and deleterious proinflammation and procoagulation responses. However, further well-established, randomized controlled trials are required to prove the presented concept.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because this is a case report. Only a retrospective data process occurred. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ÁP: Writing – original draft, Writing – review and editing. AL: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We want to express our deep gratitude to our colleague, Norbert Frei MD, consultant of anesthesiology and intensive care, for his guidance in introducing extracorporeal organ support techniques in our department.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2023.1323180/full#supplementary-material
References
1. Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. (2014) 384(9952):1455–65. doi: 10.1016/S0140-6736(14)60687-5
2. Sauaia A, Moore EE, Wade CE, Holcomb JB. Epidemiology of hemorrhage-related mortality. In: Moore HB, Neal MD, Moore EE, editors. Trauma Induced Coagulopathy. Cham: Springer International Publishing (2021). p. 13–27. doi: 10.1007/978-3-030-53606-0_2
3. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. (2003) 16(2):153–68. doi: 10.1016/s1521-6926(03)00021-5
4. Deneux-Tharaux C, Bonnet M-P, Tort J. Epidemiology of postpartum haemorrhage. J Gynecol Obstet Biol Reprod (Paris). (2014) 43(10):936–50. doi: 10.1016/j.jgyn.2014.09.023
5. Mehrabadi A, Hutcheon JA, Liu S, Bartholomew S, Kramer MS, Liston RM, et al. Contribution of placenta accreta to the incidence of postpartum hemorrhage and severe postpartum hemorrhage. Obstet Gynecol. (2015) 125(4):814–21. doi: 10.1097/AOG.0000000000000722
6. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Heal. (2014) 2(6):e323–33. doi: 10.1016/S2214-109X(14)70227-X
7. Kietaibl S, Ahmed A, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology and intensive care: second update 2022. Eur J Anaesthesiol. (2023) 40(4):226–304. doi: 10.1097/EJA.0000000000001803
8. Rossaint R, Afshari A, Bouillon B, Cerny V, Cimpoesu D, Curry N, et al. The European guideline on management of major bleeding and coagulopathy following trauma: sixth edition. Crit Care. (2023) 27(1):80. doi: 10.1186/s13054-023-04327-7
9. Görlinger K. Coagulation management during liver transplantation. Hamostaseologie. (2006) 26(3 Suppl 1):S64–76. doi: 10.1055/s-0037-1617084
10. Mikhail J. The trauma triad of death: hypothermia, acidosis, and coagulopathy. AACN Clin Issues. (1999) 10(1):85–94. doi: 10.1097/00044067-199902000-00008
11. Rajsic S, Breitkopf R, Bachler M, Treml B. Diagnostic modalities in critical care: point-of-care approach. Diagnostics (Basel, Switzerland). (2021) 11(12):2202. doi: 10.3390/diagnostics11122202
12. Stein P, Kaserer A, Spahn GH, Spahn DR. Point-of-care coagulation monitoring in trauma patients. Semin Thromb Hemost. (2017) 43(4):367–74. doi: 10.1055/s-0037-1598062
13. Pandolfi F, Altamura S, Frosali S, Conti P. Key role of DAMP in inflammation, cancer, and tissue repair. Clin Ther. (2016) 38(5):1017–28. doi: 10.1016/j.clinthera.2016.02.028
14. Ito T. PAMPs and DAMPs as triggers for DIC. J Intensive Care. (2014) 2(1):67. doi: 10.1186/s40560-014-0065-0
15. Weber B, Lackner I, Baur M, Gebhard F, Relja B, Marzi I, et al. DAMP-mediated cardiac dysfunction: cardiomyocytes as actors and target of innate immune response. J Immunol. (2020) 204(1_Supplement):144.18. doi: 10.4049/jimmunol.204.Supp.144.18
16. Ankawi G, Xie Y, Yang B, Xie Y, Xie P, Ronco C. What have we learned about the use of cytosorb adsorption columns? Blood Purif. (2019) 48(3):196–202. doi: 10.1159/000500013
17. Görlinger K, Kietaibl S, Görlinger K, Kozek-Langenecker SA. Economic aspects and organisation. In: Marcucci CE, Schoettker P, editors. Perioperative hemostasis: coagulation for anesthesiologists. Berlin: Springer (2015). p. 421–45.
18. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. (2009) 22(2):240–73. doi: 10.1128/CMR.00046-08
19. Nakahira K, Hisata S, Choi AMK. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal. (2015) 17:1329–50. doi: 10.1089/ars.2015.6407
20. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. (2013) 13(1):34–45. doi: 10.1038/nri3345
21. Sauaia A, Moore FA, Moore EE. Postinjury inflammation and organ dysfunction. Crit Care Clin. (2017) 33(1):167–91. doi: 10.1016/j.ccc.2016.08.006
22. Becker S, Lang H, Vollmer Barbosa C, Tian Z, Melk A, Schmidt BMW. Efficacy of CytoSorb®: a systematic review and meta-analysis. Crit Care. (2023) 27(1):215. doi: 10.1186/s13054-023-04492-9
23. Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z. Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study. J Crit Care. (2019) 49:172–8. doi: 10.1016/j.jcrc.2018.11.003
24. Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat Rev Drug Discov. (2010) 9(9):703–18. doi: 10.1038/nrd2805
Keywords: blood purification, coagulopathy, extracorporeal organ support, hemorrhagic shock, viscoelastometry
Citation: Pertich Á and Lovas A (2024) Case Report: The management of hemorrhagic shock of different origins by target-controlled coagulation and extracorporeal organ support (continuous renal replacement therapy). Front. Anesthesiol. 2:1323180. doi: 10.3389/fanes.2023.1323180
Received: 17 October 2023; Accepted: 28 December 2023;
Published: 18 January 2024.
Edited by:
Gabor Nardai, Semmelweis University, Hungary© 2024 Pertich and Lovas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: András Lovas landras@halasi-korhaz.hu
 Ákos Pertich
Ákos Pertich András Lovas
András Lovas